Magnesium deficiency suppresses cell cycle progression mediated by increase in transcriptional activity of p21Cip1 and p27Kip1 in renal epithelial NRK-52E cells
Abstract
Lack of magnesium suppresses cell growth, but the molecular mechanism is not examined in detail. We examined the effect of extracellular magnesium deficiency on cell cycle progression and the expression of cell cycle regulators in renal epithelial NRK-52E cells. In synchronized cells caused by serum-starved method, over 80% cells were distributed in G1 phase. Cell proliferation and percentage of the cells in S phase in the presence of MgCl2 were higher than those in the absence of MgCl2, suggesting that magnesium is involved in the cell cycle progression from G1 to S phase. After serum addition, the expression levels of p21Cip1 and p27Kip1 in the absence of MgCl2 were higher than those in the presence of MgCl2. The exogenous expression of p21Cip1 or p27Kip1 increased the percentage in G1 phase, whereas it decreased that in S phase. The mRNA levels and promoter activities of p21Cip1 and p27Kip1 in the absence of MgCl2 were higher than those in the presence of MgCl2. The phosphorylated p53 (p-p53) level was decreased by MgCl2 addition. Pifithrin-α, a p53 inhibitor, decreased the p-p53, p21Cip1 and p27Kip1 levels, and the percentage in G1 phase in the absence of MgCl2. Rotenone, a mitochondrial respiratory inhibitor, decreased ATP content and increased the p-p53 level in the presence of MgCl2. Together, lack of magnesium may increase p21Cip1 and p27Kip1 levels mediated by the decrease in ATP content and the activation of p53, resulting in the suppression of cell cycle progression from G1 to S phase in NRK-52E cells. J. Cell. Biochem. 112: 3563–3572, 2011. © 2011 Wiley Periodicals, Inc.
Abbreviations:
CDKs, cyclin-dependent kinases; CDKIs, cyclin-dependent kinase inhibitors; DMEM, Dulbecco's modified Eagle's medium; Mg2+, magnesium ion; PCR, polymerase chain reaction.
Magnesium is the second most abundant divalent cation in mammalian cells and is an essential cofactor for hundreds of enzymes. Metabolism of carbohydrate, lipid, protein, and DNA is controlled at the millimolar level of magnesium ion (Mg2+). Adequate serum Mg2+ concentration may be necessary to maintain renal functions and protect the kidney from various injuries. Hypomagnesemia is often found in the patients with renal failure [Kelepouris and Agus, 1998; Alexandridis et al., 2003]. Magnesium deficiency enhances drug-induced nephrotoxicity [Lajer et al., 2005]. On the contrary, magnesium supplementation protects against post-ischemic acute renal failure [de Araujo et al., 2005], cisplatin-induced cellular injury [Carvalho da Costa et al., 2003], cyclosporine A-induced nephrotoxicity [Pere et al., 2000], and hyperoxaluria-induced apoptosis [Sarica et al., 2004]. Lack of magnesium may inhibit cell proliferation and enhance cellular injury.
Cell proliferation through cell cycle is regulated by sequential activation and subsequent inactivation of a series of cyclin-dependent kinases (CDKs) at different phases of the cell cycle [Draetta, 1990; Sherr, 1994; Jung et al., 2010]. In normal cells, the progression from G1 to S phases requires the activity of CDK2, CDK4, and CDK6. CDKs activity can be inhibited by two different families of cyclin-dependent kinase inhibitors (CDKIs) including INK4 and WAF1/Cip1. INK4 family including p15INK4b, p16INK4a, p18INK4c, and p19INK4d binds to monomeric CDK4/6 and prevents its association with a cyclin D. In contrast, WAF1/Cip1 family including p21Cip1, p27Kip1, and p57Kip2 binds to cyclin D–CDK2, cyclin E–CDK2, cyclin A–CDK2, and cyclin B–CDK1 complexes and inhibits its function. Among them, p21Cip1 and p27Kip1 play a central role as a negative regulator of cell cycle progression.
