Raf kinase inhibitor protein correlates with sensitivity of nasopharyngeal carcinoma to radiotherapy†
Lin Ruan and Guo-Liang Wang contributed equally to this work.
Abstract
Raf kinase inhibitory protein (RKIP) is a metastasis suppressor whose expression is reduced in nasopharyngeal carcinoma (NPC) tissues and is absent in NPC metastases. To investigate the effect of RKIP on radiosensitivity of NPC, high metastatic 5-8F with low RKIP expression and non-metastatic 6-10B with high RKIP expression were stably transfected with plasmids that expressed sense and antisense RKIP cDNA. Overexpression of RKIP sensitized 5-8F cells to radiation-induced cell death, G2-M cell cycle arrest and apoptosis. In contrast, downexpression of RKIP in 6-10B cells protected cells from radiation-induced cell death, G2-M cell cycle arrest and apoptosis. In addition, RKIP expression altered the radiosensitivity of NPC cells through MEK and ERK phosphorylation changes of Raf-1/MEK/ERK signaling pathway. We further investigated the RKIP expression in NPC patients and its association with patients' survival after radiotherapy. Downexpression of RKIP was significantly correlated with advanced clinical stage, lymph node metastasis and radioresistance. Furthermore, survival curves showed that patients with RKIP downexpression had a poor prognosis and induced relapse. Multivariate analysis confirmed that RKIP expression was an independent prognostic indicator. The data suggested that RKIP was a potential biomarker for the radiosensitivity and prognosis of NPC, and its dysregulation might play an important role in the radioresistance of NPC. J. Cell. Biochem. 110: 975–984, 2010. © 2010 Wiley-Liss, Inc.
It is well known that radiotherapy is the mainstay treatment for nasopharyngeal carcinoma (NPC). About 60% of 5-year survival rate can be obtained by radiotherapy alone [Yeh et al., 2005]. However, there is a fairly large proportion of local failures and distant metastases because individual tumors can exhibit widely differing susceptibility to radiotherapy. Radioresistance of NPC cells is one of the main reasons for the failure of the therapy.
Raf kinase inhibitor protein (RKIP), a member of the phosphatidylethanolamine-binding protein (PEBP) family, is a widely expressed and highly conserved protein that does not share significant homology with any known protein family [Bollengier and Mahler, 1988; Grandy et al., 1990; Hori et al., 1994; Perry et al., 1994; Seddiqi et al., 1994; Banfield et al., 1998]. Its effect on the ERK pathway was the first to be discovered [Yeung et al., 1999, 2000]. In recent years, it was found that RKIP had its ability to influence intracellular signaling cascades, cell cycle regulation, the suppression of metastasis, neurodegenerative processes, the modulation of emotions, and reproduction [Keller et al., 2004]. RKIP loss or depletion has been associated with a number of metastatic tumors and has been implicated as a metastasis suppressor through regulation of one or more of these signaling cascades [Hagan et al., 2005; Park et al., 2005; Al-Mulla et al., 2006]. Chatterjee et al. 2004 suggested that RKIP can regulate apoptosis and, therefore, regulate growth and some DNA damaging agents can upregulate RKIP and induce apoptosis.
In our previous study, RKIP was found as an invasion suppressor protein in NPC by proteomic analysis [Chen et al., 2008]. Differential RKIP expression in two NPC cell lines 5-8F and 6-10B was found. 5-8F cell was a metastatic NPC cell line that expresses relatively small amounts of RKIP, but 6-10B cell was a nonmetastatic NPC cell line had high level of RKIP. To examine the role of RKIP in radiosensitivity of NPC cell lines, we regulated RKIP expression in NPC cells by stably transfecting 5-8F and 6-10B with sense and antisense RKIP vector, and then measured the radiosensitivity of the parental. Our results showed that overexpression of RKIP sensitized 5-8F cells to radiation-induced cell death, G2-M cell cycle arrest and apoptosis. In contrast, downexpression of RKIP in 6-10B cells protected cells from radiation-induced cell death, G2-M cell cycle arrest and apoptosis. And RKIP expression altered the radiosensitivity of NPC cells through MEK and ERK phosphorylation changes of Raf-1/MEK/ERK signaling pathway. We further investigated the RKIP expression in NPC patients and its association with patients' survival after radiotherapy. Downexpression of RKIP was significantly correlated with advanced clinical stage, lymph node metastasis and radioresistance. Furthermore, survival curves showed that patients with RKIP downexpression had a poor prognosis and induced relapse. Multivariate analysis confirmed that RKIP expression was an independent prognostic indicator. These results would help to understand the role of RKIP implicated in radiosensitivity of this tumor as well as new molecular targets for individual treatment of this malignancy.
