The cytologic criteria of malignancy
Abstract
Cytology and cell biology are two separate fields that share a focus on cancer. Cancer is still diagnosed based on morphology, and surprisingly little is known about the molecular basis of the defining structural features. Cytology uses the smallest possible biopsy for diagnosis by reducing morphologic “criteria of malignancy” to the smallest scale. To begin to develop common ground, members of the American Society of Cytopathology Cell Biology Liaison Working Group classify some of the “criteria of malignancy” and review their relation to current cell biology concepts. The criteria of malignancy are extremely varied, apparently reflecting many different pathophysiologies in specific microenvironments. Criteria in Group 1 comprise tissue-level alterations that appear to relate to resistance to anoikis, alterations in cell adhesion molecules, and loss of apical–basal polarity. Criteria in Group 2 reflect genetic instability, including chromosomal and possibly epigenetic instability. Criteria in Groups 3 are subcellular structural changes involving cytoplasmic components, nuclear lamina, chromatin and nucleoli that cannot be accounted for by genetic instability. Some distinct criteria in Group 3 are known to be induced by cancer genes, but their precise structural basis remains obscure. The criteria of malignancy are not closely related to the histogenetic classification of cancers, and they appear to provide an alternative, biologically relevant framework for establishing common ground between cytologists and cell biologists. To understand the criteria of malignancy at a molecular level would improve diagnosis, and likely point to novel cell physiologies that are not encompassed by current cell biology concepts. J. Cell. Biochem. 110: 795–811, 2010. © 2010 Wiley-Liss, Inc.
One would be hard-pressed to find an example of a biologic entity in which structure could be altered without altering function. The relationship between structure and function is so important in biology that physiologies typically cannot be discovered without understanding morphology. Cancer cells often, but not always, show structural alterations at the light microscope level compared to the normal cells from which they arise. These defining characteristics are referred to as “criteria of malignancy.” Many of the criteria are not characterized at a molecular level.
What do Cytologists know that needs to be characterized, and how can a collective expertise be brought to bear on the cancer problem? The Cell Biology Liaison Working Group of the American Society of Cytopathology (ASC) is trying to bridge the gap between these fields by conveying as simply as possible the criteria that Cytologists apply. The cell biology literature relevant to the criteria is briefly discussed to help expose fruitful areas for collaborative research.
WHO ARE CYTOLOGISTS AND WHAT DO THEY DO?
Cytotechnologists (who hold at least a Bachelor of Science degree with 1 year of specialized training and pass a rigorous credentialing examination) analyze cellular preparations and search for specific morphologic characteristics to locate abnormal cells. Cytopathologists (MD or equivalent) then make definitive diagnoses of cancer based on the abnormal cells identified by Cytotechnologists. Throughout this essay, “Cytologists” is used as an encompassing word that includes both Cytotechnologists and Cytopathologists.
Cytologists commonly use a Papanicolaou stain to display diagnostic features. Details of performing a Papanicolaou stain, and information about other cytologic techniques are available at www.cytopathology.org. The Papanicolaou stain was named after the Cytologist George Papanicolaou 1883–1962 who invented the stain that is still used for the cervical cancer screening “pap” test. The Papanicolaou stain is a modified hematoxylin and eosin (H&E) stain in which the eosin (a non-specific protein stain) was modified to have a translucent blue-green hue, and a third orange stain is used to mark dense proteins. Hematoxylin stains nucleic acids. The three colors give a very broad overview of nuclear and cytoplasmic organization unmatched by phase contrast or differential interference microscopy. DAPI approximates hematoxylin staining, but hematoxylin by itself is not enough to see the variety of morphologic features diagnostic of cancer.
THE DISTINCTION BETWEEN THE CRITERIA OF MALIGNANCY AND THE HISTOGENETIC CLASSIFICATION OF CANCER
Cancers are traditionally classified according to histogenesis (the cell of origin), but the criteria for actually diagnosing cancer are not directly related to the histogenetic classification. From the same cell of origin, there can be different types of structural changes diagnostic of different cases of cancer. Conversely, very similar criteria can sometimes be applied for diagnosing tumors that arise from different cell types. The criteria of malignancy appear to provide an independent means of describing cancers. It may be useful for researchers to try to relate cancer genes (in this essay “cancer gene” is used to include oncogenes and tumor suppressor genes) to a particular cell structural change rather than to a particular histogenetic type of cancer.
THE DISTINCTION BETWEEN THE CRITERIA OF MALIGNANCY AND THE HALLMARKS OF CANCER MODEL
With the important exception of genetic instability, the hallmarks of cancer model [Hanahan and Weinberg, 2000] bears surprisingly little relation to the criteria of malignancy. The hallmarks of cancer model is based on the known or expected functional alterations in cancer cells, and it does not attempt to account for structural alterations of cancer cells. With only a few exceptions, objective measures of mitosis or apoptosis are not useful criteria of malignancy. Normal cells typically have a shorter cell cycle than the cancers that emerge [Baserga, 1965; Tannock, 1978], and the finding of dead cells is a feature that predicts the presence of a malignant tumor. Clonal expansion can certainly take place without speeding up cell cycle kinetics or through resistance to apoptosis (reviewed in [Fischer et al., 2004b]). The lack of understanding of the precise structural basis of the criteria hinders identification of new physiologies that appear likely to contribute to clonal evolution.