Cell proliferation is attenuated by a decrease in extracellular magnesium in capillary endothelial cells [Banai et al., 1990], breast epithelial MCF-7 cells [Sgambato et al., 1999], and leukemic HL-60 cells [Covacci et al., 1998]. Extracellular magnesium deficiency increases p27Kip1 in HC11 breast epithelial cells [Sgambato et al., 1999] or p21Cip1 in microvascular 1G11 cells [Bernardini et al., 2005]. The growth of Lewis lung carcinoma tumor is suppressed in magnesium-deficient mice concomitant with the increases in p21Cip1 and p27Kip1 [Maier et al., 2007]. These negative regulators of cell cycle progression may be involved in the inhibition of cell proliferation caused by magnesium deficiency. However, it is unknown whether magnesium deficiency increases the expression of p21Cip1 and p27Kip1 in renal tubular epithelial cells. Furthermore, the regulatory mechanism of p21Cip1 and p27Kip1 expression has not been examined well. In the present study, we examined the effect of extracellular magnesium deficiency on cell cycle progression and the transcriptional activities of p21Cip1 and p27Kip1 using NRK-52E cells derived from rat renal tubule.
MATERIALS AND METHODS
Materials
Mouse anti-p21Cip1, p27Kip1, and p53 antibodies were obtained from Thermo Fisher Scientific (Fremont, CA). Rabbit anti-phosphorylated-p53 (p-p53) antibody was from Merck Chemicals (Darmstadt, Germany). Goat anti-β-actin antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Pifithrin-α (PFT-α) was from Enzo Life Sciences (Plymouth Meeting, PA). 3,8-Diamino-5-[3-(diethylmethylammonio)propyl]-6-phenylphenanthridinium diiodide (PI) was from Dojindo Laboratories (Kumamoto, Japan). Rotenone was from Sigma–Aldrich (Saint Louis, MO). All other reagents were of the highest grade of purity available.
Cell Culture
NRK-52E cells (IFO50480) were obtained from the Japanese Collection of Research Biosciences (Osaka, Japan) and were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal calf serum (FCS, HyClone, Logan, UT), 0.14 mg/ml streptomycin sulfate, and 0.07 mg/ml penicillin G potassium in a humidified environment of 5% CO2–95% air at 37°C. DMEM contains 1 mM MgCl2. Medium without MgCl2 (nominally Mg-free medium) was prepared according to the composition of DMEM. To synchronize cells in G1 phase and reduce intracellular magnesium content, the cells were cultured in the medium containing 0.5% FCS in the absence of MgCl2 for 24 h. Then the cells were cultured in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2 for the period of time indicated.
Measurement of Cell Proliferation and Injury
Cell proliferation was measured with a Cell Counting Kit (Roche Diagnostics, Basel, Switzerland). WST-1 (10 µl) was added in the medium (100 µl) and incubated at 37°C for 60 min. The absorption of WST-1 was measured at 450 and 630 nm using a Multilabel Counter 1420 ARVOsx (Perkin Elmer, Wellesley, MA). The cell proliferation was represented relative to the control values. The culture medium was collected and LDH activity was assessed using a Cytotoxicity Detection Kit (Roche Diagnostics). To measure total LDH activity, viable cells were solubilized with a 1% Triton X-100. Cell injury was represented as percentage of the LDH activity in the culture medium relative to total LDH activity.
Flow Cytometry
After fixation with 70% ethanol, the cells were incubated with 0.2 mg/ml RNase at 37°C for 30 min. The cells were next incubated with 20 µg/ml PI at room temperature for 30 min. Each sample was analyzed by a BD FACSCant II Flow Cytometer (BD Biosciences) and the percentage of the cells in the G1, S, and G2/M phases of the cell cycle was determined using FlowJo (Ashland, OR).
Preparations of Cell Lysates and Western Blotting
Preparation of total cell extract and SDS–polyacrylamide gel electrophoresis were carried out as described previously [Ikari et al., 2001]. Proteins were blotted onto a PVDF membrane and incubated with each primary antibody followed by a peroxidase-conjugated secondary antibody. Finally, the blots were stained with an ECL Western blotting kit from GE Healthcare Bio-Science (Amersham Place, England) or a LumiGLO Reagent and Peroxide from Cell Signaling Technology (Beverly, MA). Representative images were shown from at least three independent experiments. The band density of Western blotting was quantified with Doc-It LS image analysis software (UVP, Upland, CA).