MATERIALS AND METHODS
Cell Lines and Primary Tumors of NPC
Highly metastatic 5-8F and non-metastatic 6-10B NPC cell lines were routinely cultured in RPMI-1640 medium (Gibco BRL, Grand Island) supplemented with 10% fetal bovine serum (FBS) (Gibco) and antibiotics (50 U/ml penicillin and 50 µg/ml streptomycin) at 37°C in a humidified 5% CO2 incubator.
The paraffin-embedded archival tissue specimens from 93 NPC patients who were treated by radiotherapy of a 60–70 Gy total dose were provided by Xiangya Hospital of Central South University, China from 2000 to 2005 and after approval by the local ethics committee. The clinical stage of tumors were classified according to the 1992 NPC staging system of China [Qian et al., 2002]. Fifty-seven radiosensitive and 36 radioresistant patients were recruited in our study. Radiosensitive choosing standards of NPC patients were ones without the local residual lesions at more than 6 weeks after radiotherapy and recurrence at more than 2 months after radiotherapy. Distant metastases were eliminated by skeletal, thoracic, and upper abdominal imaging before radiotherapy. Radioresistant standards of NPC patients were defined as with persistent disease (incomplete regression of tumor) at more than 6 weeks after completion of radiotherapy, or ones with recurrent disease at the nasopharynx and/or neck nodes at more than 2 months after completion of radiotherapy.
Stable Transfection
Plasmids, pcDNA3.1(+)-ssRKIP (i.e., sense RKIP vector) and pcDNA3.1(−)-asRKIP (i.e., antisense RKIP vector), were constitutively expressed the RKIP cDNA in the sense and antisense orientations, respectively, as well as empty vectors, pcDNA3.1(+) and pcDNA3.1(−). They were kindly provided by Dr. Keller E.T., University of Michigan, USA [Fu et al., 2003]. According to the manufacturer's instructions, high metastatic 5-8F NPC cells were transfected with pcDNA3.1(+) or pcDNA3.1(+)-ssRKIP, and non-metastatic 6-10B NPC cells were transfected with pcDNA3.1(−) or pcDNA3.1(−)-asRKIP with Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA). Individual G418-resistant clones were isolated and expanded after 14 days of selection in RIPM-1640 medium containing 500 mg/ml G418 (Invitrogen).
Western Blot
Cells were harvested with trypsinization and washed with PBS. Proteins were extracted with lysis buffer at 4°C for 30 min and centrifuged at 14,000 rpm for 30 min. Twenty micrograms of protein lysates were separated by 10% SDS–PAGE for electrophoresis. and transferred to a PVDF membrane. Blots were blocked with 5% nonfat dry milk for 1 h and then was incubated with rabbit polyclonal anti-human RKIP primary antibody (Santa Cruz, 1:1,000 dilution) at 4°C for overnight. The membrane was washed again and incubated again with horseradish peroxidase-conjugated goat anti-rabbit IgG. Secondary antibodies (Santa Cruz, 1:3,000 dilution) were treated with enhanced chemiluminescence developing solution (Amersham Biosciences, Stockholm, Sweden) and exposed to X-ray film. β-Actin expression was used as an loading control. To measure MEK and ERK phosphorylation, rabbit polyclonal primary antibodies against pMEK, MEK, pERK and ERK (Santa Cruz, 1:1,500 dilution) were used.
Radiotherapy
Exponentially grown cells were seeded in 60 mm dishes for about 36 h in complete medium, then cells were treated with different doses of 6 MV X-ray radiation by a 21EX accelerator (Varian Medical Systems). The radiation doses were 0, 2, 4, 6, and 8 Gy, respectively; the dose efficiency was 300 cGy/min. The medium was replaced with a fresh one 24 h after radiation.
Cell Viability Assay
Briefly, 5 × 103 cells were seeded onto 96-well plates and grown in complete medium with 10% FBS. After the treatment of different radiation dose, and the cells were incubated for an additional 24 h. Each radiation dose was applied to five times wells of cells. Cell viability assay was performed with CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega) according to manufacturer's instructions. At the end of the incubation, 20 µl of CellTiter 96 AQueous One solution was added to each well, and the plate was incubated for 4 h. Absorbance was read at 490 nm by using a ELX800 microplate reader. Cell viability was expressed as mean ± SD value of percentage of absorbance reading from treated cells vs. untreated cells. Experiments were performed in triplicate.