A PROPOSED CLASSIFICATION OF THE CRITERIA
A classification can have value if it creates common ground, incorporating perspective and vocabulary from both Cell Biology and Cytology. We propose a three-tiered classification that has value for teaching, uses a common vocabulary, and exposes areas ripe for collaboration (Table I). We exclude from discussion criteria that have an obvious or trivial basis. For example, the presence of epithelial cells in a lymph node, or non-liver cells in a liver biopsy have an obvious diagnostic value, but these criteria do not illuminate a direction for cancer researchers. This review cannot possibly cover all of the detail of cytologic diagnosis, and the interested reader is referred to one of several comprehensive textbooks [DeMay, 1996; Bibbo and Wilbur, 2008; Cibas and Ducatman, 2009].
| Cytologic criteria | Probable cell biology basis or association |
|---|---|
| Group 1: Criteria at the tissue architectural level | |
| Crowding | Loss of contact inhibition |
| Papillary formation | ? |
| Glandular complexity | Aberrant branching morphogenesis |
| Stratification of epithelial cells | Resistance to anoikis, anchorage independence |
| Intraepithelial cell migration (Pagetoid spread) | Cell migration |
| ?Loss of E-cadherin | |
| ?Loss of apical–basal polarity | |
| Invasion | Extracellular matrix remodeling |
| Apparent resistance to negative growth regulatory signals from extracellular matrix | |
| Loss of intercellular adhesion | Alterations in cell adhesion molecules |
| Group 2: Criteria related to genetic instability | |
| Abnormal mitotic figures and unpredictable variation in degree of chromasia (distinct from polyploidization) | Chromosomal instability |
| Centrosome and mitotic spindle defects | |
| Micronuclei | DNA amplification |
| Acentric chromosomes | |
| Mitotic spindle defects | |
| Overall cellular pleomorphism | Genetic instability of any type |
| Unpredictable chromatin pattern variation | ?Epigenetic instability |
| Group 3: Subcellular criteria not apparently related to genetic instability | |
| Involving intermediate filaments | |
| Koilocytes | HPV E4 mediated disassembly of intermediate filaments |
| Rhabdoid change | ?Null mutations in INI1 of SNF5/INI1 chromatin remodeling complex |
| ?Mutations in keratin 8 in malignant rhabdoid tumor | |
| Dot-like keratin in small cell carcinoma | ? |
| Pseudostratification of nuclei | ?Nuclear positioning apparatus, related to SUN–KASH proteins and the cytoskeleton |
| Loss of cell polarity | ?Disruption of apical–basal polarity |
| Cytoplasmic Auer rods | ? |
| Ringed sideroblasts | ?Related to mitochondrial iron metabolism |
| Nuclear lamina changes | |
| Nuclear grooves and inclusions in papillary thyroid carcinoma | Activation of tyrosine kinases RET/PTC and TRK/PTC, and probably activation of B-RAF |
| Nuclear grooves in granulosa cell tumor of ovary | Probably FOXL2 mutation |
| Acquired Pelger–Huet anomaly | ? (Lamin B receptor is basis for inherited Pelger–Huet anomaly) |
| Nuclear molding of small cell carcinoma | ? |
| Other forms of nuclear shape irregularity | ?Related to GATA6 and loss of emerin in ovarian cancer |
| Chromatin changes | |
| Euchromatic appearance of papillary thyroid carcinoma | Activation of tyrosine kinases (RET/PTC and TRK/PTC) and probably activation of B-RAF |
| Coarse chromatin texture | Activation of H-RAS, SRC, MOS, A-RAF, and MOX-1 in fibroblasts; activation of H-RAS in thyroid follicular cells |
| Chromatin of small cell carcinoma | ? |
| Nucleolar prominence in absence of reactive cytoplasm; high nuclear cytoplasmic ratio | ? |
There is a great deal of variation in pathologists' interpretation of the biologic significance of morphologic changes [Dey, 2010], and the present interpretation is aimed toward exposing testable hypotheses. Testing the basic hypothesis that the criteria of malignancy reflect a diverse set of abnormal physiologies will ultimately require collaboration between Cytologists and Cell Biologists, and restricting attention to particular criteria within specific microenvironments.
GROUP 1: TISSUE ARCHITECTURAL CHANGES
Tumor cells do not always show cellular-level structural changes (at least at the light microscope level in Papanicolaou stained samples [Zink et al., 2004]). For these types of cancer, diagnosis is based on large-scale tissue architectural changes. There are a wide variety of diagnostic alterations in tissue organization, too many to cover in a brief review. Many of the tissue-level diagnostic alterations span a distance of less than a few 100 µm, a size encompassed by the fine needle aspiration (FNA) or brush biopsies that are obtained and diagnosed by Cytologists. Cytology samples can either be deposited directly on a glass slide or be embedded for paraffin sectioning. Paraffin sectioning is often helpful for showing the larger-scale tissue architectural changes.
Most cancers arise from epithelial cells (called “carcinomas”), and many carcinomas arise from glandular cells (“adenocarcinomas”). Glandular epithelium is normally organized as a non-stratified, two-dimensional arrangement of evenly spaced cells lying on a basal lamina. The adhesion between an epithelial cell and the basal lamina is typically weaker than the adhesion between epithelial cells. Thus, when a biopsy brush passes over an epithelium, or when an FNA biopsy retrieves tissue, epithelial fragments are often separated from the extracellular matrix. Normal epithelial cells (Fig. 1A) are typically displayed as a two dimensional array of regularly spaced “honeycombed” epithelial cells. The association of epithelial cells to a basal lamina puts a constraint on the mechanisms that permit clonal expansion. One or a combination of the following six diagnostic changes to the organization of the epithelium would appear to be required to permit clonal expansion. Cell biology findings suggest a role for cancer gene function in at least some of these reorganizations.
