High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes
Abstract
Hyperglycemia is well-recognized and has long-term complications in diabetes mellitus and diabetic nephropathy. In podocytes, the main component of the glomerular barrier, overproduction of reactive oxygen species (ROS) in the presence of high glucose induces dysfunction and increases excretion of albumin in urine. This suggests an impaired antioxidant defense system has a role in the pathogenesis of diabetic nephropathy. We studied expression of NAD(P)H oxidase subunits by Western blotting and immunofluorescence and the activities of the oxidant enzyme, NAD(P)H, and antioxidant enzymes, superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), in mouse podocytes cultured in a high glucose concentration (30 mM). We found long-term (3 and 5 days) exposure of mouse podocytes to high glucose concentrations caused oxidative stress, as evidenced by increased expression of Nox4 and activities of NAD(P)H oxidase (Δ 182%) and SOD (Δ 39%) and decreased activities of GPx (Δ −40%) and CAT (Δ −35%). These biochemical changes were accompanied by a rise in intracellular ROS production and accumulation of hydrogen peroxide in extracellular space. The role of Nox4 in ROS generation was confirmed with Nox4 siRNA. In conclusion, high glucose concentration affects the oxidant–antioxidant balance in mouse podocytes, resulting in enhanced generation of superoxide anions and its attenuated metabolism. These observations suggest free radicals may play an important role in the pathogenesis of diabetic nephropathy. J. Cell. Biochem. 112: 1661–1672, 2011. © 2011 Wiley-Liss, Inc.
Diabetic nephropathy clinically manifests as progressively worsening albuminuria with a declining glomerular filtration rate; it is associated with a high cardiovascular mortality and is the most common cause of end-stage renal disease [Mogensen, 2003]. The factors involved in the pathogenesis of diabetic nephropathy are multifaceted [Blézquez-Medela et al., 2010]. One factor is the imbalance of pro- and anti-free radical processes and the formation of excessive free radicals in the kidney [Forbes et al., 2008]. NAD(P)H oxidase is the major source of superoxide anion (O), the predominant form of reactive oxygen species (ROS) in non-phagocytic cells including renal cells such as tubular epithelial cells, mesangial cells [Gorin et al., 2005], and podocytes [Greiber et al., 1998]. The O is metabolized intracellularly by superoxide dismutase (SOD) to H2O2, a relatively stable molecule that readily crosses cell membranes. The intracellular concentration of H2O2 is regulated through enzymatic scavenging by peroxiredoxins, glutathione peroxidases (GPx), and catalase (CAT).
The low amounts of ROS produced by NAD(P)H oxidase may function as secondary messengers to influence the redox-sensitive signal transduction pathway [Soberman, 2003; Rhee, 2006; Burgoyne et al., 2007]. However, when NAD(P)H oxidase is upregulated, the higher amounts of ROS may lead to an imbalance of redox homeostasis and to oxidative damages. Generation of ROS is augmented through the increased formation of advanced glycation end-products, hexosamine pathway flux, mitochondrial dysfunction, and NF-κB or protein kinase C activation [Onozato and Tojo, 2005; Kakehi and Yabe-Nishimura, 2008]. The podocytes play a key role in maintaining the glomerular filtrate barrier and determining its permselective properties. Several investigations have shown that, in the presence of a high glucose concentration, overproduction of ROS in podocytes induces dysfunction and increases excretion of albumin with urine [Neale et al., 1993; Susztak et al., 2006; Ren et al., 2008]. Furthermore, high glucose concentration stimulates hypertrophy of podocytes through ROS-dependent activation of ERK1/2 and Akt/PKB pathways [Gorin et al., 2005; Kim et al., 2006]. Recently, Eid et al. [2009] showed that CYP4A monooxygenases are involved in the apoptosis of podocytes exposed to high glucose concentrations. The activities of antioxidative enzymes such as CAT and Cu/Zn-SOD were enhanced in the kidneys of rat with streptozotocine-induced diabetes [Sechi et al., 1997]. The overexpression of CAT attenuated ROS generation, proapoptotic gene expression, and apoptosis in the kidneys of diabetic mice in vivo [Brezniceanu et al., 2007]. On the other hand, it has been observed that CAT deficient type 2 diabetes patients have life-long increased hydrogen peroxide concentrations, which affects insulin signaling and has cytotoxic effects on pancreatic cells [Góth, 2008].
These findings suggest an impaired antioxidant defense system plays a role in the pathogenesis of diabetic nephropathy. To the best of our knowledge, little is known about the involvement of redox imbalance in diabetic podocytopathy; therefore, we examined the influence of high glucose concentration on the oxidant–antioxidant balance in cultured mouse podocytes. We investigated the production of superoxide anion and hydrogen peroxide and the activities of cellular antioxidant enzymes (CAT, SOD, gluthatione peroxidase) in podocytes in response to high glucose concentrations.
MATERIALS AND METHODS
Cell Culture
Mouse podocytes from a conditionally immortalized cell line were cultured, as described previously [Mundel et al., 1997]. Cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/L), and streptomycin (100 µg/L) in a controlled (air—5% CO2) humidified atmosphere. To propagate podocytes, the culture medium was supplemented with 10 U/ml mouse recombinant γ-interferon (γ-INF) and the cells were cultivated at 33°C to enhance the expression of the temperature-sensitive large T antigen (permissive conditions). To induce differentiation, podocytes were maintained at 37°C without γ-INF (non-permissive conditions) for 1 week. For the different experiments, cells were cultured in normal (NG, 5.6 mM) and high (HG, 30 mM) glucose for 2 h or 1, 3, or 5 days.
