Defining the hematopoietic stem cell niche: The chicken and the egg conundrum
Abstract
Understanding the in vivo regulation of hematopoietic stem cells (HSCs) will be critical to identifying key factors involved in the regulation of HSC self-renewal and differentiation. The niche (microenvironment) in which HSCs reside has recently regained attention accompanied by a dramatic increase in the understanding of the cellular constituents of the bone marrow HSC niche. The use of sophisticated genetic models allowing modulation of specific lineages has demonstrated roles for mesenchymal-derived elements such as osteoblasts and adipocytes, vasculature, nerves, and a range of hematopoietic progeny of the HSC as being participants in the regulation of the bone marrow microenvironment. Whilst providing significant insight into the cellular composition of the niche, is it possible to manipulate any given cell lineage in vivo without impacting, knowingly or unknowingly, on those that remain? J. Cell. Biochem. 112: 1486–1490, 2011. © 2011 Wiley-Liss, Inc.
Since the advent of bone marrow transplant in the 1950s we have learnt much about cell intrinsic programs and regulators of hematopoiesis [Thomas et al., 1957; Appelbaum, 2007; Orkin and Zon, 2008]. The isolation, characterization, and purification of cytokines have added greatly to the treatment options for patients with hematological disease and to our understanding of the regulation of hematopoiesis [Bradley et al., 1967; Metcalf, 1990, 2010]. The isolation of highly purified populations of hematopoietic stem cells (HSCs) and progenitors and their in vitro culture has opened new avenues to understand the genetic regulation of HSC fate and lineage determination. All of these developments have greatly enhanced our understanding of the intrinsic regulation of hematopoiesis. The physical environment, the niche in which HSCs localize, provides extrinsic signals that tightly regulate the production of differentiated cells required for sustenance of the hematopoietic system.
Hematopoiesis in situ
HSCs emerge from the vessels of the aorta-gonads mesonephros, move to the fetal liver, and as development proceeds migrate to the bone marrow cavities coinciding with the formation of bone. Under steady-state conditions adult HSCs reside within the bone marrow cavity. This space is composed of a network of cell types of several origins including mesenchymal-derived elements such as osteoblasts and adipocytes, vasculature, nerves, and a range of hematopoietic progeny of the HSC [reviewed in greater detail in Purton and Scadden, 2008] (Fig. 1). The increasing sophistication with which genetic manipulation can be performed in the mouse has rapidly improved our knowledge of the contribution of these bone marrow elements to the regulation of HSCs in both a physiological and pathological context.
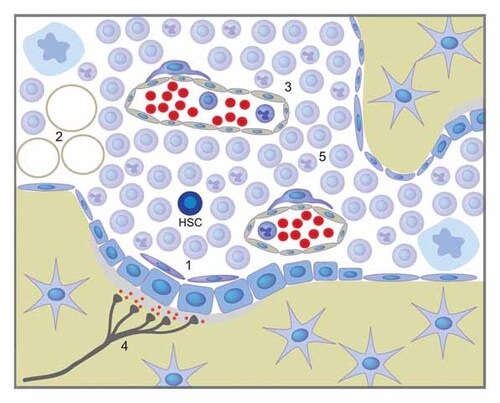
Composition of the bone marrow microenvironment. The bone marrow microenvironment is comprised of cell types of several origins including mesenchymal-derived elements like (1) cells of the osteoblastic lineage and (2) adipocytes, as well as (3) endothelial cells, (4) the sympathetic nervous system, and (5) hematopoietic accessory cells.
The bone marrow microenvironment consists of numerous niches including those that support HSCs. These niches are diverse, specialized for the development of particular blood cell lineages. For example, it was described in the 1950s that erythroid cells were found in distinctive loci in the rat bone marrow [Bessis, 1958]. These observations were confirmed by electron microscopy and by reconstructions of serial sections of rat bone by Weiss [1965] and Mohandas and Prenant [1978]. Recent three-dimensional reconstructions of the bone marrow using confocal-based imaging have revealed that the erythroblastic islands are found in the intratrabecular space and, as demonstrated in the 1970s, are always associated with a macrophage [Takaku et al., 2010]. Furthermore, specialized bone marrow niches have been described for megakaryocyte platelet shedding, and distinctive sites in the bone marrow support different stages of B-lymphoid development [Nagasawa, 2006; Junt et al., 2007]. These data demonstrate that cell–cell interactions and paracrine factors produced from a range of different cells type can have important supportive and stimulatory effects on distinct lineages.