Colony Formation Assays
Clonogenic survival was the ability of cells to maintain clonogenic capacity and to form colonies. Cells in culture were irradiated with 0, 2, 4, 6, and 8 Gy. Briefly, after exposure to radiation, cells were trypsinized, counted, and seeded for colony formation in 60 mm dishes at 200–10,000 cells/dish. After incubation intervals of 14–21 days, colonies were fixed with methanol and stained with crystal violet and manually counted. Colonies consisting of 50 cells or more were scored. Stained clones that had more than 50 cells were counted and cloning efficiency calculated as: cloning efficiency = (clone number/total cell number) × 100%, in triplicate. Furthermore, the cell survival fraction was counted out and the cell survival curve was drafted by the standard multi-target single-hit model, SF = 1 − (1 − eD/D0)N (SF, cell survival fraction; D, radiation dose; e, the bottom of the natural logarithm; D0, the mean death dose; N, extrapolate number). Finally, the survival curves were established by plotting the log of the surviving fraction versus the irradiation dose. All survival data were analyzed using survival curve program with Sigma-Plot 11.0 software.
Cell Cycle Analysis
After the treatment of different radiation dose, and the cells were incubated for an additional 24 h. The cell pellets were fixed with ice-cold 70% ethyl alcohol in PBS at 20°C for 1 h and then centrifuged at 1,500 rpm for 5 min. The pellets were suspended and incubated with 0.5% Triton X-100 and 0.05% RNase in 1 ml PBS at 37°C for 30 min and then centrifuged at 1,500 rpm for 5 min. The cell pellets were resuspended and incubated with 40 mg/ml propidium iodide (PI) in 1 ml PBS at room temperature for 30 min. Samples were immediately analyzed by FACScan flow cytometry. The distribution of cell cycle phases was determined using Cell Quest Pro and ModiFit software. All of the samples were assayed in triplicate, and the fraction of every cell cycle phase was calculated.
Apoptosis Analysis
Apoptosis was determined by either double staining of Annexin V and propidium iodide(PI) or cleavage of procaspase-3 according to the manufacturer's instructions (BD Pharmingen). The cells were suspended in 100 µl of binding buffer (10 mM of HEPES, pH 7.4, 150 mM of NaCl, 2.5 mM of CaCl2, 1 mM of MgCl2). The cells were stained with 5 µl of Annexin V-FITC and 10 µl of PI (50 µg/ml). After 15 min incubation in the dark at room temperature, stained cells were immediately analyzed by FACScan flow cytometry. All of the samples were assayed in triplicate, and the cell apoptosis rate calculated as: apoptosis rate = (apoptotic cell number/total cell number) × 100%. The six NPC cell lines were treated with or without 4 Gy radiation. To examine Caspase-3 activity, rabbit Caspase-3 polyclonal antibody (Santa Cruz) were used for Western blot.
Immunohistochemistry and Scoring Method
The paraffin-embedded clinical specimens were done using a standard immunohistochemical technique and sections were blindly evaluated by two investigators in an effort to provide a consensus on staining patterns by microscopy. Negative control sections were incubated with PBS instead of primary antibody and positive control sections were paraffin-embedded human liver tissue. Evaluation of staining was done as previously described by us [Cheng et al., 2008]. Each case was rated according to a score that added a scale of intensity of staining to the area of staining. At least 10 high-power fields were chosen randomly, and >1,000 cells were counted for each section. The intensity of staining was graded on the following scale: 0, no staining; 1+, mild staining; 2+, moderate staining; 3+, intense staining. The area of staining was evaluated as follows: 0, no staining of cells in any microscopic fields; 1+, <30% of tissue stained positive; 2+, between 30% and 60% stained positive; 3+, >60% stained positive. The minimum score when summed (extension + intensity) was, therefore, 0, and the maximum, 6. A combined staining score (extension + intensity) of ≤2 was considered to be a negative staining (low staining); a score between 3 and 4 was considered to be a moderate staining; whereas a score between 5 and 6 was considered to be a strong staining.
