Directed neural differentiation of duck embryonic germ cells†
Lin-Feng Li and Chun-Yu Bai contributed equally to this article.
Abstract
Although the avian primordial germ cells (PGCs) have been used to produce transgenic birds, their characteristics largely remain unknown. The isolation, culture, biological characterization, and directed neural differentiation of duck EG cells were assayed in this study. The Results showed that the EG cells were got by isolating embryonic gonad and surrounding tissue from 7-day-old duck embryo. The PGCs co-cultured with their gonadal somatic cells were well grown. After passaging, the EG cells were incubated in medium with cytokines and Mitomycin C on inactivated duck embryonic fibroblasts (DEFs) feeder layers. After several passages, alkaline phosphatase (ALP) and periodic acid-Schiff (PAS) resulted positive, cellular markers detection positive for SSEA-1, SSEA-4, TRA-1–60, and TRA-1–81. Karyotype analysis showed the EG cells kept diploid condition and the hereditary feature was stable in accordance with varietal characteristics of duck. These cells grew continuously for 11 passages on DEFs. Under induction of medium with BME, RA, and IBMX, the EG cells lost undifferentiated state, large amount of neural cells appeared with the formation of neural cells networks. Special Nissl body was found by toluidine blue stain after induced for 7 days. Immunofluorescence staining results indicated that differentiated EG cells expressed Nestin, NSE, and GFAP positive. The expression of Nestin, NSE, and GFAP mRNA were positive by RT-PCR. The results revealed that RA can obviously promote the directed differentiation of duck EG cells into neural lineage. The duck EG cells will be useful for the production of transgenic birds, for cell replacement therapy and for studies of germ cell differentiation. J. Cell. Biochem. 112: 1514–1523, 2011. © 2011 Wiley-Liss, Inc.
Primordial germ cells (PGCs) are embryonic precursors of the gametes of adult animals. PGCs originate from the epiblast [Eyal-Giladi et al., 1981] are located at the center of the area pellucida at stage X, and are translocated anteriorly to the germinal crescent [Tagami and Kagami, 1998]. They then migrate through the developing blood vascular system to the germinal ridges (future gonads). PGCs are separated and placed on a feeder layer with cytokines, they become cultured pluripotent cell lines called embryonic germ (EG) cells. Like embryonic stem (ES) cells, EG cells are capable of proliferation and self-renewal and have the capacity to differentiate in vitro into all cell types, as they can also contribute to the germ line of chimeras.
The isolation of pluripotent cells has generated enormous interest as a novel resource with which to investigate aspects of early development and pursue ambitious strategies of organ regeneration and transplantation [Bruce et al., 2007]. Because PGCs can in principle serve as a source of many cell types, it could yield highly effective in vitro models for use in drug discovery programs, and provide a renewable source of cells for use in transplantation therapy. In recent years, PGCs have been widely used as an ideal vector to produce transgenic avian and chimera [Yasuda et al., 1992; Ono et al., 1998; Jeong and Han, 2002]. But the limited source of PGCs greatly influenced the efficacy of subsequent studies. Therefore, how to get a great number of PGCs is critical to success of these studies.
The avian embryo represents an important model system in developmental and cell biology because of its ease of manipulation and its similarity to mammalian development. In addition, the production of transgenic birds using early embryos is an important technology in both fundamental and applied avian biology. The simple composition of the egg proteins ensures the feasibility of the avian as a bioreactor. Furthermore, the avian has a low maintenance cost, small body size, short generation time, and the size of the avian egg permits ease of manipulation in vitro, therefore, the avian is an ideal animal model for diverse types of neurological research.
It is well known that one of the most important challenges in stem cell research is to understand and control cell-differentiation processes. To date, it has been proven that ES cells are capable of directed differentiation into neural precursors and progenies in mammals [Schulz et al., 2003; Bibel et al., 2004; Dhara et al., 2008]. However, similar studies on EG cells in mammals and other species are few. Unfortunately, other than some handful reports of spontaneous differentiation in chick, in vitro directed differentiation of avian EG cells has not to be fully exploited [Pain et al., 1996; Wang et al., 2009].