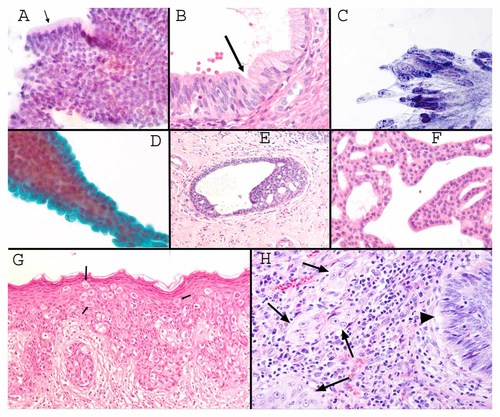
A: Normal glandular epithelium breaks away from a basal lamina in brush or FNA cytology samples and is deposited on a slide as a flat, honeycombed group of regularly spaced cells. Note the regular thickness of the apical cytoplasm (arrow) in part of the epithelium that is displayed perpendicular to the slide (Pap stain 200×). B: A paraffin-embedded 5 µm thick section of endocervical glandular mucosa is shown at the junction (arrow) between an endocervical adenocarcinoma (lower left) and normal endocervical epithelium with basally positioned nuclei to the upper right. Note the crowding of the adenocarcinoma cells that maintain very thin connections to the basal lamina (H&E stain, 200×). C: Pap test showing crowded, pseudostratified endocervical adenocarcinoma cells that are directly deposited on the slide without histological sectioning (Pap stain 600×). D: Papillary fragment in a breast FNA showing a layer of epithelial cells covering a thin cylinder of supporting fibrovascular matrix (Pap stain 200×). E: Histological section of a breast biopsy showing “usual ductal hyperplasia” characterized by stratification of cells that “mature” or change predictably depending on how far the cells are away from the basal lamina. Toward the center of the duct, the cells show increased heterochromatinization. There is also a tendency for adjacent cells to share polarity. (H&E 100×). F: Histological section of an FNA sample showing changes diagnostic of at least ductal carcinoma in situ. Compare to Figure 1E, there is no predictable alteration in the cellular appearance in spite of free stratification of the ductal cells, and there is no tendency for shared polarity. Basal lamina is not evident in this section. Cellular-level features are not particularly useful for diagnosing this lesion; diagnosis is based on larger-scale features (H&E 150×). G: Histological section showing Pagetoid spread of ductal carcinoma in situ that has extended out of the breast to colonize the periareolar skin. In spite of spreading for many centimeters, there is no “invasion” (H&E 100×). H: Histological section of invasive squamous cell carcinoma of cervix. The arrow head shows non-invasive carcinoma, and the arrows show clusters of invasive carcinoma that grow in an architectural pattern without reference to tissue landmarks. Note that the invasive tumor cells show larger nucleoli, more euchromatin, and more cytoplasm compared to the non-invasive tumor cells (see text, H&E 200×).
Crowding (Growth With Less Basal Lamina Contact)
Clonal expansion can take place if cells can survive with less basal lamina surface area (Fig. 1B). Cytologists refer to this as crowding (Fig. 1C), and it may bear a relation to “loss of contact inhibition.” Crowded cells often become more columnar in shape, for example, in a tubular adenoma of the colon and endocervical adenocarcinoma in situ. Crowded cells show less cytoplasm between nuclei (they show a criterion of “overlapping nuclei”) when viewed en face. Extremely crowded clones often show elongation of the nucleus within the thin columnar cytoplasm. Pseudostratification (nuclei at random positions relative to the basal aspect of the cells—see Group 3 below) is also typical.
Papillary Formation (Induction of New Basal Lamina)
A second mechanism for clonal expansion would be for an evolving neoplasm to induce more basal lamina surface area to grow on. Cytologists distinguish two types of increased basal lamina surface area. In “papillary” growth, new basal lamina surface area, supported by fibrovascular tissue, projects away from the pre-existing extracellular matrix and becomes lined on the outside by epithelium (Fig. 1D). Papillary growth patterns are typical of cutaneous human papilloma virus (HPV) warts, papillary thyroid carcinoma (PTC), a subset of breast neoplasms, early stages of some pancreatic cancers, the most common ovarian carcinomas (serous carcinomas), early stages in the evolution of most colonic neoplasms, and the most common urothelial neoplasms of the bladder. The mechanisms for an evagination of new basal lamina in papillary growth have not apparently received much attention. A papillary organization of some epithelia (e.g., urothelium or mammary epithelium) is not a recapitulation of any normal developmental process and is virtually diagnostic of neoplastic growth (including both benign and malignant papillary neoplasms).
Glandular Complexity
An outward branching of ducts, in which the epithelium is surrounded by a basal lamina and extracellular matrix is another apparent mechanism for tumor cells to increase the physical space needed for clonal expansion. Pathologists typically refer to this as glandular complexity and this is a prominent feature of the early development of several kinds of adenocarcinomas [Mutter et al., 2007]. Unlike papillary growth patterns, glandular outpouchings appear to recapitulate aspects of normal branching morphogenesis of development. The reciprocal interactions between stroma and epithelium in branching morphogenesis appear likely to be exploited by cancer cells in the process of increasing lamina surface area for clonal expansion [Wiseman and Werb, 2002; Yamada and Cukierman, 2007].
Stratification of Epithelial Cells
Loss of a requirement for attachment of epithelial cells to the basal lamina constitutes a fourth mechanism for clonal expansion within the geographic confines of an epithelium. The growth of epithelial cells without attachment to a basal lamina gives rise to three-dimensional “stratified” clusters of epithelial cells.
Stratification appears to be a manifestation of the cell biology concepts of anchorage independent growth or resistance to anoikis [Frisch and Screaton, 2001; Debnath et al., 2002], a phenotype induced by a growing number of cancer genes [Pratap et al., 2009]. Cytologists distinguish different degrees of apparent resistance to anoikis. Mammary ductal cells stratify (survive independent of a basal lamina connection) in a low-risk lesion termed “usual ductal hyperplasia” (Fig. 1E). In usual ductal hyperplasia, an admixture of ductal cells and myoepithelial cells typically maintain some degree of shared polarity (defined below) or they tend to show subtle predictable differences in their activity depending on how far they are from the basal lamina [Weaver et al., 1996]. When cells appear as if they grow with a random polarity to each other, and grow equally well whether or not they are next to a basal lamina (i.e., their nuclear and cytoplasmic features cannot be predicted based on how far away they are from the basal lamina) carcinoma in situ (non-invasive) is diagnosed (Fig. 1F). The distinction of the hyperplasias from ductal carcinoma in situ is a problem area in cytology for which insight from cell biologists would be valuable.