Statistical Analysis
All statistical analyses were performed by using SPSS17.0 (SPSS Inc., Chicago). Results were shown as the mean ± standard deviations. Follow-up by telephone was carried out to obtain the information of patients' outcomes. The follow-up period lasted up to 72 months. Kruskal–Wallis H-test were used to evaluate the relationships between RKIP expression and clinicopathological factors. Relapse-free probability curves and overall survival curves were obtained by the Kaplan–Meier method and log-rank testing was used to evaluate the statistical significance of differences. Univariate and multivariate Cox regression analysis was used to evaluate the prognostic significance of clinicopathological factors. P < 0.05 was considered statistically significant.
RESULTS
Isolation of Stable High-Level Expression RKIP Transductants of 5-8F ssRKIP Cells and Low-Level Expression RKIP Transductants of 6-10B asRKIP Cells
To examine the radiosensitive role of RKIP in NPC cell lines, we monitored RKIP expression in NPC cells by stably transfecting 5-8F cells with sense RKIP vector and 6-10B cells with antisense RKIP vector. 6-10B cell showed stronger RKIP expression than 5-8F cell. RKIP transductants of 5-8F and 6-10B cells were selected in multiple steps for growth in levels of G418 and for high and low RKIP expression. Transfection of sense RKIP vector into 5-8F cells increased RKIP expression, and transfection of antisense RKIP vector into 6-10B cells decreased RKIP expression compared with the corresponding control and empty vector-transfected cells (Fig. 1).
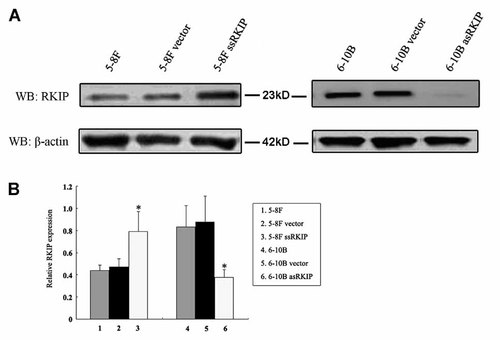
Analysis of RKIP expression in stable transductants and parental cell lines by Western blot. A: Western blots showed RKIP expression levels in stable transductants and parental cell lines. β-Actin was used for the same loading. B: Histogram shows the relative expression levels of RKIP in stable transductants and parental cell lines by densitometric analysis (P < 0.05). 5-8F: 5-8F cells without transfection, 5-8F vector: 5-8F cells transfected with empty vector, 5-8F ssRKIP: 5-8F cells transfected with sense RKIP vector; 6-10B: 6-10B cells without transfection, 6-10B vector: 6-10B cells transfected with empty vector, 6-10B asRKIP: 6-10B cells transfected with antisense RKIP vector.
Sensitivity of NPC Cell Lines with RKIP to Radiation-Induced Cell Death
To assess the sensitivity against radiotherapy, 5-8F, 5-8F vector, 5-8F ssRKIP, 6-10B, 6-10B vector and 6-10B asRKIP cells were seeded and treated with different radiation dose onto 96-well microplates. Cells were incubated for 24 h after radiotherapy and cellular viability was determined by a cell viability assay (Fig. 2). Cell viability rate of 5-8F ssRKIP cell against radiotherapy relative to 5-8F and 5-8F vector cells was a approximately 23%, 42%, 65%, 80% decrease with radiation dose 2, 4, 6, or 8 Gy. Interestingly, cell viability rate of 6-10B asRKIP cell against radiotherapy relative to 6-10B and 6-10B vector cells was a approximately 33%, 45%, 49%, 34% increase with radiation dose 2, 4, 6, or 8 Gy. Here, our data suggest that RKIP overexpression can enhance cell death of 5-8F cells induced by radiotherapy and RKIP downexpression can enhance the cell survival of 6-10B cells against radiotherapy.
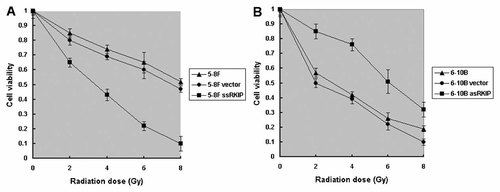
Effects of RKIP overexpression and downexpression on NPC cell viability cycle with and without radiotherapy. A: Reduced cell viability rate of 5-8F ssRKIP cells relative to 5-8F and 5-8F vector cells (P < 0.05). B: Increased cell viability rate of 6-10B asRKIP cells relative to 6-10B and 6-10B vector cells (P < 0.05).