Measurement of Intracellular Reactive Oxygen Species
ROS generation was measured with the fluoroprobe, 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA), as described previously [Piwkowska et al., 2010]. This compound is rapidly taken up by the cell and converted by intracellular esterases to 2',7'-dichlorodichydrofluorescein (DCF). The fluorescence emission of DCF was measured using a spectrofluorometer (LS55, Perkin Elmer) with excitation/emission wavelengths set at 485/525 nm. The results of intracellular ROS generation are expressed in arbitrary units (AU).
Extracellular Concentration of Hydrogen Peroxide
The concentration of hydrogen peroxide was measured according to the method described by Mohanty et al. [1997], based on conversion of 10-acetyl-3,7-dihydroxyphenoxazine in the presence of peroxidase and H2O2 to resorufin, the red oxidation product with maximal absorbance at 550 nm.
Podocytes were exposed either to NG or HG for 2 h or 1, 3, or 5 days. Afterwards, the podocytes were washed twice with warm PBS buffer and the reaction mixture containing 50 µM 10-acetyl-3,7-dihydroxyphenoxazine and 0.1 U/ml HRP was added for 20 min (37°C, air—5% CO2 in the dark). The reaction mixture without HRP was used as a background value. For each experiment, the amount of extracellular H2O2 production was determined using a H2O2 standard curve at substrate concentrations ranging from 0.1 to 5 µM.
Antioxidant Enzyme Activities
Podocytes were exposed either to NG or HG for 2 h or 1, 3, or 5 days. Afterwards, podocytes were washed twice with cold PBS and lysed on ice with agitation in 50 mM potassium phosphate, 0.1 mM EDTA, 0.1% Triton X-100 (pH 7.8). Extracts were centrifuged at 12,000 × g for 20 min at 4°C and supernatants were used to measure the enzyme activities. Protein content was measured with the Lowry method.
CAT (EC 1.11.1.6) activity was assayed according to Aebi [1984] by measuring the absorbance decrease (60 s) in a reaction medium containing 10 mM H2O2, 50 mM potassium phosphate, 0.1 mM EDTA, pH 7.0. Calculations were based on ε = 0.0436 mM/cm for H2O2 at 240 nm. Total SOD (EC 1.15.1.1) activity was determined by the method by Ukeda et al. [1997] by inhibition of tetrazolium salt WST-1 reduction to formazan at 440 nm with xantine/xantine oxidase used as a superoxide generator. One unit of SOD was defined as the amount of protein that inhibits the rate of tetrazolium salt WST-1 reduction by 50%. GPx (EC 1.11.1.9) activity was determined following the method of Paglia and Valentine (1967). Podocyte homogenates (50–100 µg protein) were pre incubated for 10 min (at 37°C) in buffer (pH 7.6) containing 50 mM Tris–HCl, 5 mM EDTA, 1 mM reduced glutathione, 1U glutathione reductase, 1 mM NaN3, 0.15 mM NADPH. The reaction was started by the addition of 0.15 mM H2O2. The change in absorbance within the first 5 min of the reaction was monitored. Calculations were based on ε = 6.22 mM/cm for NADPH at 340 nm. The non-enzymatic rate of NAD(P)H consumption was correspondingly assessed in each assay.
NAD(P)H Oxidase Assay
NAD(P)H oxidase activity in podocytes was measured by the lucigenin-enhanced chemiluminescence method [Münzel et al., 2002] with modifications [Piwkowska et al., 2010]. To measure superoxide anion production, 200 µl cells homogenates (50 µg protein) were added to 290 µl of PBS buffer, containing 1 mM EDTA and 20 µM lucigenin. The assay was initiated by adding 100 µM NADH (10 µl). Photon emission, in terms of relative light units, was measured every 30 s for 12 min in a FB12 luminometer (Berthold). There was no measurable activity in the absence of NADH. The enzyme accepts both NADH or NADPH [Greiber et al., 1998; Shiose et al., 2001]. The amounts of superoxide were calculated by integrating the area under the signal curve. These values were compared with a standard curve generated using xanthine/xanthine oxidase, as described previously [Münzel et al., 1996]. Protein content was measured with the Lowry method.
To verify the specificity of the assay system we used various inhibitors of ROS production. Incubation podocytes with 100 µM apocynin (NAD(P)H oxidase inhibitor) decreased activity of NAD(P)H oxidase about 45% (from 3.28 ± 0.29 to 1.82 ± 0.08 nmol O/mg protein/min; Figure 1, P < 0.05). The changes of NAD(P)H oxidase activity were not detected in the presence of other enzymes inhibitors; 100 µM allopurinol (xanthine oxidase inhibitor), 100 µM L-NAME (nitric oxide synthase inhibitor), or 50 µM rotenone (mitochondrial respiratory chain complex I inhibitor, Fig. 1).
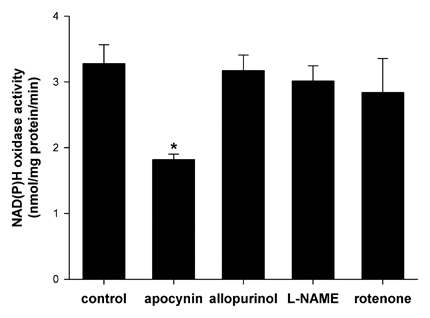
The effect of various inhibitors of reactive oxygen species production on NAD(P)H oxidase activity in mouse podocytes. Cells were incubated with normal glucose concentration (5.6 mM) for 2 h in the presence of apocynin (100 µM), allopurinol (100 µM), L-NAME (100 µM), rotenone (50 µM), or buffer (control). Activity of NAD(P)H oxidase was measured by lucigenin-enhanced chemiluminescence. Values are the means ± SEM (n = 3). *P < 0.05 versus control.