Intraepithelial Cell Migration (Pagetoid Spread)
A fifth mechanism for clonal expansion is “pagetoid spread” (named after the 19th century surgeon/pathologist James Paget) in which a pre-invasive clone of cells appear to find room by migrating intraepithelially away from its origins. Pagetoid spread can allow an intraepithelial spread of neoplastic cells over many centimeters, for example, starting with the breast duct system and extending out into the peri-areolar skin in Paget's disease (Fig. 1G). Pagetoid spread is easier to appreciate in histologic sections. It is common feature in early melanoma development (particularly lentigo maligna) and is also relatively common in the development of breast carcinomas. In breast carcinomas it is virtually universal in lobular carcinoma in situ, a tumor in which E-cadherin expression is specifically lost [Contreras and Sattar, 2009]. However, ductal carcinomas of breast (that at least have immunohistochemically detectable E-cadherin) can also sometimes show pagetoid spread, and it is ductal rather than lobular carcinoma that extends out onto periareolar skin. Targeted overexpression of ERK 1/2 in matrigel acinar cultures of MCF-10A human breast ductal cells produces an apparently pagetoid phenotype in which cell contacts are loosened by migrating cells, associated with partial loss of membranous E-cadherin staining but without other manifestations of epithelial mesenchymal transition, and without alteration in the topography of the basal lamina [Pearson and Hunter, 2007]. Pagetoid spread necessarily requires cell migration as well as at least transient dissociation of cells from a physical connection to the basal lamina, implying that resistance to anoikis is part of the process.
Invasion
The defining feature of invasion is growth of cells without a predictable relation to pre-existing tissue landmarks established during development (Fig. 1H). To discern these large-scale architectural features generally requires a histologic section. Mitotic activity is irrelevant to the diagnosis of invasion, and it is not paradoxical that invasive tumors can show fewer mitoses than in situ carcinoma or even the normal cells from which they arose [Fischer et al., 2004b]. An important and overlooked observation of pathologists is that it is insufficient to ascribe “invasion” to a digestive process whereby cells break down basal lamina or extracellular matrix components. The process of performing an incisional biopsy necessarily breaks through the basal lamina and physically displaces non-invasive tumor cells into the extracellular matrix, and yet incisional biopsies do not transform an in situ tumor into an invasive tumor. In situ tumor cells displaced into the presumably growth factor-rich extracellular matrix of a healing biopsy wound can be sometimes be seen to involute in subsequent excisional biopsies. Though ECM modeling and breakdown is clearly experimentally important for invasion of cancer cells [Wiseman and Werb, 2002], pathology observations suggest that invasion also requires loss of responsiveness to negative growth regulatory signals from extracellular matrix.
The previous six criteria appear to bear a direct relation to solving the problem of clonal expansion within the confines of epithelial organization. The functional significance of the following criterion, for the mechanism of clonal expansion is not as easy to envision.
Loss of Intercellular Adhesion
Cytologists are able to gauge the degree of cohesiveness of adjacent epithelial cells. The brushing or fine needle biopsy procedure exposes planes of cleavage that are not evident from histologic examination of intact paraffin-embedded biopsies. Loss of intercellular adhesion compared to normal cells is a common finding in cancers of diverse origin (illustrated in examples in Figs. 2 and 3). Not all cancers (even starting from the same cell of origin) show loss of adhesion, and loss of adhesion is sometimes seen in benign tumors or even benign non-neoplastic diseases, and thus the diagnostic value of loss of cohesion is modest.
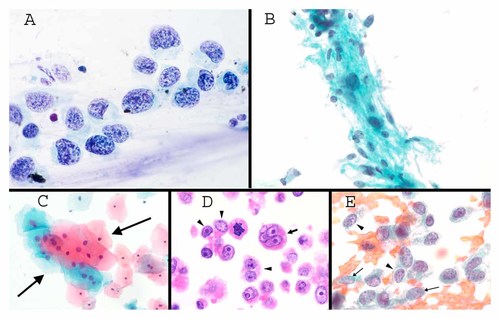
A: Pap test with squamous cell carcinoma in situ. The total amount of hematoxylin staining varies in every one of the cells, evidence for chromosomal instability. Note also the variation from cell to cell in the amount and density of cytoplasm. Note also the discohesive nature of the cells (Pap stain 1,000×). B: FNA of a benign schwannoma directly deposited on a slide without sectioning. The marked variability of DNA content (total amount of hematoxylin) is characteristic of polyploidization, with many cells showing a normal amount of hematoxylin, moderate numbers of cells showing twice normal and a few showing four or eight times normal amount of hematoxylin (Pap stain 300×). C: Benign squamous atypia in a Pap test (group marked by arrows). This type of atypia is common and may represent polyploidization. Note the commensurate increase in cytoplasm compared to the increased amount of hematoxylin in each cell compared to the normal cells above and to the right (Pap stain 100×). D: Highly genetically unstable ovarian cancer, as seen in a histological section. The arrow shows a micronucleus near the edge of the cytoplasm. Note the unpredictable variation in cytoplasmic features from cell to cell, and the unpredictability of nucleolar enlargement compared to amount of cytoplasm (see text in Group 3; H&E 400×). E: FNA of a poorly differentiated carcinoma with unpredictable variation from cell to cell in heterochromatin packaging. Note the coarse texture in the two cells marked by arrowheads compared to finer texture in the two cells marked with arrows (Pap stain 400×).