Extending on these observations was the concept of a hematopoietic inductive microenvironment which could influence the fate of progenitors/HSCs [Wolf and Trentin, 1968; Wolf, 1979]. These represented specific structures within the hematopoietic organs that supported and regulated blood cell production. Through a series of studies using electron microscopy and transplantation approaches it was demonstrated that the inductive environment varied between bone marrow, spleen, and thymus and that there was a spatial organization which characterized hematopoiesis in the bone marrow [Curry et al., 1967; Wolf and Trentin, 1968; Weiss, 1976]. They also provided evidence that the microenvironments within these organs were not only supportive of hematopoiesis, but also were instructive and provided positive regulatory cues to the outcome of HSC activity.
HOW DO WE DEFINE THE HSC NICHE?
Elegant studies in the Drosophila germ line have provided an in vivo model of the niche. Somatic cap cells provide the niche to support the germ cells, with evidence of bidirectional communication between the cells [Davies and Fuller, 2008]. The analysis of stem cell–niche interactions in the Drosophila germ line very clearly defines a single niche cell type supporting a single stem cell. This model appears unlikely to be conserved in the hematopoietic system.
An important consideration in any discussion of the composition of the HSC niche is how to define the niche? Is localization of purified HSC/progenitor fractions at early time points postinjection sufficient to identify a location as a niche? In the case of these approaches in non-conditioned recipients there is no evidence of functional engraftment of these cells based on contribution of transplanted cells to hematopoiesis [Nilsson et al., 1997; Czechowicz et al., 2007]. In such a scenario, is the transplanted cell occupying a niche if there is no evidence of function? Furthermore, studies localizing the HSCs at early time points after transplantation into irradiated recipients may also not reveal the true niche, as the bone marrow microenvironment cells are rapidly and dramatically altered by the irradiation procedure [DeGowin et al., 1981; Wathen et al., 1981; Wathen et al., 1982] (M.A and L.P. unpublished work). A more stringent criterion to define the niche, such as the location where a cell is functionally regulated and can contribute to hematopoiesis, may be required to define the in vivo environment. Experimentally the later is technically difficult and may not be currently possible. In vivo genetic labeling strategies and in vivo imaging techniques are rapidly improving and now allow monitoring of transplanted cells over days and a number of divisions [Lo Celso et al., 2009]. These approaches are most likely to conclusively yield the functional HSC microenvironment, the most correct definition of the HSC niche.
NON-HEMATOPOIETIC ELEMENTS OF THE HSC NICHE
The introduction of long-term bone marrow cultures of HSCs and primitive progenitors gave indication that non-hematopoietic elements collectively termed “stroma” were able to positively regulate and sustain HSCs [Dexter et al., 1973]. The nature and cellular origin of these stromal cells was not well-defined, but later work highlighted the in vitro supportive capacity of cells of the osteoblastic lineage [Taichman and Emerson, 1994]. These and many subsequent studies gave evidence that cells of the mesenchymal lineage, in particular cells of the osteoblastic lineage [reviewed in Askmyr et al., 2009] and more recently mesenchymal stem/progenitor cells [Sacchetti et al., 2007; Mendez-Ferrer et al., 2010], are involved in the regulation of HSCs.
Evidence for an in vivo role of osteoblastic lineage cells has also been presented using a range of approaches. Expansion of the osteoblastic lineage by stimulation of the parathyroid hormone receptor pathways, either genetically or pharmacologically, leads to increased frequencies of HSCs [Calvi et al., 2003; Adams et al., 2007]. Genetic mutations in osteoblastic cells that leads to loss or altered function are associated with negative effects on HSC homeostasis and retention in the marrow space [Zhang et al., 2003; Visnjic et al., 2004]. Whilst these genetic models have provided support for the in vivo roles of osteoblastic cells in the niche, evidence that osteoblasts are not required is also present. Biglycan-deficient animals have significantly reduced bone mass and an osteoporosis-like phenotype. However, despite these changes to the skeletal contribution to the bone marrow environment the mice do not show any significant changes in hematopoiesis or HSC function or frequency [Kiel et al., 2007]. It was recently proposed that developmentally endochondrial ossification, bone formation that proceeds through a cartilage intermediate, is necessary to form an HSC supportive microenvironment [Chan et al., 2009]. These observations were made using kidney capsule grafts of isolated skeletal cell populations. Contrasting these results is the presence of HSCs and robust hematopoiesis in the calvaria, which evolves through a process of intramembranous ossification [Lo Celso et al., 2009].