To date, prolonged propagation of undifferentiated ES cells has historically relied on mouse embryonic fibroblasts (MEFs) feeder layer or in conditioned medium from MEFs feeder layer. Removal of feeder layer or at least MEFs conditioned medium, results in spontaneous, unpredictable differentiation of ES cells. Recently, our group purified a population of EG cells from duck embryos. These EG cells can be supported on MEFs and have typical EG cells characteristics such as continuous growth, expression of cell surface markers, and normal karyotype [Guan et al., 2010]. However, duck EG cells cultured on MEFs have been contaminated with heterogeneity cell, protein, and unknown pathogenic microorganism in culture. In order to eliminate the risk of contamination, attempts had been made to find the same species feeder layer substitute for the MEFs. Following up our prior work, in this study, we used duck embryonic fibroblast (DEFs) as a feeder layer instead of MEFs to support in vitro growth of EG cells. The establishment and differentiation of EG cell line will provide a paradigm for studying the molecular basis of reproductive engineering in vitro. Thereby, the aim of this study was to discuss the influencing factors of isolation and culture, induce duck EG cells to differentiate into neural lineage. To characterize the differentiated EG cells, we examined their morphology and analyzed several neural markers at different passages after induction by means of histochemistry and immunofluorescence methods, which provides a theoretical foundation and a technological method for the utilization of EG cells. They provide an in vitro model of early embryonic differentiation and create new opportunities to manipulate the genome of duck for agricultural and pharmaceutical applications.
MATERIALS AND METHODS
Experimental Materials
Fertilized eggs were obtained from Beijing Duck maintained at the Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, China. All ducks were treated in accordance with NIH and USDA guidelines for the use of animals in research and all experimental procedures involving ducks were conducted in accordance with the protocols and guidelines for agricultural animal research imposed by the Committee for Ethics of Beijing, China.
Preparation of Duck Embryonic Fibroblasts (DEFs) Feeder Layer
Fibroblast cells were isolated from 9 to 11 days duck embryos and cultured with Dulbecco's modified Eagle's medium (DMEM, Invitrogen) containing 10% fetal bovine serum (FBS, Gibco BRL, Germany). The dissociation method was same to that for PGCs. Subculture was carried out after 24 h primary culture when fibroblast cells reached confluence at the bottom of the plate. The medium was removed from culture after three-generation subculture, and cells were treated for 1–2 h with mitomycin C (Sigma) at 10 mg/ml. Cells were rinsed five times with PBS and then culture again with fresh medium. The mouse embryo fibroblast cells (MEFs) were used as a control.
Isolation, Culture, and Biological Characterization of Duck EG Cells
The isolation, culture, and biological characterization of duck EG cells (including evaluation of alkaline phosphatase (ALP) activity, Periodic Acid-Schiff Staining, Growth dynamical analysis, karyotype analysis, immunofluorescence staining of surface antigens, and formation of embryoid bodies (EBs) and spontaneous differentiation in vitro and in vivo) were operated according to the methods of Guan et al. [2010].
Induced Differentiation of EG Cells Into Neural Lineage
After EBs were formed with DMEM, 20% FBS and 5.5 × 10−5 M β-mercaptoethanol (Sigma), EBs were cultured in neural stem cell selective medium with 2% B27 (Invitrogen) and 20 µg/L basic fibroblast growth factor (bFGF, Sigma), for 7 days, then cells were cultured in neural stem cell differential medium with DMEM, 10% FBS, 5.5 × 10−5 M β-mercaptoethanol, 1.0 × 10−6 mol/L RA (Sigma), and 2.5 mg/ml Insulin (Invitrogen). The morphological changes of EBs were monitored every 12 h, half of the medium was replaced daily with fresh medium. After 5 days, EBs of diameter 250–350 µm were dissociated and re-plated 20 EBs/well on a fresh 24-well plate as monolayer with neural stem cell differential medium.
Characterization of Induced Neural Lineage
A combination of methods, including immunofluorescence staining, Nissl body, and RT-PCR was used to characterize neural cells.
The immunofluorescence staining was the same as that mentioned above. The primary antibodies were Nestin, NSE, CNP, and GFAP (Chemicon). The secondary antibodies were conjugated with FITC and Cy5 (Chemicon), respectively. For the control group, 0.01 M PBS was used to replace primary Nestin, NSE, and GFAP antibodies. Ten non-overlapped visions (×100) were randomized from induced stained cells, followed by the same data processing as that mentioned above.