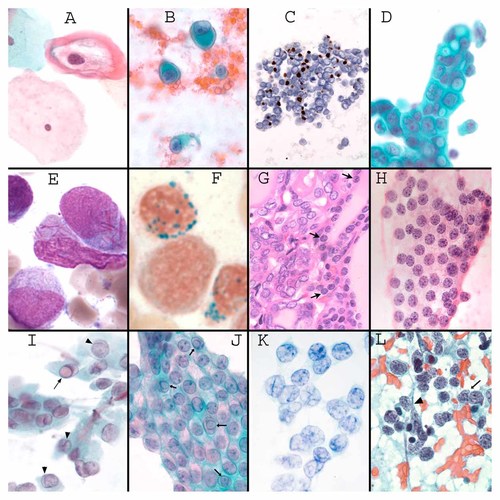
A: Pap test showing a koilocyte in which the keratin collapses peripherally through the binding of HPV E4, leaving a clear halo around the nucleus (Pap stain (600×). B: FNA of a melanoma showing cells with “rhabdoid” collapse of vimentin into a perinuclear ball (Pap stain 400×). C: Merkel cell tumor of skin (a kind of small cell carcinoma) immunostained for keratin 20 (brown) showing “dotlike” keratin. (Paraffin section of an FNA sample 200×). D: Breast FNA with adenocarcinoma, based on randomization of cell polarity, as judged on the basis of the long axis of the nuclei and the random orientation of cytoplasmic vacuoles. These types of vacuoles with a central dot are also highly specific for a diagnosis of mammary carcinoma (usually of the lobular type) (Pap stain 400×). E: Auer rods (magenta colored needle-like crystals) in acute myelogenous leukemia (Giemsa stain of a bone marrow aspirate, 1,000×, image courtesy Dr. Hongbo Yu, University of Massachusetts). F. Ringed sideroblasts in myelodysplasia (Prussian blue stain for iron, 1,000×, image courtesy Dr. Hongbo Yu). G: Histological section of the interface between a papillary thyroid carcinoma (left) and normal thyroid (delineated with arrows). Note the pale chromatin, and irregular shapes of some of the papillary thyroid carcinoma cells (H&E 200×). H: FNA of a follicular neoplasm. Like normal thyroid, follicular neoplasms have round nuclei and aggregates of heterochromatin (H&E 400×). I: FNA of papillary thyroid carcinoma. In comparison to follicular neoplasms, the heterochromatin is finely dispersed, and nuclei tend to be irregular in shape, often with formation of long shallow grooves (arrow heads) or intranuclear cytoplasmic inclusions (arrow) (see text) (Pap stain 400×). J: FNA of a pre-malignant cystic mucinous neoplasm of pancreas showing multiple intranuclear cytoplasmic inclusions (arrows) (Pap stain 400×). J. FNA of granulosa cell tumor of ovary, showing prominent longitudinal inward “nuclear grooves” of the lamina (Pap stain 600×). L. Small cell carcinoma of lung showing heterochromatin aggregates that fill the nucleus and do not appear to be organized with respect to the nuclear lamina. The lamina is also fragile, resulting in easy rupture of the nuclei (arrow head) and “nuclear molding” in which the nuclei passively conform to each other (arrow). Note also the lack of prominent nucleoli, and discohesion (FNA 300×).
Loss of adhesion is obviously a non-specific phenotype that could reflect alteration of the diverse physiologies related to integrins, desmosomes, adherens junctions, and/or tight junctions [Burridge and Tsukita, 2001]. Such “adhesion physiologies” are inter-related to apoptotic signaling/anoikis [Gilmore et al., 2009], wnt signaling [Adler and Lee, 2001], epithelial–mesenchymal transition [Thiery et al., 2009], and tumor cell migration and invasion [Mercurio et al., 2001]. There is also extensive cross-talk between many of these adhesion physiologies and the regulators of apical–basal cell polarity [Feigin and Muthuswamy, 2009] as discussed in group 3.
GROUP 2: CRITERIA RELATED TO GENETIC INSTABILITY
Abnormal Mitotic Figures and Unpredictable Variation in Degree of Chromasia
Genetic instability was apparent to pathologists studying cancer since the early 1900s (reviewed in [Bignold, 2002]). Many familiar criteria of malignancy amount to recognizing genetically unstable cells, for example, tripolar mitoses and asymmetric metaphase plates. In interphase cells, the criteria that relate to genetic instability involve comparisons between cells, as opposed to looking for a change within a single cell. It is the unpredictable variation amongst cells that seems characteristic of genetic instability, rather than the occurrence of a specific structural feature within a population of tumor cells.
DNA content can be roughly estimated by the total integrated amount of hematoxylin in the nucleus [Schulte and Fink, 1995], at least when comparing adjacent cells of the same cell type. Some commercialized hematoxylin staining protocols are sufficiently stoichiometric that hematoxylin is used for computerized screening based at least partly on DNA content abnormalities in cervical Pap tests (Hologic, Incorporated, Marlborough, MA). Such an estimate of DNA content takes into account both nuclear volume and the darkness of staining. Thus, resting lymphocytes that have a small heterochromatic (dark staining) nucleus have the same DNA content as neurons that have large, pale euchromatic nucleus. DNA content is harder to estimate in tissue sections than it is in direct smears of cytology samples, because tissue sections can cut off part of the nucleus.
Many forms of cancer show obvious variation from cell to cell in DNA content. A rapidly dividing normal population will typically have fewer than about 25% of cells in G2 (with twice normal DNA content) and S-phase. Thus, the majority of cells will have a discernable uniform diploid DNA content. Except that cells in G2 have a twice normal overall nuclear size and a commensurate approximate doubling of cytoplasmic volume, there are typically no specific differences that distinguish a G1 cell from a G2 cell in terms of chromatin morphology or cytoplasmic features in cytology material. In many tumors, a high degree of genetic instability is present, and for these tumors, a modal DNA content is not evident with hematoxylin staining (Fig. 2A). The majority of aneuploid tumors have an overall increased average DNA content, and accordingly, overall “hyperchromasia” is typical. Variation in the degree of chromasia (or objectively measured DNA content) is relatively subtle, apparently reflecting gains or loss of individual chromosomes—chromosomal instability. The amount of variation suggests that for many cancers, the majority of chromosome segregations are unequal?
Cytologists are incorporating objective markers of chromosomal instability into clinical practice, including especially fluorescent in situ hybridization (FISH) for chromosome numeration. Supernumerary centrosomes, or abnormally shaped centrosomes are other potential markers [Pihan et al., 2003]. HPV infection itself appears to induce genetic instability [Munger et al., 2004]. The correlation of increased p53 immunostaining (reflecting loss of p53 function) [Tomasini et al., 2008] and FISH abnormalities is substantantial, and in some highly unstable tumors, p53 status and aneuploidy can often be predicted based only on the H&E or Papanicolaou staining appearance [Cho, 2009]. Aneuploidy is not always apparent based on hematoxylin staining, and the presence of aneusomy detected by FISH can be useful for diagnosing cancer [Halling et al., 2000; Brankley et al., 2006]. Aneusomy has been shown to be a marker for risk of cancer development in histologically normal cells at a considerable distance from the site of subsequent evolving neoplasms [Bronner et al., 2008].