Survival Curves of NPC Cells After Radiation
The survival curve of NPC cells after radiation was shown in Fig. 3. The radiobiological parameters of 5-8F ssRKIP cell treated with radiation were D0 = 2.45, Dq = 1.01, SF2 = 0.32 while those of 5-8F cells were D0 = 1.88, Dq = 2.98, SF2 = 0.87 and those of 5-8F vector cells were D0 = 1.99, Dq = 2.53, SF2 = 0.80 (P < 0.05). These data suggested that 5-8F ssRKIP cell with RKIP overexpression resulted in a decline in cell survival due both to a higher initial slope of the dose–response curve and to a major decrease of the quadratic parameter. On the other hand, 6-10B asRKIP cells had radiobiological values of D0 = 2.02, Dq = 2.76, SF2 = 0.84. And respectively, 6-10B cells had radiobiological values of D0 = 1.83, Dq = 0.22, SF2 = 0.34, and 6-10B vector cells had radiobiological values of D0 = 1.86, Dq = 0.29, SF2 = 0.30. These results showed 6-10B asRKIP with RKIP downexpression had a higher survival fraction at 2 Gy and had a obvious increase of the quadratic parameter compared with 6-10B cells and 6-10B vector cells (P < 0.05). The data herein suggested the radiotherapy sensitivity of NPC cells was closely associated with RKIP expression in NPC cells.
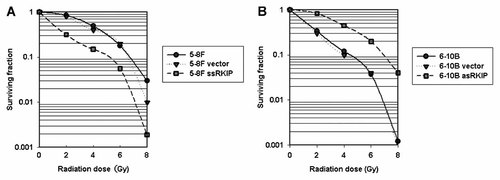
NPC cells radiosensitivity by colony formation assays. A: 5-8F ssRKIP cells conferred radiosensitivity relative to 5-8F and 5-8F vector cells (P < 0.05). B: 6-10B asRKIP cells conferred radioresistance relative to 6-10B and 6-10B vector cells (P < 0.05).
Kinetics and Cell Cycle Progression
Using flow cytometric analysis, cell cycle distribution of six NPC cell lines with and without radiation was determined. As shown in Fig. 4, compared with that of 5-8F and 5-8F vector cells, prolonged G2-M phase accumulation was induced in 5-8F ssRKIP cells. However, 6-10B and 6-10B vector cells showed G2-M cell cycle arrest and G1-phase depletion together induced by radiation, but 6-10B asRKIP cells have no significant changes of cell cycle distribution with radiation. These results suggested that RKIP overexpression can enhance radiosensitivity by increasing G2-M phase arrest of NPC cells after radiotherapy and RKIP downexpression could show radioresistance by protect cells from G2-M phase arrest after radiotherapy.

Effects of RKIP overexpression and downexpression on NPC cell cycle with and without radiotherapy. A: Increased G2-M cell cycle arrest of 5-8F ssRKIP cells relative to 5-8F and 5-8F vector cells (P < 0.05). B: Reverse G2-M cell cycle arrest of 6-10B asRKIP cells relative to 6-10B and 6-10B vector cells (P < 0.05).
RKIP Sensitized NPC Cells to Radiotherapy-Mediated Apoptosis
Fig. 5A shows the apoptosis rate induced by radiation in six NPC cell lines. Apoptosis rate induced by radiation in 5-8F ssRKIP cells were significantly higher than that of in 5-8F and 5-8F vector cells following with increasing radiation dose (P < 0.05). In contrast, we observed apoptosis rate induced by radiation in 6-10B asRKIP cells were significantly lower than that of in 6-10B and 5-10B vector cells with increasing radiation dose (P < 0.05).
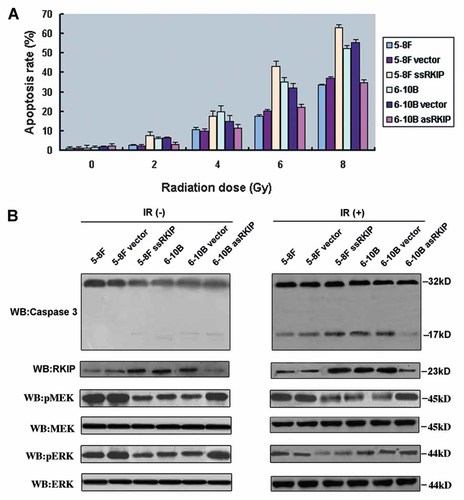
Effects of RKIP expression on NPC cell apoptosis with and without radiotherapy. A: Apoptosis rate of six NPC cell lines in different radiation dose. B: caspase 3, RKIP, and pMEK, MEK, pERK and ERK expression of six NPC cell lines without (left) and with (right) a 4 Gy radiation dose by Western blot. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We measured cleavage caspase 3 for radiation-induced apoptosis by Western blot (Fig. 5B). After 4 Gy of radiation, 5-8F ssRKIP, 6-10B, and 6-10B vector cells including high RKIP expression contained increasing caspase 3 cleavage fragments than 5-8F, 5-8F vector and 6-10B asRKIP cells by grayscale scan analysis. These results indicated that RKIP overexpression promotes apoptosis and RKIP downexpression can protect cells from apoptosis induced by radiation.