Transfection of Plasmid DNA and Fluorescence Measurement
Total RNA was isolated from NRK-52E cells using TRI reagent (Sigma–Aldrich). First strand cDNA was synthesized from total RNA using M-MLV Reverse Transcriptase and random primers. Polymerase chain reaction (PCR) was performed with initial 2 min denaturation at 94°C, followed by 35 cycles of amplification (0.5 min denaturation at 94°C, 2 min annealing at 54°C, and 2 min extension at 72°C), which final extension for 7 min at 72°C. Primers used in PCR were 5′-CCCTCGAGATGTCCGATCCTGGTGATG-3′ and 5′-CGGGATCCTCAGGGCTTTCTCTTGCAG-3′ (p21Cip1) and 5′-CTCGAGATGTCAAACGTGAGAGTGTC-3′ and 5′-GGATCCGTCTGGCGTCGAAGGCCG-3′ (p27Kip1). These primer pairs containing restriction enzyme sites of Xho I or Bam HI amplified the open reading flame of p21Cip1 (NM_080782.3) and p27Kip1 (AY623040), respectively. The PCR product was cloned into pEGFP-N1 vector (Clontech Laboratories, CA). Sequence analysis was performed by Bio Matrix Research (Chiba, Japan). The pEGFP, pEGFP/p21Cip1, and pEGFP/p27Kip1 vectors were transfected into NRK-52E cells using Lipofectamine 2000 (Invtrogen, Carlsbad, CA) as recommended by the manufacturer. After 24 h of transfection, fluorescence images of EGFP were taken using an Olympus IX70 microscope with acquisition software QCapture Pro.
Real-Time PCR (RT-PCR)
Quantitative RT-PCR was performed using FastStart Universal SYBR Green Master (Roche Diagnostics). The reaction conditions were initial 10 min denaturation at 95°C, followed by 40 cycles of amplification (10 s denaturation at 95°C and 35 s annealing and extension at 60°C). The following primers were used: 5′-CCGTGGACAGTGAGCAGTTGAG-3′ and 5′-GGCGAAGTCAAAGTTCCACCGTT-3′ (p21Cip1), 5′-CTTCCGCCTGCAGAAACC-3′ and 5′-CTTCTCCAAGTCCCGGGTTAG-3′ (p27Kip1), and 5′-CCAACCGTGAAAAGATGACC-3′ and 5′-CCAGAGGCATACAGGGACAG-3′ (β-actin). The threshold cycle (Ct) for each individual PCR product was calculated by the instrument software, and Ct values obtained for p21Cip1 and p27Kip1 were normalized by subtracting the Ct values obtained for β-actin. The resulting ΔCt values were then used to calculate relative change of mRNA expression as the ratio (R) of the mRNA expression of chemically treated cells/ the mRNA expression of the control cells according to the equation R = 2−(ΔCt(chemical treatment) − ΔCt(control)).
Luciferase Reporter Assay
The luciferase reporter vector containing p21Cip1 (pWWP) or p27Kip1 (p27PF) promoter region was kindly gifted from Dr. T. Sakai (Kyoto Prefectural University of Medicine, Japan) [Minami et al., 1997; Nakano et al., 1997]. A Renilla construct, pRL-TK vector (Promega, Madison, WI), was used for normalizing transfection efficiency. Cells were transfected with plasmid DNA using Lipofectamine 2000. After 24 h of transfection, cells were cultured in the presence and absence of magnesium for another 18 h. Then, the luciferase activity was assessed using Dual-Glo Luciferase Assay system (Promega). Luminescence of Firefly and Renilla luciferase was measured by a Multilabel Counter 1420 ARVOsx (Perkin Elmer). The relative promoter activity was represented as the fold induction when compared to the promoterless pGVB2 vector.
Measurement of ATP Content
The cells grown on 96-well plates were cultured in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2 for 3 h. Intracellular ATP content was measured with an ATP assay kit (TOYO B-Net Co., Ltd., Tokyo, Japan) and represented as percentage of that in the presence of MgCl2 relative to that in the absence of MgCl2.
Statistics
Data are reported as means ± SEM. Differences between groups were analyzed with a one-way analysis of variance, and corrections for multiple comparisons were made using Tukey's multiple comparison test. Comparison between two groups was made using Student's t-test. Significant differences were assumed at P < 0.05.
RESULTS
Increase in Cell Proliferation and G1–S Progression in the Presence of Magnesium
To synchronize cells in G1 phase and reduce intracellular magnesium content, the cells were cultured in the medium containing 0.5% FCS in the absence of MgCl2 for 24 h. Then, the cells were cultured in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2 for 24 h. The percentage of cell proliferation was significantly increased by the addition of MgCl2 (Fig. 1A). To examine the effect of magnesium deficiency on cellular injury, we measured the LDH activity in the culture medium. The percentage of LDH release was 15.5 ± 0.6% and 13.3 ± 1.5% (n = 4) in the presence and absence of MgCl2, respectively (Fig. 1B). These results indicate that magnesium deficiency suppressed cell proliferation without affecting cellular injury. Next, we examined the effect of magnesium on cell cycle progression. The percentage in G1 phase was decreased by the addition of 5% FCS in a time-dependent manner, whereas that in S phase was increased (Fig. 1C–E). The addition of MgCl2 enhanced the change of the percentage in G1 and S phases caused by FCS. In contrast, the percentage in G2/M phase was unchanged in the presence and absence of MgCl2. These results indicate that magnesium deficiency suppresses cell cycle progression from G1 to S phase.