Distinction from polyploidization
In contrast to the relatively subtle variation in chromasia reflected in chromosomal instability, marked variation in chromasia can possibly reflect polyploidization, or completion of multiple rounds of DNA replication without a mitosis, to produce 4 n, 8 n, 16 n, etc. DNA contents. Polyploidization appears to be a relatively common benign change, seen in some normal cells and in some benign tumors. It is a prominent and characteristic of particular types of benign tumors, for example, schwannoma [Takeshita et al., 1985] (Fig. 2B). Polyploidization is characterized by commensurate increase in both nuclear chromasia and cytoplasic volume without unpredictable segregation of these two features. It is as if polyploid cells were merely bigger versions of diploid cells, with a similar chromatin pattern from cell to cell in spite of polyploidization. Polyploidization is physiological in megakaryocytes, and it has been well-documented in aging liver cells and cardiomyocytes subjected to mechanical stress (e.g., in hypertension or following a heart attack which decreases total cardiomyocyte content) (reviewed in [Ravid et al., 2002]). Tetraploidy is commonly observed in otherwise benign urothelial cells examined in FISH based assays [Bubendorf et al., 2001]. The occurrence of polyploidization has not been actively explored in other body sites, but it appears likely that tetraploid cells are common in many organs. For example, based on Papanicoalou-stained morphology, some terminally differentiated squamous cells from Pap tests of perimenopausal women appear commonly to be tetraploid (Fig. 2C). Such changes have no relation to HPV infection, and are without apparent risk of progression to cervical cancer [Cibas et al., 2005]. Some malignant tumors, however, seem to show a combination of polyploidization and chromosomal instability.
Polyploidization has been proposed as a precursor to chromosomal instability, and is proposed to require altered p53 for continued cell cycling [Storchova and Pellman, 2004; Ganem et al., 2007], but the apparent widespread nature of polyploidization puts this proposal into question. If polyploidization is a precursor to chromosomal instability, it would appear that the rate of progression would have to be very low.
Micronuclei
Micronuclei are sometimes seen in genetically unstable tumor cells as a diagnostic trait (Fig. 2D). Amplified DNA in tumor cells is known to be able to become extruded from the nucleus during S-phase, enclosed by a nuclear envelope as a “micronucleus” [Shimizu et al., 1998; Shimizu et al., 2007]. Micronuclei also form from acentric chromosomes, or lagging chromosomes in cells with mitotic spindle abnormalities [Rao et al., 2008].
Overall Cellular Pleomorphism
Regardless of the type of genetic instability, unpredictable variation from cell to cell in the amount and character of cytoplasm (Fig. 2A,D) is an important criterion that seems to reflect a genetically unstable population prone to tumor progression.
Unpredictable Chromatin Pattern Variation
Unpredictable variation in the chromatin texture in adjacent cells is a criterion of malignancy (Fig. 2E). Large-scale chromatin packaging patterns evident to pathologists appear likely to be determined by histone modification (see also below) [Zinner et al., 2006], and it is tempting to hypothesize that epigenetic instability is a characteristic of some tumors [Stein et al., 2009]. Evidence for epigenetic instability in X chromosome silencing in breast cancers has been reported [Pageau et al., 2007].
GROUP 3: SUB-CELLULAR CRITERIA NOT APPARENTLY RELATED TO GENETIC INSTABILITY
Unpredictable variation between cells is characteristic of genetic instability. When a specific diagnostic subcellular structural feature is shared by the cells of a tumor (and are not present in the cells from which the tumor arises), it is reasonable to infer that the structural trait has been selected for. Conserved features in genetically unstable cancers would appear likely to have a distinct heritable basis (induced by cancer genes), and have functional significance. It is useful to convey this point through analogy with Darwinian evolution. In Darwinian evolution, conserved morphologic features that distinguish related species are due to the genetic differences between the two species. For example, a broader beak shape in one of Darwin's finches is due to a selection for genes that affect beak morphology. Importantly, the altered beak morphology provides the mechanism for clonal expansion [Abzhanov et al., 2004]. By analogy, diagnostic cellular-level criteria of malignancy, conserved in genetically unstable tumors, should bear an important relation to cancer genes and altered cell physiology [Fischer et al., 2004b]. Given the broad range of morphologic findings in different cancers (even when starting from the same cell of origin and growing in the same microenvironment), one would have to predict that a large number of genetic events can contribute in complex ways to cancer development.
A few criteria in Group 3 have been directly linked to activation of cancer genes. These few criteria were documented in stages of tumor growth in which genetic instability is not a prominent feature, or in vitro model systems in which the genes could be studied in isolation from genetic instability. The background of genetic instability in so many tumors probably contributes to the masking of an underlying genetic basis for many of the other diagnostic traits in Group 3. The hypothesis that cellular-level criteria of malignancy are related to the function of oncogenes has been difficult to test because the cytology of Cell Biologists' model systems is not commonly documented.
Group 3 is organized by subcellular compartments involving cytoskeleton, cytoplasm, nuclear lamina, chromatin, and nucleoli. It is important to note that the same compartment (e.g., the nuclear lamina) can have distinctly different types of changes in clinically different types of tumors. In addition, distinctive changes in one cellular compartment can occur together with distinctive changes in other compartments.
Disorganization of Intermediate Filaments
HPV and koilocytes
The history of the discovery of a viral etiology of cervical cancers is a testament to the importance of using cellular morphologic clues to understand cancer (reviewed in [Stoler, 2000]). A peripheral collapse of intermediate filaments in infected squamous cells had been recognized to be characteristic of viral warts. Normal terminally differentiating squamous cells have a smooth regular distribution of keratins extending from the nuclear envelop to the cell periphery. The term “koilocyte” (“hollow cell”) was coined by Cytologists [Purola and Savia, 1977] for the appearance of the squamous cells of warty lesions, and it consists of a cell having intermediate filaments collapsed centrifugally away from the nucleus toward the periphery of the cell, with formation of a clear perinuclear space (Fig. 3A). Cytologic findings demonstrated an association of this distinctive koilocytic change with progression to invasive cervical cancer, supporting a common etiology with viral warts [Purola and Savia, 1977].