For Nissl bodies detection, the cells were fixed for 30 min with 4% (w/v) paraformaldehyde/PBS, and then stained for 3 min with 0.5% toluidine blue.
Total RNA was extracted from the differentiated EG cells using a Qiagen RNeasy kit (Qiagen). Standard reverse transcription reactions were performed with 300 ng of total RNA with random hexamers using SuperScripte First-Strand Synthesis System (Invitrogen). The PCR was performed using a taq PCR master mix (Takara, Japan). Primer sequences (forward and reverse) and the length of the amplified products are listed in Table I. After incubation for 5 min and initial denaturation for 30 s at 94°C, 30 amplification cycles were performed (annealing for 30 s at 61°C, extension for 30 s at 72°C), and this was followed by a final extension for 7 min at 72°C. Successful PCR was confirmed by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining. To exclude the possibility of the presence of genomic DNA, we performed control reactions without the RT step. In RT-PCR assay, the RNA isolated from brain of 12.5 days duck embryo was used as a positive control.
| Gene | Primers | Products (bp) |
|---|---|---|
| Nestin | F: GAATCGCTGCAGATGTGGGA | 900 |
| R: CAGGCTGAGGGACATCTTGAG | ||
| NSE | F: GCCATCTTGGGTGTATCTC | 345 |
| R: CCAGGGCTTCTTTATTCT | ||
| GFAP | F: CAGGAGACCGAGGAGTGGT | 530 |
| CTCCTGCTTGGACTCCTTGA | ||
| β-Actin | F: TCTTGGGTATGGAGTCCTG | 331 |
| R: TAGAAGCATTTGCGGTGG |
- F, forward primer; R, reverse primer. β-Actin (331 bp) was used as inner control.
Statistical Analysis
Statistical analyses of the data were performed with a one-way ANOVA followed by the Tukey–Kramer honestly significant difference (HSD) test for the three sets of results. A P-value of less than 0.05 was considered significant. Statistical analyses were done with a JMP® Statistical Discovery Software (SAS Institute, Cary, NC).
RESULTS
Isolation, Culture, and Characterization of Duck PGCs and EG Cells
PGCs were characterized by larger size, larger nucleus and high glycogen content, compared with somatic cells. Most EG cells exhibited an ES-like phenotype, namely, a large translucent nucleus, a dome-shaped colonies containing a large nucleus with prominent nucleoli and a relatively small amount of cytoplasm. All EG cells attached to each other and generally grew in colonies with a well-defined border.
DEFs displayed as a typical long spindle shape (Fig. 1A). The primary cell growth slowed, after passaging, growth accelerated. MEFs also showed a fibroblast-like morphology, but the growth of MEFs was slower than DEFs after passaging. The EG cells cultured on DEFs were successfully grown as MEFs. Furthermore, MEFs could maintain EG cells in an undifferentiated state only until passage 7, whereas DEFs can be used to support the growth of duck EG cells until passage 11.
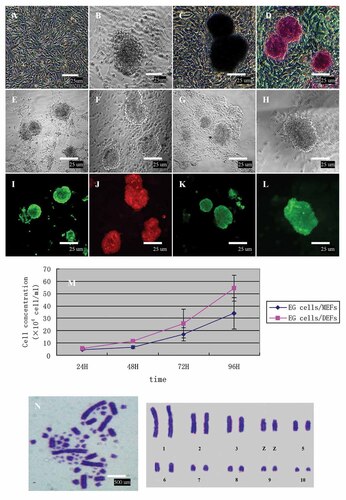
Morphology and biological characterization of duck EG cells. A: DEFs. DEFs displayed as a typical long spindle shape. B: Duck EG cells derived from PGCs after culture 24 h. EG cells were a dome-shaped colonies, the colonies are uniformly round, multilayered, and well delineated. C: Positive ALP staining of EG cells colonies (BCIP/NBT). D: Positive PAS staining of EG cells colonies. E–H: Phase-contrast micrograph of EG cells at 3rd passage. I–L: Immunofluorescence staining of EG cells at 3rd passage surface markers, SSEA-1, SSEA-4, TRA-1–60, and TRA-1–81 staining in a colony formed, respectively. All cells in the colony appeared to be positive, these results indicated duck EG cells were in an undifferentiated state. Scale bar = 25 µm. M: The growth curves of EG cells were observed for comparison. The growth curve of the EG cells on different-feeder layer appeared as a typical “S” shape. The population doubling time of EG cells/DEFs and EG cells/MEFs were 29.69 ± 0.24 and 32.76 ± 0.11 h, respectively (P < 0.05). N: Chromosome at metaphase (left) and karyotype (right) of EG cells (♂). 2n chromosomes number of EG cells was 78, consisting of 10 pairs of macrochromosomes and 29 pairs of microchromosomes, while the sex chromosome type was ZZ (♂). Scale bar = 500 µm.