Effect of magnesium deficiency on cell proliferation and G1–S progression. NRK-52E cells were cultured in the medium containing 0.5% FCS in the absence of MgCl2 for 24 h. A,B: The cells were incubated in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2 for 24 h. Cell proliferation was measured using WST-1 and expressed relative to the cells incubated in the absence of MgCl2. Cell injury was represented as percentage of the LDH activity in the culture medium relative to total LDH activity. C,D: The cells were incubated in the medium containing 5% FCS in the presence and absence of MgCl2 for period indicated. The percentage in S and G1 phases was analyzed by flow cytometer. E: The cells were incubated in the medium containing 5% FCS in the presence and absence of MgCl2 for 24 h. The percentage in G1, S, and G2/M phases was analyzed by a flow cytometer. **P < 0.01. NS, not significant. n = 4.
Effect of Magnesium on p21Cip1 and p27Kip1 Expression Levels
The effect of magnesium on the expression levels of cell cycle regulator factors, p21Cip1 and p27Kip1, was examined by Western blotting. After the addition FCS, the expression level of p21Cip1 was constant for 24 h and that of p27Kip1 slightly decreased in the absence of MgCl2 (Fig. 2). The expression level of p21Cip1 and p27Kip1 were significantly decreased in the presence of MgCl2 compared with that in the absence of MgCl2. These results indicate that magnesium is involved in the regulation of p21Cip1 and p27Kip1 expression levels in renal epithelial cells.
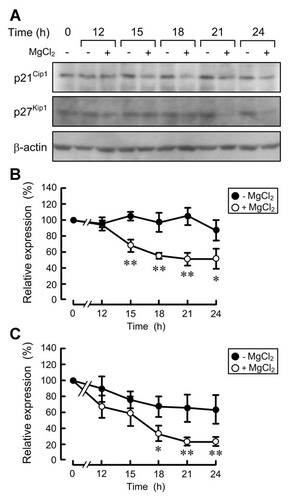
Effect of magnesium deficiency on p21Cip1 and p27Kip1 expression levels. Cells were cultured in the medium containing 0.5% FCS in the absence of MgCl2 for 24 h. Then the cells were cultured in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2. The total cell lysates were prepared in the period indicated and immunoblotted with anti-p21Cip1 and p27Kip1 antibodies. β-actin serves as an internal control for normalization purposes. The relative abundance of proteins was quantified and 0 h was set as 100%. **P < 0.01 and *P < 0.05 compared with the cells in the absence of MgCl2. n = 3.
Decrease in G1–S Progression by Exogenous Expression of p21Cip1 OR p27Kip1
To clarify that p21Cip1 and p27Kip1 play as key regulators of cell cycle progression, EGFP/p21Cip1, or EGFP/p27Kip1 was transiently expressed into NRK-52E cells. The expressions of EGFP (mock), EGFP/p21Cip1, and EGFP/P27Kip1 were identified by a fluorescence microscopy (Fig. 3A). Only the cells expressing EGFP were selected and the percentage in G1, S, and G2/M phases were examined using a flow cytometer. The percentage in G1 phase was increased by the expression of p21Cip1 or p27Kip1, whereas that in S phase was decreased by the expression of these proteins (Fig. 3B,C). In contrast, the percentage in G2/M phases was unchanged by the expression of these proteins. These results indicate that the increase in p21Cip1 or p27Kip1 expression level suppresses cell cycle progression from G1 to S phases.
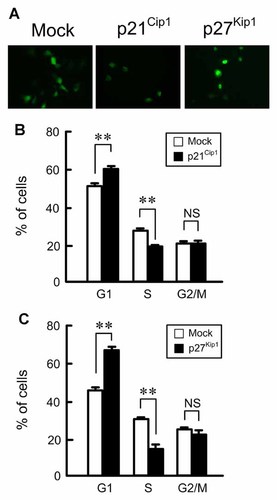
Decrease in the percentage in S phase by the overexpression of p21Cip1 or p27Kip1. A: The EGFP (mock), EGFP/p21Cip1, or EGFP/p27Kip1 vector was transfected in NRK-52E cells using Lipofectamine 2000. After 24 h, the expression of EGFP was determined using a fluorescence microscopy. B,C: The percentage in S, G1, and G2/M phases was analyzed by a flow cytometer in the EGFP, EGFP/p21Cip1, or EGFP/p27Kip1-expressing cells. **P < 0.01. NS, not significant. n = 3.