For HPV 16, the collapse of intermediate filaments (keratins specifically, and not vimentin or lamins) was shown to be due to the E4 protein of HPV [Doorbar et al., 1991]. The E4 protein is transcribed from a spliced transcript that includes codons for the first five amino acids of the E1 gene. In tissue culture cells, binding of phosphorylated HPV type 16 E4 with keratins results in collapse into a single paranuclear aggregate, whereas in tissue, the disassembled keratins complexed to E4 collapse centrifugally away from the nucleus [Wang et al., 2009]. The difference between the in vitro and in vivo morphology may be due to the circumferential peripheral tethering of keratins to desmosomes in tissue. Koilocytic change probably reflects the HPV-specific need to disrupt cellular integrity to facilitate shedding of virions from infected cells [Stoler, 2000]. Other localizations and functions of various E4 transcripts have also been described [Roberts et al., 2003]. Koilocytic change is commonly lost in the more advanced pre-cancerous lesions and invasive squamous carcinomas associated with HPV infection.
Rhabdoid change and dot-like keratin distribution
A collapse of intermediate filaments (keratins and/or vimentin) into a single perinuclear hyaline mass (dense and waxy-appearing by H&E or Papanicolaou stains) is a reliable criterion of malignancy in tumors of diverse cellular origins (Fig. 3B). The collapse into a perinuclear mass is referred to as “rhabdoid change,” referring to the similarity to embryonic mononuclear skeletal muscle cells laden with desmin (rhabdomyoblasts). Rhabdoid change is usually seen in highly aggressive cancers, and is relatively common in melanomas (in which case it is vimentin that collapses), as well as an occasional finding in a wide variety of other tumors.
One particular rare entity, “malignant rhabdoid tumor” is a highly aggressive tumor of infants and children of uncertain histogenesis, in which the collapsed intermediate filament are composed mostly of keratin and vimentin. Malignant rhabdoid tumor is characterized by bi-allelic null mutations in the SNF5/INI1 subunit (also known as BAF47 and SMARCB1) of one of the SWI/SNF chromatin remodeling complexes [Caramel et al., 2008]. While the association between chromatin remodeling and rhabdoid collapse of intermediate filaments has not been apparently explored, a direct relationship is suggested by several observations. A mouse model heterozygous for lack of SNF5/INI1 develops tumors with a rhabdoid phenotype [Roberts et al., 2000]. Deletion of the SNF5/INI1 locus by FISH has been demonstrated in some rhabdoid tumors other than the entity malignant rhabdoid tumor [Fuller et al., 2001], and an adult pancreatic carcinoma with rhabdoid change also showed a missense mutation in SNF5/INI1 [Cho et al., 2006].
One single paper identified missense mutations in keratin 8 in seven of seven malignant rhabdoid tumor cases, but not in a series of tumors lacking rhabdoid morphology [Shiratsuchi et al., 2002]. The missense mutations occurred in regions of the protein known to be important for filament assembly. No other molecular explanation for rhabdoid collapse has been apparently offered.
Small cell carcinoma, an aggressive tumor that can arise from many different apparently “neuroendocrine” cells, shows a feature similar to rhabdoid change. The small amount of keratin in small cell carcinoma collapses into a dot-like perinuclear aggregate as an important diagnostic feature of small cell carcinomas (Fig. 3C).
Pseudostratification of Nuclei
Normal glandular cells tend to show a basally located nucleus with a regular amount of apical cytoplasm. Pseudostratification refers to positioning of the nucleus at various planes above the basal aspect of non-stratified epithelium (Fig. 1B,C). Some types of normal epithelium (e.g., respiratory and fallopian tube) can look pseudostratified, due to the admixture of different cell types in the epithelium. For glandular epithelia composed normally of just one cell type, pseudostratification can be seen in regenerating benign epithelium, but commonly it is also an early neoplastic change preceding carcinoma in situ.
Pseudostratification may be related to the emerging cell biology concept of nuclear positioning (reviewed in [Burke and Roux, 2009]), in which there is aberrant nuclear positioning relative to the apical–basal polarized cytoplasm. The mechanism for abnormal nuclear positioning in human cancers has apparently not been explored. Pseudostratification typically precedes true stratification in the evolution of many adenocarcinomas, suggesting that aberrant nuclear positioning would typically precede loss of resistance to anoikis. Nuclear positioning involves active physiological processes during development. For example, the nuclei of developing neuroepithelial cells migrate from an apical to basal and back to apical during the cell cycle, and nuclear positioning is crucial to the arrest of neuronal nuclei in stereotyped positions in the various layers of cortex. Wound healing involves directed movement of the nucleus in relation to the microtubule organizing center [Gomes et al., 2005].
The molecular basis of nuclear positioning relative to the apical–basal polarity involves a growing number of LINC (LInker of Nucleoskeleton and Cytoskeleton) proteins that span the inner and outer nuclear membrane to form a mechanical connection extending from the cytoplasm through the two membranes of nuclear envelope to chromatin [Starr and Han, 2003; Burke and Roux, 2009]. SUN domain-containing proteins are inner nuclear membrane transmembrane proteins that associate (within the perinuclear space) with outer nuclear membrane integral membrane proteins bearing KASH domains. SUN–KASH pairs interact with microtubules (directly or via dynein or kinesin), actin, and apparently intermediate filaments (via plectin). Remarkably, a direct mechanical link mediated by a SUN–KASH pair connects the cytoskeleton (possibly microtubules) to individual meitotic chromosomes, contributing to homologous chromosome pairing prior to meiosis 1 prophase (prior to nuclear envelope breakdown; reviewed in [Hiraoka and Dernburg, 2009]). Some SUN–KASH pairs show cell-type specificity, including the KASH-containing protein Nesprin 4 which was recently suggested to maintain the normal basal localization of exocrine glandular nuclei [Roux et al., 2009].
Loss of Cell Polarity
Loss of polarity is an important large-scale criterion for cancer diagnosis. Normal cells show a predictable orientation in adjacent cells. Loss of polarity is estimated by Cytologists based on the long axis of the nucleus of a cell, or by positioning of exocrine products. Random polarity of epithelial cells is potent evidence for carcinoma (Fig. 3D), as if cells had no predictable relation to each other or to environmental cues. Stratification of carcinoma cells (see above) generally occurs coincidental to loss of predictable orientation of the long axis of adjacent nuclei, suggesting a mechanistic relation between these criteria.