The colonies of EG cells were passaged and seeded on mitomycin C-treated DEFs at an interval of 5–6 days (Fig. 1B). The EG cells colonies exhibited a mosaic appearance with loosely packed cells. These colonies were maintained for up to 11 passages.
In this study, duck PGCs and EG cells had very strong ALP activity (Fig. 1C). Duck PGCs can be easily identified using the PAS reaction which stains for glycogen in the cytoplasm. After passages, duck EG cells could still be stained by PAS reaction (Fig. 1D). Immunofluorescence staining results showed that the expressions of SSEA-1 (Fig. 1E,I), SSEA-4 (Fig. 1F,J), TRA-1–60 (Fig. 1G,K), and TRA-1–81 (Fig. 1H,L) were strongly positive in duck PGCs-derived EG cells. However, most of the colonies were lost prior to completion after 11 passages, they rapidly lost the expressions of pluripotent cell markers (data not shown).
The cell growth curve of EG cells/DEFs was similar to that of EG cells/MEFs. The cell growth curves appeared as typical “S” shape (Fig. 1M). The population doubling time of EG cells/DEFs and EG cells/MEFs were 29.69 ± 0.24 and 32.76 ± 0.11 h, respectively (P < 0.05) (Table II). These results indicated the EG cells proliferated faster on DEFs than on MEFs.
| Cell concentration (cell/ml) | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|
| EG cells/MEFs | 4.46 ± 0.38 × 104 | 6.41 ± 0.82 × 104 | 1.704 ± 0.45 × 105 | 3.401 ± 0.94 × 105 |
| EG cells/DEFs | 5.78 ± 0.19 × 104 | 1.148 ± 0.43 × 105 | 2.569 ± 0.96 × 105 | 5.435 ± 0.87 × 105 |
- The number of cells at every 24 h. EG cells/MEFs: the numbers of EG cells on MEFs. EG cells/DEFs: the numbers of EG cells on DEFs.
The karyotype of duck EG cells was evaluated. The chromosomes number of different generation EG cells was 2n = 78, consisting of 10 pairs of macrochromosomes and 29 pairs of microchromosomes, while the sex chromosome type was ZZ (♂)/ZW (♀). These results were consistent with the varietal characteristics of duck (Fig. 1N), and in accordance with our prior work [Guan et al., 2010].
Spontaneous Differentiation In Vitro and In Vivo
The ability to differentiate in vitro into EBs in suspension culture is one of characteristics of pluripotent ES cells and EG cells. These EBs can differentiate in vitro into various cell types, indicating that the stem cells have pluripotency. After approximately 7 days in suspension culture without LIF, the EG-like cells formed simple EBs. These EBs enlarged and eventually formed a structure named cystic EBs after 10 days (Fig. 2A), occasionally containing several cell types. After adherent culture of the EBs, spontaneous differentiation was observed. The epithelial-like and neural-like cells can be observed derived from ectoderm and endoderm, respectively (Fig. 2B,C). Therefore, the duck EG cells derived from gonadal PGCs were capable of differentiation in vitro. The EG cells teratomas developed in the kidney capsules of the immunodeficient SCID mice that received transplants up to the end of the 20-day experimental period (Fig. 2D). Histological examination revealed that these teratomas contained tissues of the three EG layers, such as neural tissue (ectoderm, Fig. 2E), cartilaginous tissue (mesoderm, Fig. 2F), and glandular tissue (endoderm, Fig. 2G).