RNA Interference and Cell Transfection
Small interference RNA (siRNA) for Nox4 and a non-silencing siRNA (scrambled siRNA, negative control) were synthesized by Santa Cruz Biotechnology. Podocytes were seeded at a density of 9 × 104/well on type-I collagen coated six-well plates (Becton Dickinson Labware, Beckton, UK) and cultured in RPMI 1640 supplemented with 10% FBS. One day before transfection, the culture medium was removed and cells were cultivated in antibiotic-free RPMI 1640 supplemented with 10% FBS. The cells were transfected with siRNA using siRNA Transfection Reagent (Santa Cruz Biotechnology) according to the manufacturer's instructions. Briefly, Nox4 siRNA or scrambled siRNA were diluted in Transfection Medium (final concentration, 80 nM), mixed with siRNA Transfection Reagent and incubated 30 min at room temperature. The transfection mixture was added to the Transfection Medium, mixed gently and added to the cells and after 7 h a growth medium containing twofold higher FBS and antibiotics concentration was added. The cells were incubated additional 24 h. After transfection, gene silencing was monitored at the protein level by Western blotting.
For the different experiments, transfected cells were cultured in normal (NG, 5.6 mM) and high (HG, 30 mM) glucose concentration for 5 days.
Preparation the Membrane and Cytosolic Fraction
Podocytes were washed twice with ice-cold PBS and homogenized in a lysis buffer (30 mM Tris, pH 7.5, 10 mM EGTA, 5 mM EDTA, 1 mM DTT, 250 mM sucrose) in the present of a protease inhibitor cocktail (Sigma–Aldrich). The lysates were centrifuged at 9,500 × g for 10 min at 4°C and supernatant was ultracentrifuged at 60,000 × g for 30 min at 4°C. Afterwards supernatant was used as a cytosolic fraction and pallet was resuspended with lysis buffer (without sucrose), solubilized with 1% Triton X-100 (membrane fraction). The protein expression of p47phox (membrane and cytosolic fraction) was further examined by Western blot analysis.
Western Blotting Analysis
Podocytes were treated with lysis buffer (20 mM Tris, 140 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Nonidet P-40) in the presence of a protease inhibitor cocktail (Sigma–Aldrich) and then homogenized at 4°C by scraping. The homogenates were centrifuged at 9,500 × g for 20 min at 4°C and supernatants were used as sample proteins. Equal amounts of protein, containing 20 µg, were separated on a SDS–polyacrylamide gel (10%) and transferred to a nitrocellulose membrane. The membranes were blocked for 1.5 h with Tris-buffered saline (TBS, 20 mM Tris–HCl, 140 mM NaCl, 0.01% NaN3) containing 3% non-fat dry milk, washed with TBS containing 0.1% Tween-20 and 0.1% BSA, and incubated overnight at 4°C with specific antibodies: anti-actin (1:3,000; Sigma), antibodies to antioxidant enzymes (anti-SOD1, 1:1000; anti-SOD2, 1:1000; anti-SOD3, 1:400; anti-GPx1, 1:300; anti-CAT, 1:400; Santa Cruz Biotechnology) and antibodies to NAD(P)H oxidase subunits (anti-p22phox, anti-p47phox, anti-p67phox, anti-Nox1, anti-Nox2, and anti-Nox4; 1:400; Santa Cruz Biotechnology) in TBS containing 0.05% Tween-20 and 1% BSA. Immunodetection was accomplished by incubating the membrane for 2 h with the secondary antibody labeled with alkaline phosphatase (goat anti-rabbit IgG-AP, goat anti-mouse IgG-AP, and donkey anti-goat IgG-AP; Santa Cruz Biotechnology). The bands were detected using the colorimetric 5-bromo-4-chloro-3-indolylphasphate/nitroblue tetrazolium (BCIP/NBT) system. The density of the corresponding bands was measured quantitatively using the Quantity One program (Bio-Rad). Protein content was measured using a Lowry method.
Immunofluorescence
Podocytes were seeded on type-I collagen coated coverslips (Becton Dickinson Labware) and cultured in RPMI 1640 supplemented with 10% FBS. Five days before each experiment, the culture medium was removed and cells were cultivated in RPMI 1640 containing a normal (5.6 mM) or high (30 mM) glucose concentration. After incubation, cells were fixed in PBS containing 2% formaldehyde for 10 min at room temperature. Coverslips were placed on ice and cells were permeabilized with 0.3% Triton-X 100 for 3 min and then blocked with PBSB solution (PBS containing 2% FBS, 2% BSA, and 0.2% fish gelatin) for 60 min. After blocking, cells were incubated with anti-p22phox, anti-p47phox, and anti-Nox4 antibodies in PBSB (1:100) at 4°C for 1 h. For non-specific staining, the primary antibodies were substituted with PBSB solution. Next, cells were washed three times with cold PBS and then incubated with Cy3 conjugated anti-mouse (1:150) or Alexa 488 conjugated anti-rabbit (1:75) secondary antibodies for 45 min, followed by three 5-min washes. Coverslips were attached to slides with Mowiol 4-88 diluted in glycerol-PBS (1:3 v/v) and cells were viewed under the fluorescence microscope. Double staining was achieved by incubating with primary antibodies (anti-p22phox and anti-Nox4) and with the appropriate conjugated secondary antibodies, as above.