Most recently mesenchymal stem cells (MSCs) have been posited to form a key component of the HSC microenvironment. Nestin-positive cells with characteristics of MSCs were shown to localize close to HSCs in vivo [Mendez-Ferrer et al., 2010]. Depletion of the Nestin-positive population adversely affected HSC retention in the bone marrow, and was associated with the sympathetic nervous system [Mendez-Ferrer and Frenette, 2007]. The in vivo evidence for the function of Nestin-positive cells as candidate MSCs is, however, incomplete. When using a lineage tracing approach with constitutive Nestin-Cre and a Rosa26 reporter line there were few cells of the skeletal lineage labeled, suggesting that if these cells are MSC-like cells they represent a small subset of the population. These cells also demonstrated low clonal efficiency in vitro. In fact, the Nestin-positive cells share many characteristics with osteo-adipogenic progenitors that have been shown to play a role in the HSC niche [Omatsu et al., 2010], including high expression of the HSC retaining chemokine CXCL12.
With age, human long bones become filled with adipocytes and are no longer hematopoietically active. Adipocytes, which arise from a common progenitor shared with the osteoblastic lineage, have been reported to act as negative regulators in the HSC microenvironment [Naveiras et al., 2009]. On the contrary, adipose tissue can support aspects of hematopoiesis in states of stress, as was observed in RARγ-deficient animals [Walkley et al., 2007], and have also been reported to secrete factors that positively regulate hematopoiesis [Lanotte et al., 1982]. Although the role of adipocytes and their products in the bone marrow milieu is not clearly defined, the significant negative correlation between active hematopoiesis and numbers of adipocytes would most strongly support the interpretation that under homeostasis they contribute negatively to hematopoiesis and the HSC microenvironment.
The endothelial compartment is also part of the bone marrow microenvironment of HSCs. Co-localization studies using phenotypic markers of highly purified HSCs (SLAM) demonstrated that HSCs associated with the vasculature at a greater frequency than the osteoblastic endosteal surfaces in the bone marrow [Kiel et al., 2005]. Using genetic models it has been found that changes in the endothelial compartment impact on HSCs [Butler et al., 2010; Kobayashi et al., 2010] and these studies collectively suggest that the endothelial elements in the bone marrow are contributing to the regulation of HSCs and hematopoiesis.
HEMATOPOIETIC CONTRIBUTION TO THE HSC MICROENVIRONMENT
As noted above, interlineage regulation and co-ordinated responses are a central part of the homeostatic mechanisms controlling the bone marrow microenvironment. Osteoclasts, which are hematopoietic in origin and derived from a monocytes precursor, constitute an important element regulating skeletal mass, and have been implicated to play a role in regulating the bone marrow microenvironment. This may occur through induction of bone remodeling, but may also relate to functions of the osteoclasts directly in the HSC microenvironment. Furthermore, three groups have recently shown that macrophages are important in the in vivo regulation of the HSC microenvironment [Winkler et al., 2010; Chow et al., 2011; Christopher et al., 2011]. Macrophages have been observed in close interaction with the osteoblastic cells lining the bone surface, and disruption of this interaction by depletion of the macrophages leads to HSC mobilization and egress from the marrow [Winkler et al., 2010]. This phenotype is not dissimilar to that observed with conditional ablation of the osteoblasts themselves [Visnjic et al., 2004].