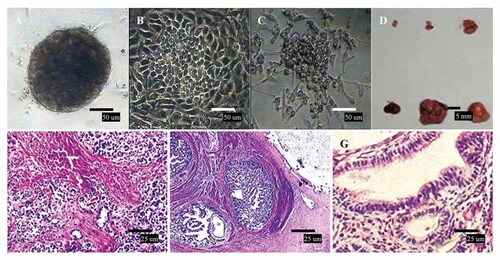
Spontaneous differentiation of duck EG cells. A: Simple EBs formation induced by suspension culture of duck EG cells without mLIF for 2–4 days in vitro. B: Epithelial-like cells. C: neural-like cells. D: teratomas. E: Neural tissue (ectoderm, HE staining). F: Cartilaginous tissue (mesoderm, HE staining). G: Glandular tissue (endoderm, HE staining).
Directed Differentiation and Characterization of Neural Lineage
In directed differentiation procedure, after the induction with neural stem cell selective medium for 3 days, a transparent cellular ring formed surrounding the induced EBs, and a compact and transparent area in the center of EBs was formed. (Fig. 3A). After incubation for 5 days, neural precursors derived from the dissociated EBs formed many small neurospheres with the morphological changes, 93% EBs formed neurospheres (Fig. 3B). The neurospheres grew much larger and became elliptical and strongly refractive after incubation for 7 days. They were identified as neural precursors by staining with Nestin. The neurospheres stained green, indicating that they were Nestin positive (Fig. 3C–F). A single cell dissociated from a neurosphere could reform a monoclonal sphere; cloning efficiency and monoclonal sphere formation rates were 65.3% and 26.3%, respectively.
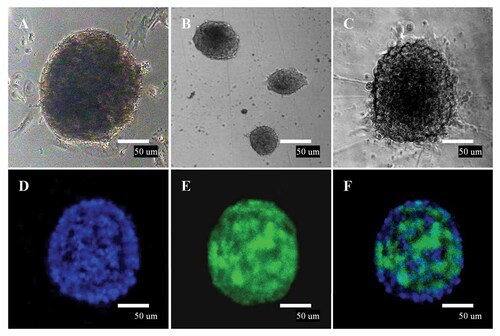
Characterization of neurospheres derived from duck EG cells. A: Cystic EBs formation induced by suspension culture of chick EG cells for 5–10 days in vitro. B: Neurosphere with regular rim and well-defined spherical shape. Ninety-three percent EBs formed neurospheres. C: Neurosphere-like colonies become flatten followed by the migration of many cells from the neurospheres to the bottom of the plate. D: Blue staining represents DAPI counterstain. E: Immunocytochemical staining of EG-derived neurospheres, EG-derived spheres expressed Nestin (green), these results indicated that they were neural precursors. F: Merge. Scale bar = 50 µm.
The cells with neuronal or glial morphology began to appear within less than 2 days after the pieces of neurospheres cultured in neural stem cell differential medium supplemented with RA and Insulin. First, the neurosphere-like colonies become flatten followed by the migration of many cells from the neurospheres to the bottom of the plate (Fig. 4A). Three to five days later, the migrated cells stretched out the neuroepithelial-like cells (Fig. 4B) and many slim protuberances (Fig. 4C) migrated out from the center to the surrounding of the neurosphere-like colonies. The potential of the clonally expanded neurospheres to differentiate into neuron, gliacyte, and oligodendrocyte was demonstrated by in vitro differentiation (Fig. 4D). These cells were fixed and proved to be neural stem cells (Nestin positive) (Fig. 4E–H), neurons (NSE positive) (Fig. 4I–L), and astrocytes (GFAP positive) (Fig. 4M–P) by immunofluorescence staining.