RKIP Enhances NPC Cells Radiosensitivity In Vitro Through Activation of Raf-1/Mek/Erk Signaling Pathway
RKIP is an inhibitor of Raf-1/MEK/ERK signaling pathway. Whether RKIP expression affected the radiosensitivity of NPC cells through phosphorylated MEK and ERK of the Raf-1/MEK/ERK signaling pathway. To test this possibility, we examined the phosphorylation status of MEK and ERK in NPC cells with and without radiotherapy (Fig. 5B). Although parental 5-8F and 6-10B cells had similar levels of total MEK and ERK, 5-8F, 5-8F vector, and 6-10B asRKIP cells had higher basal levels of the phosphorylated forms of MEK and ERK than did 5-8F ssRKIP, 6-10B, and 6-10B vector cells. 5-8F ssRKIP, 6-10B, and 6-10B vector cells after radiotherapy had less phosphorylated MEK and ERK than did these cells without radiotherapy. 5-8F, 5-8F vector, and 6-10B asRKIP cells after radiotherapy had similar phosphorylated MEK and ERK with these cells without radiotherapy. These data suggested that RKIP altered the radiation sensitization of NPC cells through MEK and ERK phosphorylation changes of the Raf-1/MEK/ERK signaling pathway.
Correlations of Relapse and Survival With RKIP Expression
Finally, we further detected the expression of RKIP using immunohistochemistry in paraffin-embedded archival clinical primary NPC tissues (Fig. 6). Forty-two (45.1%) of 93 patients had died, 48 patients (51.6%) were still alive, and three patient (3.2%) was lost to follow-up. The mean follow-up period was 46.0 ± 12.7 months. Table I shows the correlation of several clinicopathologic factors with RKIP expression in 93 cases of primary NPC. Survival curves were calculated for a total of 93 cases of NPC patients by the Kaplan–Meier method and analyzed using the log-rank test. The relapse-free probability curve and overall survival curve showed that the relapse rate and overall survival rate was significantly increased with decreasing RKIP expression (Fig. 7). Using univariate analysis from Cox's proportional hazards model (Table II), the following variables were found to be significantly associated with prognosis: clinical stage, primary tumor(T) stage, lymph node metastases, radiotherapy sensitivity, and the expression level of RKIP. In multivariate analysis, advanced clinical stage and primary tumor(T) stage, lymph node metastases, radioresistance, and decreasing RKIP expression were an independent prognostic factor of increased relapse rate and decreased overall survival rate (Table III).
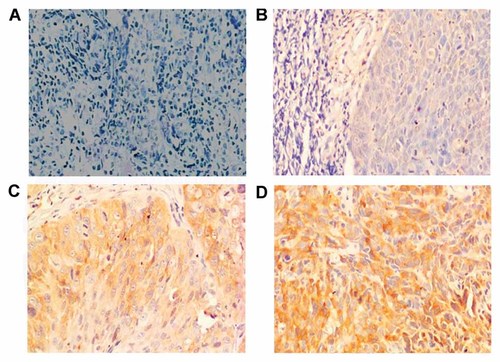
RKIP immunohistochemistry patterns in primary NPC. A: Negative staining for RKIP. B: Weak staining for RKIP. C: Moderate staining for RKIP. D: Strong staining for RKIP. Original magnification 200×. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Variables | n | Scores of RKIP expression | P | ||
|---|---|---|---|---|---|
| Low | Moderate | High | |||
| (0–2) | (3–4) | (5–6) | |||
| Gender | 0.489 | ||||
| Male | 48 | 22 | 16 | 10 | |
| Female | 45 | 18 | 15 | 12 | |
| Age | 0.443 | ||||
| ≥50 | 41 | 16 | 14 | 11 | |
| <50 | 52 | 24 | 17 | 11 | |
| Stage | 0.003* | ||||
| II | 14 | 1 | 3 | 10 | |
| III | 40 | 22 | 11 | 7 | |
| IV | 39 | 17 | 17 | 5 | |
| Primary tumor (T) stage | 0.680 | ||||
| T1 | 14 | 6 | 5 | 3 | |
| T2 | 35 | 15 | 12 | 8 | |
| T3 | 32 | 12 | 11 | 9 | |
| T4 | 12 | 7 | 3 | 2 | |
| Lymph node metastasis (N) | 0.000* | ||||
| Negative | 43 | 5 | 22 | 16 | |
| Positive | 50 | 35 | 9 | 6 | |
| Radiotherapy | 0.000* | ||||
| Sensitive | 57 | 10 | 25 | 22 | |
| Resistant | 36 | 30 | 6 | 0 | |
- P values are from Kruskal–Wallis H-test and were statistically significant.