Statistical Analysis
All data are expressed as means ± SEM. Statistical significance between groups was analyzed by one-way ANOVA followed by the Student–Newman–Keuls multiple comparison test. A P-value < 0.05 was considered significant.
RESULTS
Effect of Glucose on ROS Production in Live Podocytes
We evaluated DCF sensitive ROS production in podocytes exposed to either a normal (NG, 5.6 mM) or high (HG, 30 mM) glucose concentration. A 2-h incubation (short-term) of cells in HG led to a significant increase (∼ 36%) in ROS production (from 163 ± 4 to 219 ± 8 AU, P < 0.01, Fig. 2A). To examine the long-term effects of HG on intracellular ROS generation, three incubation times (1, 3, and 5 days) were used. A significant decrease (Δ −32%, P < 0.05) in ROS production was observed on day 1 and then intracellular ROS increased about 45% and 90% versus the control at day 3 and 5, respectively. Exposure of podocytes to mannitol for 2 h had no effect on ROS generation (Fig. 2B).
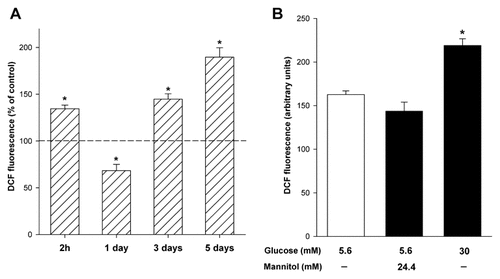
The effect of high glucose concentration on intracellular production of ROS in mouse podocytes. A: Mouse podocytes were exposed to either NG (5.6 mM) or HG (30 mM) for the indicated time periods. B: Mouse podocytes were treated with HG (30 mM) and NG (5.6 mM) with or without mannitol (22.4 mM) for 2 h. ROS generation was measured by DCF. Values are the means ± SEM (n = 5). *P < 0.05 HG versus NG. DCF, 2',7'-dichlorodichydrofluorescein; HG, high glucose concentration; NG, normal glucose concentration; ROS, reactive oxygen species.
Expression of NAD(P)H Oxidase Subunits in Podocytes Cultured in a High Glucose Concentration
Expression of NAD(P)H oxidase subunits in mouse podocytes was confirmed at the protein level using specific antibodies (Fig. 3). Furthermore, the influence of long-term (5 days) exposure of podocytes to HG on the expression of NAD(P)H oxidase subunits was evaluated. The bands detected at ∼22, ∼50, ∼90, and ∼65 kDa corresponded to the membrane-bound subunits: p22phox, Nox1, Nox2, and Nox4, respectively; bands at ∼47 and ∼67 kDa corresponded to cytosolic subunits: p47phox and p67phox. Only expression of Nox4 protein increased, about 30% (P < 0.05), in HG. There was a tendency for p22phox and p47phox proteins expression to be increased in HG; however, significance was not achieved.
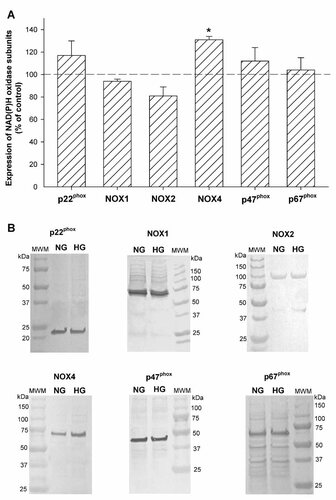
The effect of high glucose concentration on the expression of NAD(P)H oxidase subunits in mouse podocytes. Mouse podocytes were exposed to either NG (5.6 mM) or HG (30 mM) for 5 days. Expression of p22phox, Nox1, Nox2, Nox4, p47phox, and p67phox proteins were determined by Western blotting analysis on homogenized podocytes. A: Densitometric analysis of NAD(P)H oxidase subunits expression. Values are the means ± SEM (n = 3–6). *P < 0.05 HG versus NG. B: A representative immunoblots. MWM, molecular weight marker; NG, normal glucose concentration; HG, high glucose concentration.
Activation of NAD(P)H oxidase is associated with the translocation of cytosolic components to the cell membrane; therefore, we investigated the cellular distribution of p47phox protein in NG and HG. As shown in Figure 4A HG markedly enhanced p47phox protein content in membrane fraction, about 95% (P < 0.05), and decrease in cytosolic fraction, about 21% (P < 0.05). Also, as shown by fluorescence, staining for the p47phox protein was markedly increased in plasma membrane in HG (Fig. 4C) compared to NG (Fig. 4D).