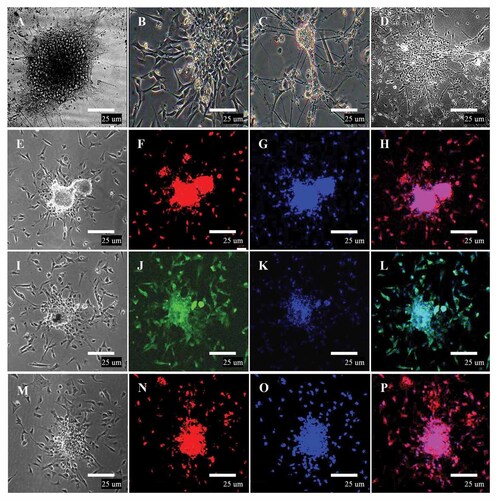
Characterization of duck EG-derived neural progenitors by immunocytochemistry. A: The migration of many cells from the neurospheres to the bottom of the plate. B: The migrated cells stretched out the neuroepithelial-like cells. C: The migrated cells stretched out many slim protuberances. D: The neural progenitors differentiated into neural, glial, and oligodendrocyte lineages. E, I, and M: Phase-contrast micrograph of neural progenitors. F, J, and N: Immunocytochemical staining of neural progenitors marker, Nestin (red), NSE (green), and GFAP (red) staining in the colony appear to be positive, respectively. G, K, and O: Blue staining represents DAPI counterstain. H, L, and P: merge. Scale bar = 25 µm.
After passaging, these cells were still cultured in neural stem cell differential medium, they were fixed after 7 days and proved to be astrocytes (GFAP, green) (Fig. 5A–D), neurons (NSE red) (Fig. 5E–H), and oligodendrocytes (CNP green) (Fig. 5I–L) by immunofluorescence staining.
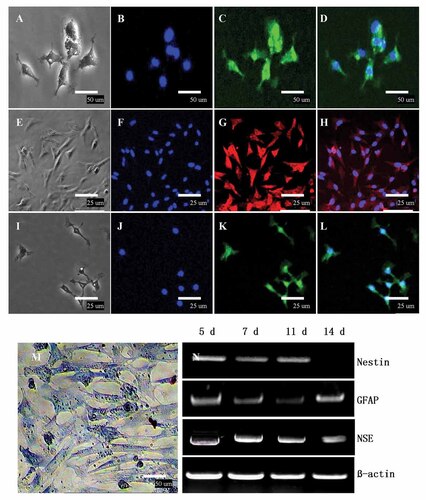
Characterization of neurons, astrocytes, and oligodendrocytes in duck EG-derived neurosphere. The cells are positive for neural cells markers, such as NSE, GFAP, and CNP. A, E, and I: Astroglia cells, neuron, and oligodendroglia cells (Phase-contrast), respectively. B, F, and J: Blue staining represents DAPI counterstain. C: GFAP (green)-expressing astrocytes. G: NSE (red)-expressing dopaminergic neurons. K: CNP (green)-expressing oligodendrocytes. D, H, and L: Merge. M: Toluidine blue stainings are positive, this indicated that Nissl bodies existed in plasma of neuron-like cells. N: RT-PCR analyses of region-specific markers. mRNA expression of duck EG-derived neural lineage cells determined by RT-PCR assays. Duck EG-derived neural lineage cells expressed Nestin, NSE, and GFAP, these results showed a time-dependent variation in expression of neural lineage markers. Lane 1: Nestin (900 bp), lane 2: GFAP (530 bp), lane 3: NSE (345 bp), and lane 4: β-actin (331 bp). β-Actin was used as inner control. A–D and M: Scale bar = 50 µm. E–L: Scale bar = 25 µm.
Toluidine blue staining indicated that Nissl bodies existed in plasma of neuron-like cells that had been induced for 7 days (Fig. 5M).
RT-PCR analyses were carried out to examine the expression of markers of neural lineage. The RT-PCR showed a time-dependent variation in expression of neural lineage markers, including markers of NSC (Nestin), neural (NSE), and astrocytes (GFAP) (Fig. 5N). The reduction in expression of the neural progenitors markers (Nestin) was associated with increased expression of neural cells markers, such as NSE and GFAP. Nestin of the neural progenitors marker was not detected after 14 days of differentiation, however, NSE and GFAP markers were observed by the second week of differentiation and became more homogenous along the progress of differentiation, which corresponded to the immunostaining results. Results showed that undifferentiated EG cells did not express Nestin, NSE, and GFAP (data not shown). Together, these results suggested that duck EG cells had neural lineage differentiation potentials after directed induction of RA.