- * P < 0.05.
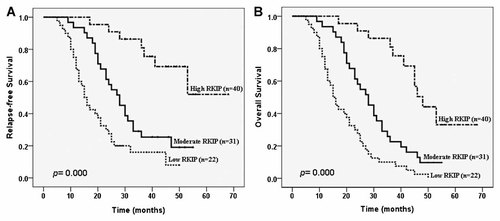
Kaplan–Meier survival plots for NPC patients according to the expression levels of RKIP. A: RKIP expression and relapse-free probability. B: RKIP expression and overall survival. P was determined using a two-sided log-rank test.
| Variables | Relapse-free probability | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender | 0.969 | 0.579 | ||
| Male | 1.000 | 1.000 | ||
| Female | 1.010 (0.621–1.643) | 0.969 | 1.133 (0.728–1.764) | |
| Age | 0.367 | 0.465 | ||
| ≥55 | 1.000 | 1.000 | ||
| <55 | 1.255 (0.766–2.058) | 0.367 | 1.181 (0.756–1.843) | 0.465 |
| Stage | 0.000* | 0.005* | ||
| II | 1.000 | 1.000 | ||
| III | 2.116 (1.521–4.460) | 0.002 | 2.553 (1.339–4.660) | 0.007 |
| IV | 3.038 (1.363–5.380) | 0.000 | 2.465 (1.474–4.125) | 0.000 |
| Primary tumor (T) stage | 0.000* | 0.000* | ||
| T1 | 1.000 | 1.000 | ||
| T2 | 1.054 (0.610–2.661) | 0.000 | 1.246 (0.932–2.260) | 0.000 |
| T3 | 5.152 (2.005–8.512) | 0.000 | 5.014 (3.546–10.002) | 0.000 |
| T4 | 6.737 (4.440–10.814) | 0.000 | 6.139 (4.110–10.027) | 0.000 |
| Lymph node metastasis (N) | 0.000* | 0.004* | ||
| Negative | 1.000 | 1.000 | ||
| Positive | 4.555 (2.712–7.650) | 0.000 | 2.330 (1.316–4.127) | 0.004 |
| Radiotherapy | 0.002* | 0.000* | ||
| Sensitive | 1.000 | 1.000 | ||
| Resistant | 2.443 (1.371–4.353) | 0.002 | 4.652 (2.890–7.488) | 0.000 |
| RKIP expression | 0.000* | 0.000* | ||
| High | 1.000 | 1.000 | ||
| Moderate | 1.145 (0.259–2.157) | 0.000 | 1.123 (0.281–3.281) | 0.000 |
| Low | 2.521 (0.609–3.214) | 0.000 | 2.024 (0.306–4.092) | 0.000 |
- HR, hazard ratio; CI, confidence interval.
- * P < 0.05.
| Variables | Relapse-free probability | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Stage | 0.014* | 0.005* | ||
| II | 1.000 | 1.000 | ||
| III | 1.929 (1.093–3.404) | 0.023 | 2.170 (1.439–3.274) | 0.006 |
| IV | 3.038 (1.363–5.380) | 0.012 | 2.590 (1.104–6.078) | 0.001 |
| Primary tumor (T) stage | 0.000* | 0.000* | ||
| T1 | 1.000 | 1.000 | ||
| T2 | 1.211 (0.743–2.008) | 0.000 | 0.994 (0.607–1.628) | 0.000 |
| T3 | 5.126 (2.971–8.844) | 0.000 | 5.014 (3.546–10.002) | 0.000 |
| T4 | 6.709 (1.536–29.294) | 0.000 | 6.406 (2.110–15.026) | 0.000 |
| Lymph node metastasis (N) | 0.026* | 0.017* | ||
| Negative | 1.000 | 1.000 | ||
| Positive | 2.491 (1.116–5.560) | 0.026 | 2.652 (1.194–5.892) | 0.017 |
| Radiotherapy | 0.002* | 0.000* | ||
| Sensitive | 1.000 | 1.000 | ||
| Resistant | 2.443 (1.371–4.353) | 0.002 | 4.555 (2.712–7.650) | 0.000 |
| RKIP expression | 0.011* | 0.000* | ||
| High | 1.000 | 1.000 | ||
| Moderate | 1.252 (0.054–3.701) | 0.029 | 1.317 (0.046–5.524) | 0.001 |
| Low | 2.033 (0.415–3.206) | 0.000 | 2.143 (0.703–4.092) | 0.000 |
- HR, hazard ratio; CI, confidence interval.