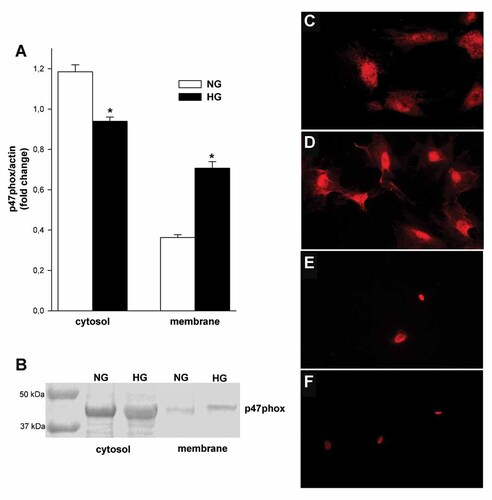
The influence of high glucose concentration on expression and translocation of p47phox protein in mouse podocytes. A: Results of Western blot analysis of cytosolic and membrane fractions. Values are the means ± SEM (n = 4). *P < 0.05 versus NG. B: Representative immunoblots for cytosolic and membrane fractions. Representative photomicrographs of immunostaining for p47phox in 5.6 mM (C) and 30 mM (D) glucose after 5 days incubation. Non-specific staining of podocytes after the substitution of primary antibodies with blocking buffer in 5.6 mM (E) and 30 mM (F) glucose, as described in the Materials and Methods section. NG, normal glucose concentration.
Double labeling of p22phox and Nox4 showed co-localization of these molecules in the cytoplasm and plasma membrane in NG (Fig. 5A–C) and HG (Fig. 5D–F).
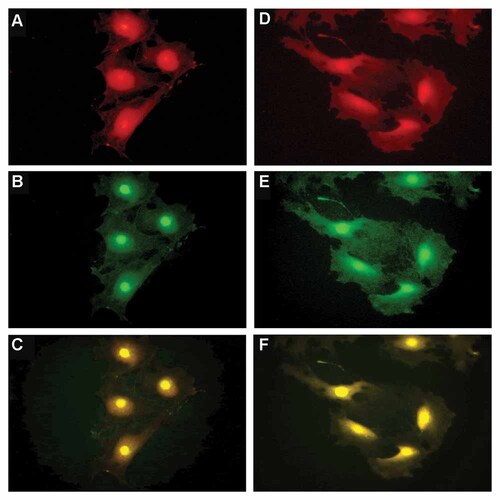
The influence of high glucose concentration on subcellular distribution of p22phox and Nox4 in mouse podocytes—double immunolabeling. Double staining of p22phox (red) and Nox4 (green) in 5.6 mM (A–C) and 30 mM (D–F) glucose, as described in the Materials and Methods section.
Nox4 siRNA Downregulates Nox4 Protein Expression in Podocytes
To investigate the role of Nox4 in HG-induced ROS production, Nox4 protein expression was knocked down by small-interfering RNA (siRNA). The significant decrease in Nox4 protein expression was observed in podocytes transfected with Nox4 siRNA in NG (∼46%) and HG (∼40%; Fig. 6, P < 0.05) compared to scrambled siRNA. Nosilencing siRNA (scrambled siRNA) had no effect on Nox4 expression in NG and HG.
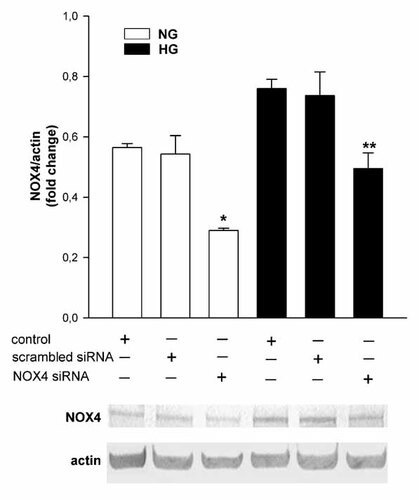
Effect of Nox4 small-interfering RNA (siRNA) and scrambled siRNA on NOX4 protein expression in mouse podocytes after 5 days incubation in normal (NG) and high (HG) glucose concentration. Densitometry of Nox4 expression is normalized to that of actin. Values are the means ± SEM (n = 4). *P < 0.05 versus other groups in NG. **P < 0.05 versus other groups in HG (upper panel). Representative immunoblots of Nox4 and actin expression in the homogenate from transfected podocytes (lower panel). NG, normal glucose concentration; HG, high glucose concentration.
Generation of Superoxide Anion by NADP(H) Oxidase
We tested whether exposure of podocytes to HG for various time periods (2 h—short-term, 1, 3, and 5 days—long-term exposure) influenced NAD(P)H oxidase-dependent superoxide production. Short-term incubation (2 h) induced a rapid increase in NAD(P)H oxidase-dependent O production by 117%, compared to NG (Fig. 7A, P < 0.05). The longer incubations in HG caused augmentation of O production by 176%, 182%, and 177% (Fig. 7A, P < 0.05) on day 1, 3, and 5, respectively. Therefore, we determined the effect of Nox4 siRNA on NAD(P)H oxidase activity in podocytes exposed to either NG (5.6 mM) or HG (30 mM) for 5 days. Dowregulation of Nox4 was associated with decrease activation of NAD(P)H oxidase by 39% and 66% (Fig. 7B, P < 0.05) in NG and HG, respectively.
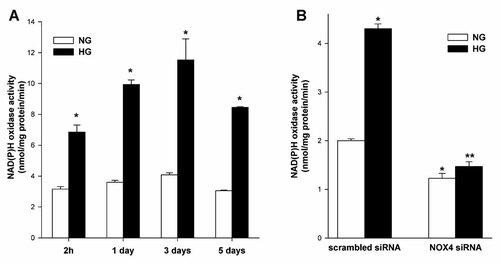
The effects of high glucose concentration (A) and downregulation of Nox4 (B) on NAD(P)H oxidase activity measured by lucigenin-enhanced chemiluminescence in mouse podocytes. A: Mouse podocytes were exposed to either NG (5.6 mM) or HG (30 mM) for the indicated time periods. Values are the means ± SEM (n = 4). *P < 0.05 HG versus NG. B: Nox4 small-interfering RNA (siRNA) or scrambled siRNA transfected podocytes were exposed to either NG (5.6 mM) or HG (30 mM) for 5 days. Values are the means ± SEM (n = 3). *P < 0.05 versus scrambled siRNA NG, **P < 0.05 versus scrambled siRNA HG. NG, normal glucose concentration; HG, high glucose concentration.