No Effect of Magnesium Deficiency on the Stability of p21Cip1 and p27Kip1 Proteins
The expression levels of p21Cip1 and p27Kip1 are regulated by the phosphorylation and ubiquitination-dependent degradation [el-Deiry et al., 1994; Slingerland and Pagano, 2000; Bloom et al., 2003]. The phosphorylation level of Thr187 of p27Kip1 in the absence of MgCl2 was not different from that in the presence of MgCl2 (data not shown). To examine the stability of p21Cip1 and p27Kip1 proteins, the expression levels of these proteins were examined in the presence of cycloheximide, a translational inhibitor. The expression levels of p21Cip1 and p27Kip1 were decreased in a time-dependent manner after the addition of 5% FCS (Fig. 4). The rates of decrease in p21Cip1 and p27Kip1 levels in the absence of MgCl2 were not different from those in the presence of MgCl2. These results indicate that magnesium has no effect on the stability of p21Cip1 and p27Kip1 proteins.
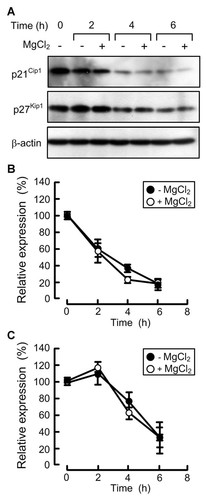
Effect of magnesium deficiency on the stability of p21Cip1 and p27Kip1 proteins. Cells were cultured in the medium containing 0.5% FCS in the absence of MgCl2 for 24 h. Then the cells were cultured in the medium containing 5% FCS and 3 µM cycloheximide in the presence and absence of 1 mM MgCl2. A: The total cell lysates were prepared in the period indicated and immunoblotted with anti-p21Cip1, p27Kip1, and β-actin antibodies. B,C: The band densities of Western blotting were quantified, and then expressed relative to value at 0 h. Open and closed circles show p21Cip1 (B) and p27Kip1 (C) expression in the presence and absence of MgCl2, respectively. β-Actin serves as an internal control for normalization purposes. n = 4.
Decrease in p21Cip1 and p27Kip1 mRNA Levels and Promoter Activities by Magnesium
After synchronizing in the medium containing 0.5% FCS in the absence of MgCl2, the cells were cultured in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2 for 12 h. The mRNA levels of p21Cip1 and p27Kip1 decreased in the presence of MgCl2 compared with those in the absence of MgCl2 (Fig. 5A). These results suggest that magnesium deficiency affects the transcriptional activities of p21Cip1 and p27Kip1. Therefore, we examined the effect of magnesium deficiency on the promoter activities of p21Cip1 and p27Kip1. The promoter activities in the absence of MgCl2 were higher than those in the presence of MgCl2 (Fig. 5B), indicating that the transcriptional activities of p21Cip1 and p27Kip1 are up-regulated by magnesium deficiency.
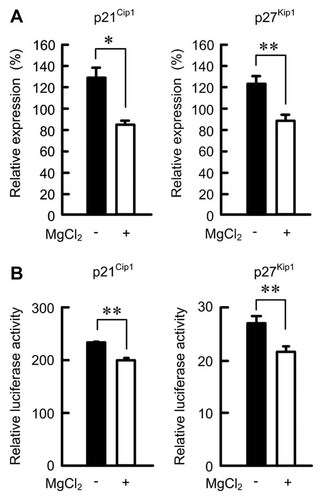
Effect of magnesium deficiency on mRNA levels and promoter activities of p21Cip1 and p27Kip1. A: Cells were cultured in the medium containing 0.5% FCS in the absence of MgCl2 for 24 h. Then the cells were cultured in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2 for 12 h. After isolation of total RNA, quantitative RT-PCR was performed using specific primers for p21Cip1, p27Kip1, and β-actin. The mRNA levels of p21Cip1 and p27Kip1 were normalized to β-actin level and expressed relative to value at 0 h. B: The mock, pWWP, or p27PF vector was co-transfected with pRL-TK vector in NRK-52E cells using Lipofectamine 2000. After 24 h, the cells were cultured in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2 for 24 h. The Firefly luciferase activity of each construct was normalized using Renilla luciferase activity. The relative promoter activity was represented as the fold induction compared to mock. **P < 0.01 and *P < 0.05. n = 4.