How other cells of the hematopoietic lineage contribute to the HSC microenvironment remains largely unknown. However, both T-cells and monocytes have been described as accessory cells contributing to the niche by producing factors that positively and negatively influence HSC fate [reviewed in Mayani et al., 1992]. These cells also compete for growth factors, a scenario that was recently proposed as part of the mechanism of HSC regulation in mice with elevated numbers of megakaryocytes and platelets [de Graaf et al., 2010]. This last observation is intriguing and use of better genetic models where lineage restricted deletions are used will allow separation of the role for the gene in HSC intrinsic maintenance programs and progenitor phenotypes.
CAN WE ISOLATE THE ROLE OF THE DIFFERENT COMPONENTS OF THE MICROENVIRONMENT IN VIVO?
An important issue to the interpretation of these murine models of the niche is that in vivo there is a tight nexus coupling osteoblastic, adipogenic, MSCs, and vasculature elements of the bone marrow microenvironment. Developmentally, and during tissue repair, the modeling of the marrow space is a co-ordinated process involving both vessels and mesenchymal elements [Schipani et al., 2009; Maes et al., 2010]. There is a close proximity between bone, vessels, and hematopoietic cells, making it hard to separate the roles of the different niche components (Fig. 2). In vivo imaging of mouse calvaria demonstrates that HSCs localize within functional proximity to bone surfaces and endothelial cells [Lo Celso et al., 2009]. These observations, coupled with the extensive vascularization of the bone, suggest that this may also be true in the long bones where the majority of HSC localize in murine models.
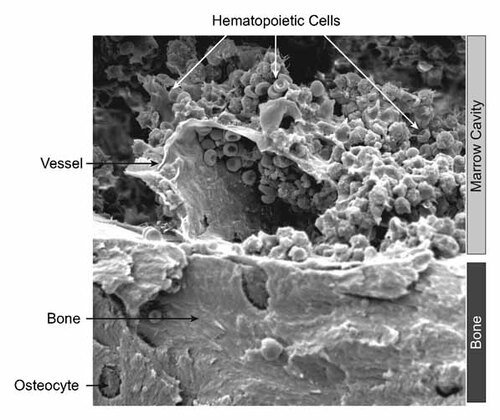
Scanning electron microscopy of the murine bone marrow. Analysis of hematopoiesis in situ in the bone marrow of mice, demonstrating close proximity of the different components of the bone marrow microenvironment including bone, vessels, and mature hematopoietic cells.
Interpretation of results generated using models where lineage restricted deletions have not been used needs to be cautioned, and the roles for mutated genes in HSC intrinsic maintenance programs cannot be discounted. Importantly, it remains unclear how modulating one cell lineage, such as genetic modulation of the osteoblastic lineage, will impact on the function and supportive capacity for HSCs of the other cell types within the bone marrow microenvironment. Changes to the cytokine/growth factor milieu, systemic factors produced in response to local changes and changes in cellular composition of the microenvironment could all impact on both the HSCs as well as the other cellular components of the microenvironment. Furthermore, how the hematopoietic supportive capacity of these cells is affected by changes in the levels of growth factors, cytokines, and chemokines in the local environment induced in these genetic models is yet unknown and largely uncharacterized.
CONCLUSIONS
The increasing sophistication of in vivo imaging techniques and genetic manipulation in the mouse has rapidly improved our knowledge of the HSC microenvironment and the contribution of several niche elements including osteoblasts, adipocytes, and MSCs, as well as vasculature, nerves, and a range of hematopoietic cells to the regulation of HSCs. However, HSCs are very rare and it remains technical difficult to prove that the HSCs localized in proximity to different niche elements are actually functionally regulated and can contribute to hematopoiesis. Furthermore, an important issue to the interpretation of murine models of the niche is that there is a tight nexus coupling the separate elements of the bone marrow microenvironment in vivo. It remains to be determined how modulating one niche component will in turn impact on the function and HSC supportive capacity of the other cell types within the niche.
Acknowledgements
We apologize to those whose work could not be cited due to reference limits. We thank Jack Martin for helpful discussion and comment. Research support from: Swedish Research Council (S.S. and M.A.); Swedish Tegger Foundation (S.S.); NHMRC Senior Research Fellow (L.P.); NHMRC Career Development Award (C.W.); Leukaemia Foundation Phillip Desbrow Senior Research Fellowship (C.W.); NHMRC (L.P. and C.W.), Baker Foundation (C.W.), Association for International Cancer Research (L.P.).