- * P < 0.05.
DISCUSSION
It is a challenging task of radiotherapy of nasopharyngeal carcinoma due to the complex mechanics of the tumor and the several pivotal and functional structures surrounding the tumor. The nasopharyngeal cavity is surrounded by critical neural tissues and sensitive structures such as auditory apparatus, temporomandibular joints, and parotid glands whose normal functioning is essential for maintenance of the patients' overall life quantity [Taheri-Kadkhoda et al., 2008]. Then it is stringent to develop a new strategy to enhance the radiosensitivity of NPC tumor cells while lower the side-effects of radiation.
More and more investigations suggest that there is a significant relationship with cell cycle, apoptosis rate and radiosensitivity of tumor cells, and apoptosis enhancing genes. Multiple pathways are involved in maintaining the genetic integrity of cells after their exposure to ionizing radiation. Cell cycle regulation and apoptosis are perhaps the most important determinants of ionizing radiation sensitivity [Pawlik and Keyomarsi, 2004]. In this report, we have demonstrated that NPC cells with RKIP overexpression sensitizes cell death, cell cycle arrest and apoptosis with radiation. In contrast, RKIP downexpression protects cells from radiation-induced cell death, G2-M cell cycle arrest and apoptosis. Likewise, the loss of RKIP, as seen in primary prostate cancer tumors and metastases, conferred protection against radiation-induced apoptosis. Therefore, it was conceivable that the loss of RKIP confers a growth advantage on PCa cells, because the loss of RKIP would decrease apoptosis, favoring proliferation [Woods Ignatoski et al., 2008].
Overexpression of RKIP in metastatic cell lines, derived from hepatoma [Lee et al., 2006], melanoma [Schuierer et al., 2004], and prostate [Fu et al., 2003, 2006] diminished their invasiveness and motility in vitro. Our previous results suggest that RKIP may be a NPC metastasis suppressor, and decreased RKIP expression is associated with the increased invasive capability of NPC cells possibly through the activation of Raf-1/MEK/ERK pathway [Chen et al., 2008]. Whether RKIP also enhances radiosensitivity of NPC through activation of Raf-1/MEK/ERK pathway. Our results suggest RKIP overexpression enhances radiosensitivity of NPC cells and RKIP downexpression induces NPC cells radioresistance against radiation. RKIP enhances the radiation sensitivity in NPC cells through decreasing MEK and ERK phosphorylation of Raf-1/MEK/ERK signaling pathway.
RKIP loss was significantly observed in prostate [Keller et al., 2005], breast cancer [Chatterjee et al., 2004; Hagan et al., 2005] and melanoma [Schuierer et al., 2004]. Our data suggest RKIP downexpression significantly correlates with advanced clinical stage, lymph node metastasis and radioresistance in primary NPC patients. Survival curves show that patients with RKIP downexpression has a poor prognosis and induced relapse.
In summary, our data demonstrate that RKIP downexpression conferred a growth advantage of NPC cells and protect cells from cell cycle arrest and apoptosis after radiation. But RKIP overexpression brings about cell death, cell cycle arrest and apoptosis of NPC cells after radiation. RKIP enhances the radiation sensitivity in NPC cells through decreasing MEK and ERK phosphorylation of Raf-1/MEK/ERK signaling pathway. RKIP downexpression significantly correlated with advanced clinical stage, lymph node metastasis and radioresistance in primary NPC patients. We suggest that RKIP may be a biomarker for the radiation sensitivity and prognosis of NPC. The results could help to provide new adjuvant therapies with radiotherapy for individual radioresistant NPC patient.
Acknowledgements
We thank the members of the Key Laboratory of Cancer Proteomics of Chinese Ministry of Health for critical reading of the manuscript and helpful comments.