Inhibition of Magnesium Deficiency-Induced p21Cip1 and p27Kip1 Elevation by PFT-α
The expression level of p21Cip1 is increased by various stresses including ultra violet, oxidation, and ischemia [Gartel and Tyner, 1999]. The activation of p53 is involved in the increase in p21Cip1 expression. The p-p53 level in the absence of MgCl2 was higher than that in the presence of MgCl2 (Fig. 6A). The activation of p53 is inhibited by PFT-α [Komarov et al., 1999]. PFT-α decreased the p-p53 level in the absence of MgCl2. Similarly, PFT-α decreased the expression levels and promoter activities of p21Cip1 and p27Kip1 caused by magnesium deficiency (Fig. 6B–D). Next, we examined the effect of PFT-α on cell cycle progression from G1 to S phases. PFT-α decreased the percentage in G1 phase, whereas it increased that in S phase in the absence of MgCl2. These results indicate that the activation of p53 by magnesium deficiency is involved in the elevation of p21Cip1 and p27Kip1 expression levels and in the suppression of cell cycle progression from G1 to S phases.
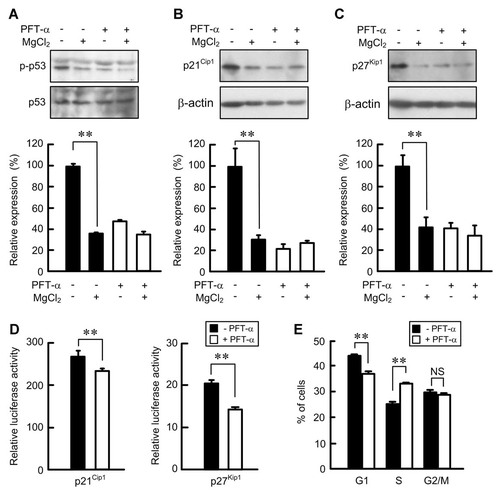
Effect of PFT-α on p-p53 level, the expression and promoter activities of p21Cip1 and p27Kip1, and cell cycle. Cells were cultured in the medium containing 0.5% FCS in the absence of MgCl2 for 24 h. A: The cells were cultured in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2 and PFT-α for 8 h. The total cell lysates were immunoblotted with anti-p-p53 and p53 antibody. The relative abundance of proteins was quantified and control was set as 100%. B,C: The cells were cultured in the medium containing 5% FCS in the presence and absence of MgCl2 and PFT-α for 18 h. The total cell lysates were immunoblotted with anti-p21Cip1 and p27Kip1 antibodies. β-Actin serves as an internal control for normalization purposes. The relative abundance of proteins was quantified and control was set as 100%. D: The mock, pWWP, or p27PF vector was co-transfected with pRL-TK vector in NRK-52E cells. After 24 h, the cells were cultured in the medium containing 5% FCS in the presence and absence of MgCl2 for 18 h. The relative promoter activity was represented as the fold induction compared to mock. E: The cells were cultured in the medium containing 5% FCS in the presence and absence of magnesium for 18 h. The percentage in G1, S, and G2/M phases was analyzed by a flow cytometer. **P < 0.01. NS, not significant. n = 3.
Increase in p-p53 Level by Reduction of ATP Content
The elevation of p-p53 level is induced by DNA damage, hypoxia, oxidative stress, and low ATP content [Kastan et al., 1991; Levine and Oren, 2009; Maclaine and Hupp, 2009]. Intracellular ATP content in the absence of MgCl2 was lower than that in the presence of MgCl2 (Fig. 7A). Rotenone, an inhibitor of the mitochondrial respiratory complex I, decreased ATP content in the presence of MgCl2. Next, we examined the effect of rotenone on p-p53 level. Rotenone increased p-p53 level in the presence of MgCl2 similar to that in the absence of MgCl2 (Fig. 7B,C).