Extracellular Concentration of Hydrogen Peroxide in Podocytes Incubated in a High Glucose Concentration
The basal extracellular concentration of H2O2 was 0.81 ± 0.04 µM. To analyze whether HG exerts an effect on extracellular H2O2 concentration, podocytes were incubated for 2 h and 1, 3, and 5 days in HG. The short-term (2 h) incubation did not influence the extracellular H2O2 concentration, but longer incubation (>1 day) led to an increase in the extracellular concentration of H2O2. The concentration of H2O2 increased about 303% (3.61 ± 0.05 vs. 0.86 ± 0.04 µM, P < 0.05, Fig. 8A) on day 5. Next, we examined the effect of Nox4 siRNA on the production of hydrogen peroxide in podocyte. RNA interference knockdown of Nox4 decrease (Δ 21%, P < 0.05, Fig. 8B) extracellular concentration of H2O2 induced by high glucose.
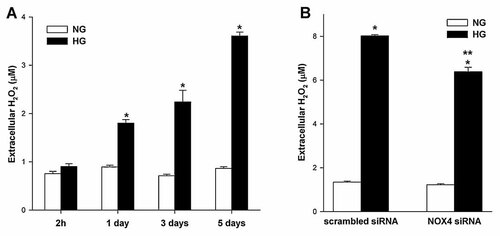
The effects of high glucose concentration and downregulation of Nox4 expression on the extracellular concentration of hydrogen peroxide in mouse podocytes. A: Mouse podocytes were exposed to either NG (5.6 mM) or HG (30 mM) for the indicated time periods. Values are the means ± SEM (n = 5). *P < 0.05 HG versus NG. B: Nox4 small-interfering RNA (siRNA) or scrambled siRNA transfected podocytes were exposed to either NG (5.6 mM) or HG (30 mM) for 5 days. Values are the means ± SEM (n = 4). *P < 0.05 versus scrambled siRNA NG, **P < 0.05 versus scrambled siRNA HG. NG, normal glucose concentration; HG, high glucose concentration.
Influence of High Glucose Concentration on the Protein Expression and Activity of Antioxidant Enzymes
Expression of antioxidant enzymes in mouse podocytes was confirmed at the protein level using specific antibodies (Fig. 9). Furthermore, the influence of long-term (5 days) exposure of podocytes to HG on the expression of superoxide dismutase (SOD-1, cytosolic; SOD-2, mitochondrial, SOD-3, extracellular), glutathione peroxidase (GPx-1), and CAT was evaluated. Only expression of CAT protein decreased about 42% (P < 0.05) in HG. Subsequently, we examined the effect of Nox4 siRNA on the CAT expression level in HG. It was found that downregulation of Nox4 significantly blocked decrease of CAT expression in HG (Fig. 10), suggesting the contribution of Nox4 on the regulation of CAT expression.
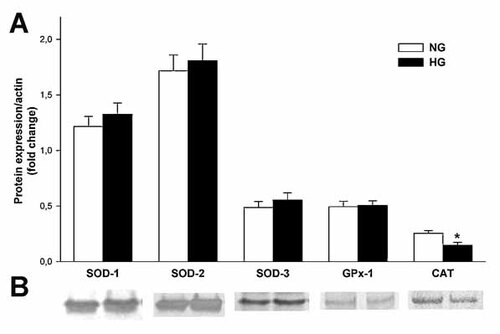
The effect of high glucose concentration on the expression of antioxidant enzymes in mouse podocytes. Mouse podocytes were exposed to either NG (5.6 mM) or HG (30 mM) for 5 days. Expression of superoxide dismutases (SOD-1, SOD-2, SOD-3), glutathione peroxidase (GPx-1), and catalase (CAT) were determined by Western blotting. A: Densitometric analysis of antioxidant enzymes expression. Values are the means ± SEM (n = 4–6). *P < 0.05 versus NG. B: A representative immunoblots for SOD-1 (23 kDa), SOD-2 (25 kDa), SOD-3 (32 kDa), GPx-1 (92 kDa), and CAT (64 kDa). NG, normal glucose concentration; HG, high glucose concentration.
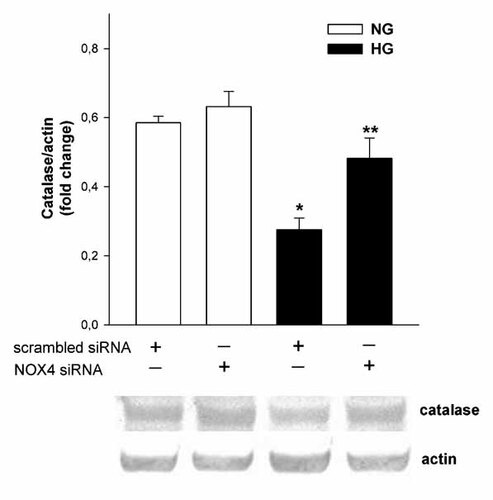
The effect of Nox4 gene silencing on the catalase protein expression in mouse podocytes after 5 days incubation in normal (NG; 5.6 mM) and high (HG; 30 mM) glucose concentration. Analysis of catalase protein levels in Nox4 small-interfering (siRNA) and scrambled siRNA transfected podocytes. Values are the means ± SEM (n = 4). *P < 0.05 versus other groups. **P < 0.05 versus scrambled siRNA HG (upper panel). Representative immunoblots of catalase and actin expression in the homogenate from transfected podocytes (lower panel). NG, normal glucose concentration; HG, high glucose concentration.