DISCUSSION
The purpose of this work was to set up culture conditions allowing the growth and characterization of putative avian EG cells. Although the occurrence of pluripotent cells in early avian embryos has been demonstrated, these cells have not yet been identified and maintained in culture. In this report, the culture conditions, which included the use of DEFs and the inclusion of LIF, IL-11, SCF, bFGF, and IGF-1 in the medium, facilitated the proliferation of cells with an undifferentiated phenotype during more than 11 passages. Therefore, the culture conditions described above are likely to promote the growth of a specific kind of cells endowed with extended growth potential like ES cells.
Recently, our group purified a population of EG cells from duck embryos. MEFs could maintain duck EG cells in an undifferentiated state only until passage 7 [Guan et al., 2010]. MEFs are associated with risks such as outbred strains animals cells, viral infection, and pathogen transmission when the EG cells are used in preparation of transgenic chimeras. In this study, we developed DEFs feeder layer avoid to occur species cross-contamination. The EG cells cultured on DEFs were successfully grown as MEFs. Furthermore, MEFs can maintain EG cells in an undifferentiated state only until passage 7, whereas DEFs can be used to support the growth of EG cells until passage 11.
We found the amplified EG cells on DEFs appeared to be the morphology of typical undifferentiated clones. Our EG cells established on DEFs grew in tight colonies composed of cells with a high nuclear-to-cytoplasmic ratio, with each cell typically having one or more prominent nucleoli like DEFs cultured on MEFs. In addition, after several months in culture, the EG cells on DEFs remained a normal karyotype. Also, the EG cells differentiated successfully into EBs. After the EG cells were injected into immunodeficient SCID mice for about 3 weeks, teratoma formation with various cell types of the three EG layers in mice was examined. Therefore, our results suggest that DEFs can support EG cells growth, as previously described. However, there were some differences in growth behavior between EG cells cultured on DEFs and MEFs, the population doubling time of EG cells/DEFs and EG cells/MEFs were 29.69 ± 0.24 and 32.76 ± 0.11 h, respectively (P < 0.05). The EG cells proliferated faster on DEFs than on MEFs. These results suggest that DEFs provides appropriate extracellular matrix components, cytokines and growth factors and promotes cell–cell and cell–extracellular matrix interactions. We supposed that the various factors expressed in each cell type may have an important role in inducing the growth and inhibiting the differentiation of EG cells. Many factors that regulate the growth of cells are also expressed from different feeder layer cells [Imagawa et al., 2002; Palmieri et al., 2003]. During the work, we observed that the plating efficiency of duck EG cells were low. In our opinion, one way to approach this problem for avian stem cells research is to find out a better feeder cells from a kind of avian somatic cells or even a genetically engineered one, which could provide a much better microenvironment for the survival and proliferation of duck EG cells than the present-day used DEFs and MEFs.
Since EG cells are highly similar to ES cells in their characteristics, PGCs may provide an alternative source of pluripotent stem cells [Horii et al., 2003]. Compared with the traditional methods for isolating ES cells from ICM cells, PGCs are available in large numbers per embryo [Resnick et al., 1992]. It has been reported that the enrichment of gonadally derived PGCs is more efficient than blood derived PGCs because gonadal preparations contain much more PGCs per embryo compared with blood cell preparations [Mozdziak et al., 2005]. This is consistent with our observations, thus, to facilitate the following studies in the current article, the duck 7-day-old embryonic gonads were used to obtain enough PGCs.
The main finding of this study is that the duck EG cells are prone to spontaneous differentiation into flat or fibroblast-like cells if plated at low density, whereas mouse ES cells can grow in culture after disassociation into single cells and seeding at low density [Xu et al., 2001], indicating that duck EG cells require cell–cell contacts to control their fate.
Markers for pluripotent cells are often useful to identify stem cells in culture. Expression of ALP has been demonstrated in ES and ES-like cells in many mammals [Wobus et al., 1984; Talbot et al., 1995]. ALP activity has also been detected in murine and chick EG cells [Stewart et al., 1994; Wang et al., 2009]. In the present study, ALP activity was consistently expressed in primary cultures and subcultures of duck EG cells. With in vitro differentiation of EG cells, ALP activity was rapidly lost. In conjunction with morphological evaluation of EG cell colonies, ALP expression was a convenient marker to identify undifferentiated stem cells in culture.