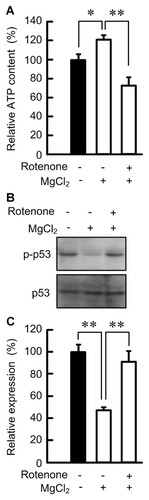
Effects of magnesium deficiency and rotenone on ATP content and p-p53 level. Cells were cultured in the medium containing 0.5% FCS in the absence of MgCl2 for 24 h. A: The cells were cultured in the medium containing 5% FCS in the presence and absence of 1 mM MgCl2 and 5 µM rotenone for 3 h. Intracellular ATP content was represented as percentage relative to that in the absence of MgCl2. B,C: The cells were cultured in the medium containing 5% FCS in the presence and absence of MgCl2 and rotenone for 8 h. The total cell lysates were immunoblotted with anti-p-p53 and p53 antibodies. The relative abundance of proteins was quantified and control was set as 100%. **P < 0.01 and *P < 0.05.
DISCUSSION
Intracellular Mg2+ plays an important role on the metabolism of carbohydrate, lipid, protein, and DNA. Magnesium deficiency inhibited cell proliferation in NRK-52E cells (Fig. 1). Similar results are reported in capillary endothelial cells [Banai et al., 1990], breast epithelial MCF-7 cells [Sgambato et al., 1999], leukemic HL-60 cells [Covacci et al., 1998], and Lewis lung carcinoma cells [Maier et al., 2007]. The percentage in G1 phase in the presence of MgCl2 was lower than that in the absence of MgCl2, whereas that in S phase in the presence of MgCl2 was higher. These findings raise the possibility that magnesium deficiency causes cellular injury, resulting in the decrease in the percentage in S phase. However, LDH release assay revealed that magnesium deficiency has no effect on cell injury. We recently reported that the inhibition of magnesium influx suppresses cell proliferation and cell cycle progression from G1 to S phases [Ikari et al., 2008]. We suggest that the decrease in intracellular Mg2+ concentration suppresses cell cycle progression from G1 to S phases.
Cell cycle is rigorously controlled by the protein expression levels of cell cycle regulators. At the check point of G1–S transition, the activities of CDK2, CDK4, and CDK6 are activated by the binding of cyclin, whereas they are negatively regulated by CDK inhibitors such as p21Cip1 and p27Kip1. We found the expression levels of p21Cip1 and p27Kip1 in the absence of MgCl2 are higher than those in the presence of MgCl2 (Fig. 2). Similarly, it has been reported that extracellular magnesium deficiency increases p27Kip1 in HC11 breast epithelial cells [Sgambato et al., 1999] or p21Cip1 in microvascular 1G11 cells [Bernardini et al., 2005]. Furthermore, cDNA array shows that a magnesium-deficient diet increases the expression levels of p21Cip1 and p27Kip1 in Lewis lung carcinoma tumors grown in mice [Maier et al., 2007]. p21Cip1 is induced by various biological situations including cell cycle arrest [Brugarolas et al., 1995], differentiation [Di Cunto et al., 1998], and apoptosis [Marches et al., 1999]. Maintenance of high p21Cip1 level prevents the activation of cyclin E-Cdk2 [Foster et al., 2001] that function in the progression from G1 to S phases. Overexpression of p21Cip1 inhibits progression from G1 to S phases in vascular smooth muscle cells [Chang et al., 1995] and human diploid fibroblasts [Harper et al., 1993]. Similarly, p27Kip1 has a role on the inhibition of the progression from G1 to S phases. Overexpression of p27Kip1 inhibits progression from G1 to S phase in Saos-2 cells [Toyoshima and Hunter, 1994] and 293 human embryonic epithelial kidney cells [Hurteau et al., 2002]. Our results showed that the percentage in G1 phase is increased by overexpression of p21Cip1 or p27Kip1 in NRK-52E cells (Fig. 3). We suggest that magnesium deficiency maintains an elevated expression of p21Cip1 and p27Kip1, resulting in the suppression of cell cycle progression from G1 to S phases.
The expression levels of p21Cip1 and p27Kip1 are controlled by the transcriptional [Jung et al., 2010; Khattar and Kumar, 2010] and post-transcriptional regulation [el-Deiry et al., 1994; Slingerland and Pagano, 2000; Bloom et al., 2003]. p21Cip1 and p27Kip1 are degraded by proteasome mediated via its phosphorylation and ubiquitination. In the presence of cycloheximide, the expression levels of p21Cip1 and p27Kip1 decreased in a time-dependent manner (Fig. 4). The rates of degradation of these proteins were unaffected by magnesium deficiency, indicating that magnesium is not involved in the post-transcriptional regulation of p21Cip1 and p27Kip1 expression. In the promoter assay, the transcriptional activities of p21Cip1 and p27Kip1 were enhanced by magnesium deficiency (Fig. 5). These results suggest that magnesium deficiency increases p21Cip1 and p27Kip1 levels mediated by the increase in the transcriptional activity, but not by the decrease in the degradation in NRK-52E cells.