The activities of superoxide anion radical-scavenging enzymes, SOD, GPx, and CAT are presented in Figure 11. The basal enzymes activities in NG were: SOD; 2.78 ± 0.06 U/mg protein, GPx; 24.53 ± 0.53 nmol/mg protein/min, and CAT; 8.12 ± 0.19 µmol/mg protein/min.
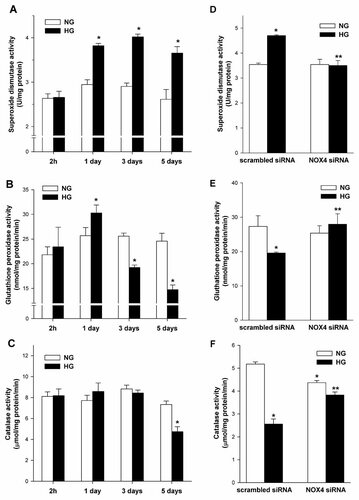
The effects of high glucose concentration and downregulation of Nox4 expression on the activity of antioxidant enzymes in mouse podocytes. The activity of superoxide dismutase (A,D), gluthatione peroxidase (B,E), and catalase (C,F) were measured as described in the Material and methods section. Mouse podocytes were exposed to either NG (5.6 mM) or HG (30 mM) for the indicated time periods (left panel) and Nox4 small-interfering RNA (siRNA) or scrambled siRNA transfected podocytes were exposed to either NG (5.6 mM) or HG (30 mM) for 5 days (right panel). Values are the means ± SEM (n = 3). *P < 0.05 versus scrambled siRNA NG, **P < 0.05 versus scrambled siRNA HG. NG, normal glucose concentration; HG, high glucose concentration.
Then, we evaluated the influence of HG on the activities of SOD, GPx, and CAT in podocytes incubated for various time periods (2 h and 1, 3, and 5 days). The SOD activity increased about 30% (3.82 ± 0.05 vs. 2.95 ± 0.11 U/mg protein, P < 0.05) on day 1 and was maintained at a similar level on days 3 and 5 (Fig. 11A). In response to HG, GPx activity increased about 118% (30.3 ± 1.6 vs. 25.6 ± 1.6 nmol/mg protein/min, P < 0.05) on day 1. The longer incubation times reduced the activity of GPx about 25% (19.2 ± 0.5 vs. 25.6 ± 0.6 nmol/mg protein/min, P < 0.05) and 40% (14.7 ± 0.9 vs. 24.6 ± 1.6 nmol/mg protein/min, P < 0.05) on day 3 and 5, respectively (Fig. 11B). The CAT activity in podocytes incubated in HG did not change significantly after 3 days of incubation (Fig. 11C), but decreased about 35% (4.74 ± 0.47 vs. 7.33 ± 0.34 to µmol/mg protein/min; P < 0.05) by day 5.
Finally, we examined the effect of downregulation of Nox4 on the activity of these enzymes after 5 days incubation in HG. It was found that HG significantly increased SOD activity and this effect was blocked by Nox4 siRNA (Fig. 11D). RNA interference knockdown of Nox4 also significantly blocked the HG-induced decrease activity of gluthatione peroxidase and catalse in podocytes (Fig. 11E,F).
DISCUSSION
The increased production of ROS, associated with hyperglycemia, plays an important role in the development and progression of diabetic nephropathy. This is represented, among other effects, by morphological and functional changes in podocytes [Lee et al., 2003; Forbes et al., 2008]. The final concentration of ROS in cell compartments and/or extracellular space is dependent on the balance between the rate of ROS generation and its degradation—(oxidant–antioxidant balance). The oxidant arm of this balance is formed mainly by NAD(P)H oxidase generating superoxide anion (O) and the antioxidant arm is formed by SOD, GPx, and CAT. Disturbance of the oxidant-antioxidant balance may affect intracellular and extracellular signaling pathways and finally cell function.
The present study demonstrated that long-term exposure of mouse podocytes to high glucose concentration causes oxidative stress, as indicated by increased activities of NAD(P)H oxidase (Δ 182%) and SOD (Δ 39%) and decreased activities of GPx (Δ −40%) and CAT (Δ −35%). Together, these biochemical changes led to an overall rise in intracellular ROS production and accumulation of H2O2 in podocytes. We also demonstrated the importance of Nox4 as a source of ROS in normal and high glucose concentration, and potential role of Nox4 in regulation of antioxidant enzymes in podocytes.