Compared with ES, the identification of EG must include an additional marker, namely, the result of PAS staining, to confirm the origin of PGCs [Matsui et al., 1992]. In the present study, ALP activity, PAS staining and markers of SSEA-1, SSEA-4, TRA-1–60, and TRA-1–81 were consistently expressed in primary cultures and subcultures of EG cells. However, the time of colony formation and the characteristic of immunostaining are quite different in Park's report [Park and Han, 2000].
Like ES, the induction of EG cells differentiation in vitro generally occurs through an intermediate step of the formation of EBs, which are complex three-dimensional cell aggregates. Continued culturing can result in more differentiated cell types [Ku et al., 2004]. Specialized cells derived through differentiation procedures may thus be useful in the future treatment of diseases. However, it is obvious that the efficiency of spontaneous differentiation is very low [Clark et al., 2004]. As a result, understanding how to directed differentiate ES and EG cells into a specific lineage pathway and generate appropriate cell types robustly is very important for the study of developmental biology.
Cellular differentiation by the vitamin A derivative RA has been studied with undifferentiated pluripotent embryonic carcinoma cells [McBurney et al., 1988] and mouse ES cells [Rohweddel et al., 1999]. In vivo, RA was identified as a morphogenic, teratogenic compound and, furthermore, as a signaling molecule regulating gene expression. Neuronal and glial differentiation of mouse EG cells has been described by Bain et al. [1995] with standardized protocols.
Among the many factors used to induce ES cell differentiation, retinoic acid (RA) has a pivotal role. Although efficient neural differentiation of ES cells can be achieved without RA [Okabe et al., 1996], many protocols employ this compound for neural induction in ES-cell-derived 3D cell aggregates called EBs [Bain et al., 1995]. RA-treated EBs have been shown to give rise to a large variety of neural cells, including excitatory and inhibitory neurons and glia [Strübing et al., 1995]. Although much work has been published extolling the potential of ES cells, equivalent reports on EG cells have, by comparison, been lacking. In partial redress of this imbalance, Nevertheless, no studies have been published about the neural differentiation of duck EG cells. We have developed a chemically defined system, investigated in this paper that directed differentiation in vitro of duck EG cells into neural cells was also possible and practicable.
In this study, our results suggest that RA acts by inducing all the EG cells in the aggregated cultures to differentiate and that these cells are committed to form a limited variety of cell types, namely neurons, astrocytes, and oligodendrocytes which were confirmed by the immunostaining of NSE, GFAP, CNP, respectively. Consequently, a conclusion can be drawn that the efficiency of neural differentiation with RA is much higher in directed process than in spontaneous process, indicating that RA can, indeed, promote duck EG cells differentiate into neural cells. It is also possible that cells in multicellular aggregates are at various developmental stages due to their complex microenvironments; some cells are more developmentally advanced than others, so they may develop into gliacytes when most of the cells are still neurons. This finding lays down a conceptual and technical foundation for duck EG cells directed differentiation.
CONCLUSION
In conclusion, we showed in this work that cells derived from stage 28 ducks genital ridge exhibit a morphology and epitope profiles that are similar to those of chicken ES and EG cells after extended periods in culture. The duck EG cells cultured on DEFs were successfully grown as MEFs. DEFs can be used to support the growth of EG cells until passage 11. In addition, the duck EG cells proliferate faster on DEFs than on MEFs. The duck EG cells can differentiate into ectodermally derived tissues in vitro. This paper provide evidence that, with the induction of RA, duck EG cells can also differentiate into neuron-glia precursor cells and then into mature neurons, astrocytes, and oligodendrocytes in vitro. However, this study was limited to duck EG cells only, the situation in EG cells of other species and the function of derived neural cells in vivo should be further investigated. This differentiation system could provide a simple experimental model for developing optimal cultures of neurons, astrocytes, and oligodendrocytes suitable for implantation studies in animal models of PD and possible therapeutic applications.
Acknowledgements
This research was supported by the “863” National Major Research Program (2006AA10Z198, 2007AA10Z170), National Infrastructure of Natural Science and Technology Program (2006BAD13B08), and National Scientific Foundation of China (30671539).