The transcriptional activity of p21Cip1 is up-regulated by p53 or other transcriptional factors including Sp1, Sp3, and p300 [Gartel and Tyner, 1999]. In contrast, the regulatory mechanism of transcriptional activity of p27Kip1 is not understood well. p53 up-regulates gene expression by directly binding to a p53-responsive element in the p21Cip1 promoter elements. Since p53 is a sensor for various extrinsic stresses including DNA damage and oxidative stress, these stress signals transcriptionally up-regulate p21Cip1 expression. The p-p53 level decreased in the presence of MgCl2 compared with that in the absence of MgCl2 (Fig. 6). Furthermore, PFT-α decreased the p-p53 level, the promoter activities of p21Cip1 and p27Kip1, and the percentage in G1 phase in the absence of MgCl2. We suggest that magnesium deficiency increases p21Cip1 and p27Kip1 levels mediated by the elevation of the p-p53 level. In HEK293 cells derived from human embryonic kidney, cell proliferation was increased by the addition of MgCl2 and the levels of p-p53, p21Cip1, and p27Kip1 decreased in the presence of MgCl2 compared with those in the absence of MgCl2 (Supplementary Fig. 1). Magnesium deficiency must inhibit cell proliferation concomitant with the increase in the expression levels of cell cycle regulators including p-p53, p21Cip1, and p27Kip1 in renal tubular cells. At present, we do not know whether p53 is directly involved in the regulation of p27Kip1 level.
Various stresses including DNA damage, hypoxia, oxidative stress, and low ATP content induce the elevation of p-p53 level [Kastan et al., 1991; Levine and Oren, 2009; Maclaine and Hupp, 2009]. We recently reported that magnesium deficiency does not increase the production of reactive oxygen species and cellular injury in renal tubular Madin–Darby canine kidney cells [Ikari et al., 2010]. In the present study, we found that intracellular ATP content in the absence of MgCl2 is lower than that in the presence of MgCl2 (Fig. 7). The inhibition of mitochondrial respiration by rotenone decreased intracellular ATP content and increased p-p53 level. We suggest that the decrease in ATP content may be involved in the activation of p53 in the absence of MgCl2. Figure 8 shows a tentative scheme of suppression of cell cycle progression caused by magnesium deficiency.
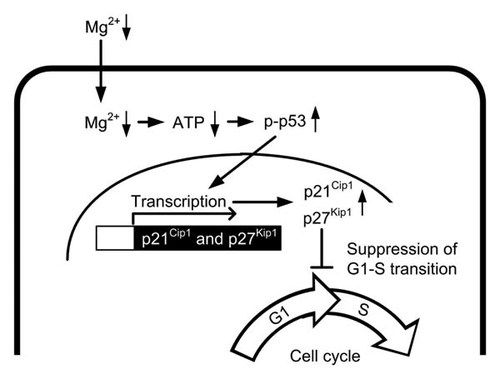
A tentative scheme of suppression of cell cycle progression caused by magnesium deficiency. Magnesium deficiency decreases intracellular ATP content and increases p-p53 level. The transcriptional activities of p21Cip1 and p27Kip1 are increased by p-p53. The elevation of p21Cip1 and p27Kip1 expression levels suppresses cell cycle progression from G1 to S phase.
In conclusion, magnesium deficiency suppressed cell proliferation in NRK-52E cells concomitant with the increase in the percentage in G1 phase and the decrease in that in S phase. The expression levels and promoter activities of p21Cip1 and p27Kip1 in the absence of MgCl2 were higher than those in the presence of MgCl2. Hypomagnesemia is often found in patients with renal failure, which is accompanied by tubular injury [Kelepouris and Agus, 1998; Alexandridis et al., 2003]. In in vivo model, p21Cip1 and p27Kip1 expression levels increases in the renal cells after injury [Megyesi et al., 1996; Park et al., 2000]. Magnesium supplementation protects against post-ischemic acute renal failure and drug induced renal failure [Pere et al., 2000; Carvalho da Costa et al., 2003; de Araujo et al., 2005]. We suggest that adequate Mg2+ is necessary to proliferate tubular cells and restore the damage of renal tubule.
Acknowledgements
This work was supported in part by KAKENHI (20790175), grants from the Salt Science Research Foundation (no. 1124), and Mochida Memorial Foundation for Medical and Pharmaceutical Research (to A.I.).