We observed that podocytes are able to produce ROS in a normal glucose concentration. It has been previously shown that Nox4, a member of the NAD(P)H oxidase protein family, is highly expressed and constitutively active in kidneys [Brown & Griendling, 2009]. Nox4 requires p22phox for enzyme activity and we have shown co-localization of Nox4 and p22phox in the podocyte plasma membrane. Moreover, with the significant rise in ROS generation (mainly O) in high glucose concentration medium, we also observed enhanced expression of Nox4 protein. This is in accordance with recent findings that Nox4 might be inducible [Serrander et al., 2007]. To confirm that high glucose concentration stimulates ROS generation through Nox4-based NAD(P)H oxidase, Nox4 was downregulated using small-interfering RNA. This strategy resulting in about 66% decrease in Nox4 protein expression in podocytes was sufficient to prevent HG-stimulated activation of NAD(P)H oxidase and production of ROS. Moreover, Nox4 siRNA downregulates NAD(P)H oxidase activity in normal glucose concentration supporting the hypothesis for its constitutive function. We also detected other proteins of the Nox family, that is, Nox1 and Nox2, in podocytes. For full enzyme activity, these proteins need to associate with cytoplasmic proteins, mainly p47phox and p67phox [Nauseef, 2008]. We confirmed expression of both proteins in mouse podocytes. Furthermore, our results suggest that high glucose concentration induces translocation of p47phox from cytoplasm to the membrane.
It is proposed that increased production of O, under pathophysiological conditions, is compensated for by the enhanced ability of cells to metabolize O . SOD is located intracellular (SOD-1; cytosolic, SOD-2; mitochondrial matrix) and extracellular (SOD-3), and catalyzes the dismutation of superoxide anion to H2O2. Subsequently, it is converted to H2O in peroxisomes by CAT and in the cytoplasm by GPx. We have shown that total SOD activity is increased in HG conditions and this effect is prevented by Nox4 siRNA; however, we were not able to detect significant changes in protein expression of SOD-1, SOD-2, and SOD-3 in HG. Taken together, our results suggest allosteric regulation of SOD activity in HG, probably due to compensation the increased NAD(P)H oxidase-dependent O generation. The threshold of cell protection against the increased production of O varies dramatically as a function of activities of SOD, GPx, and CAT [Michiels et al., 1994]. Interestingly, cells with increased levels of SOD are hypersensitive to oxidative stress rather than able to protect against it [Michiels et al., 1994]. In high concentrations of glucose, increased SOD activity led to an approximately fourfold enhanced formation of H2O2, which was not efficiently converted to H2O, as indicated by inadequate activity of CAT and GPx in podocytes. Thus, it seems possible that in vivo podocytes might be an important source of hydrogen peroxide in urine. It has been shown that the urinary hydrogen peroxide level is significantly increased in diabetic mice and adiponectin plays an important role in kidney protection, especially in podocytes, during oxidative stress [Sharma et al., 2008].
Expression of antioxidant enzymes is particularly high in the kidney compared with several other organs [Marklund, 1984; Kakkar et al., 1995]. However, oxidative injury has been reported in several models of renal diseases [Nath et al., 1994], especially in glomerular diseases [Shah, 1989], suggesting glomeruli are a main target for oxygen radical attack.
We investigated the dynamics of the change in activities of key antioxidant enzymes, GPx and CAT [Matés et al., 1999], in response to high glucose concentrations in podocytes. We observed the significant increase in GPx activity on day 1 of incubation in high glucose concentration. This effect is probably responsible for the decrease (Δ −32%) in overall intracellular ROS production for this incubation time. Furthermore, GPx activity significantly decreased on day 5 of incubation, however, we did not detect changes in GPx protein expression. The effect on GPx activity was prevented by Nox4 downregulation. The short-time increase in GPx activity suggests there could be compensatory mechanisms in response to initial oxidative stress in the cells. An increase in GPx mRNA in the presence of high concentrations of H2O2 has been reported [Shull et al., 1991], but under non-diabetic conditions. It should be noted that there are contradictory reports regarding the effect of diabetes-induced hyperglycemia on GPx activity in kidneys [Tagami et al., 1992; Kakkar et al., 1995; Catherwood et al., 2002; Limaye et al., 2003]. The differences are probably consequences of various degrees of diabetes.
Many studies have been published on the role of CAT in the antioxidant defense system [Brezniceanu et al., 2007; Góth, 2008]. CAT expression is highly tissue specific with the highest levels found in the liver, kidney, and blood [Brezniceanu et al., 2007]. In our study, we have detected CAT protein expression in podocytes and its activity was about 8 µmol H2O2/min/mg protein. The basal CAT activity varies from 250 to 300 µmol H2O2/min/mg protein in mouse kidney tissue [Kang et al., 1996; Brown-Borg & Rakoczy, 2000]. This indicates that CAT activity is about 30-fold higher in kidney tissue than in mouse podocytes, suggesting podocytes are particularly susceptible to oxidative damage. The results of our experiments with downregulation of Nox4 have provided evidence for the key role of CAT in antioxidant defense. Although, Nox4 knockdown by siRNA prevented NAD(P)H oxidase-dependent O generation, the extracellular H2O2 concentration was only slightly decreased in HG. Moreover changes of SOD and GPx in HG were also prevented by Nox4 siRNA in HG. Of importance, CAT protein expression and its activity were only partially affected by Nox4 siRNA. Taken together, these results suggest that antioxidant balance, at least in mouse podocytes under high glucose concentration, is determined rather by processes of ROS utilization than ROS production. This suggestion is supported by findings where overexpression of CAT in experimental models of type 2 diabetic nephropathy appears to be protective [Brezniceanu et al., 2007].
In conclusion, we have shown that high glucose concentration affects the oxidant–antioxidant balance in mouse podocytes, resulting in enhanced generation of superoxide anion and its attenuated metabolism. These observations suggest that free radicals may play an important role in the pathogenesis of diabetic nephropathy.