It seems likely that Cytologists' concept of polarity is related to cell biologists' concept of apical–basal polarity. If so, objective markers of apical and basal cytoplasm, or assessment of the determinants of apical–basal polarity should have diagnostic value. Loss of basal–apical polarity is induced in vitro by a number of cancer genes commonly mutated in a wide variety of human tumors. Loss of basal–apical polarity contributes to resistance to anoikis [Aranda et al., 2006] (reviewed in [Feigin and Muthuswamy, 2009]), further suggesting a mechanistic relation between loss of basal–apical polarity and stratification. One of the apical–basal determinants, Scribble, functions as a tumor suppressor in mammary tumorigenesis [Zhan et al., 2008]. The interplay between loss of basal–apical polarity and carcinogenesis is further highlighted by the ability of the transforming gene HPV E6 to induce degradation of apical–basal polarity determinant Scribble, and mutations in E6 that lack the ability to degrade Scribble are defective in “transforming” cells [Nakagawa and Huibregtse, 2000].
Cytoplasmic Auer Rods
Auer rods are elongated, needle-like cytoplasmic crystalline inclusions in immature myeloid cells (Fig. 3E). They are composed of fused lysosomes containing myeloperoxidase and acid and alkaline phosphatase—normal components of differentiating myeloblasts—and are virtually diagnostic of either acute myeloid leukemia or clonal pre-leukemic myelodysplastic syndromes (reviewed in [Yoshida et al., 2009]). They are hard to detect by H&E staining or Papanicolaou staining, but are readily apparent by a Giemsa stain used on air-dried fixed smears (see www.cytopathology.org for protocol). They are found in at least some cells of about 50% of acute myelogenous leukemia patients, and when present, their number can vary from many per cell to rare [Zittoun et al., 1984]. The genetic basis for their formation and functional significance are obscure [Yoshida et al., 2009].
Ringed Sideroblasts
Abnormal iron accumulation in the mitochondria of erythroid precursors, is the defining characteristic of sideroblastic anemias [Sheftel et al., 2009]. Iron is detectable with cytochemical stains and the mitochondria are (normally) arranged in a perinuclear ring (Fig. 3F). Several hereditary forms of sideroblastic anemias have been defined genetically, characterized by mutations in the pathways of heme synthesis (which takes place in mitochondria). Heme synthesis has to be completed before iron is incorporated, and feedback loops cause accumulation of iron in the mitochondria. Acquired forms of sideroblastic anemias have been described, for example, due to drugs or vitamin B6 deficiency that interfere with heme synthesis. These acquired forms as well as the hereditary forms of sideroblastic anemia are not clonal or pre-malignant. However, an idiopathic acquired form of sideroblastic anemia is characteristic of a clonal, pre-leukemic myelodysplastic syndrome called refractory anemia with ringed sideroblasts (RARS). Of the heterogeneous groups of myelodysplastic syndromes, RARS has a relatively low rate of progression to acute leukemia. The genetic basis of sideroblast formation in RARS remains unclear, and there are no clear biochemical clues as to why sideroblasts are associated with clonal evolution only in RARS. Heme synthesis in RARS is rarely impaired, suggesting that a more downstream mitochondrial event is affected, such as reduction of ferric to ferrous iron, incorporation of ferrous iron into the heme, or possibly export of iron-bound heme from the mitochondria for incorporation into cytoplasmic hemoglobin [Gattermann, 2000]. Sideroblasts are evident at earlier stages of erythyroid differentiation in RARS compared to the other non-clonal forms of sideroblastic anemia [Sheftel et al., 2009], and mitochondrial autophagy is more pronounced and occurs at earlier stages of erythroid differentiation in RARS as well as other myelodysplastic patients compared to normal subjects [Houwerzijl et al., 2009].
Nuclear Lamina Alterations
There are a wide variety of nuclear lamina changes diagnostic of various types of tumors. Many of the distinctive lamina alterations are apparently independent of genetic instability, due to their conservation through clonal evolution, or their appearance in relatively genetically stable tumor cells. Some nuclear lamina alterations appear to be a consequence of activation of particular cancer genes. However, a precise structural explanation for any of the nuclear lamina alterations in cancer is lacking. What follows is a morphologic description of different types of nuclear lamina alteration and the cancer genes that apparently signal for a reorganized lamina. At the end is a brief summary of the possible structural basis of lamina alterations.
Nuclear grooves and cytoplasmic inclusions in papillary thyroid carcinoma
Papillary thyroid carcinoma (PTC) provides an example of diagnostic criteria being directly induced by cancer genes. Normal thyroid epithelium gives rise to two types of tumors: follicular neoplasms and PTC (reviewed in [Rosai et al., 1992]). PTC commonly shows a diploid DNA content [Kondo et al., 2006]. These two types of tumors are distinctive morphologically and clinically, and they bear different activated cancer genes (reviewed in [Kondo et al., 2006; Nikiforov, 2008]). PTC has a distinctive nuclear morphology consisting of both a strong tendency for irregularity of the nuclear lamina and a dispersal of heterochromatin into smaller-sized aggregates that appear to result in a smoother heterochromatin layer associated with the lamina (Fig. 3G). The peculiar chromatin changes are discussed below, though they may bear a relation to the lamina changes. Both normal thyroid epithelial cells and follicular neoplasms have a spherical nuclear lamina and small blocks of heterochromatin many of which have a lamina association (Fig. 3H).
The lamina of PTC tends to be folded into long “grooves”, and they have a strong tendency to develop “intranuclear cytoplasmic inclusions” (Fig. 3I) [Fischer et al., 2001; Papotti et al., 2004]. Intranuclear cytoplasmic inclusions are rare in follicular neoplasms and virtually non-existent in normal follicular cells, and thus they have very high predictive value for a diagnosis of PTC. These inclusions consist of spherically shaped partial invaginations of cytoplasm, into the nucleus (Fig. 3I). Intranuclear cytoplasmic inclusions are also seen in a variety of other neoplasms (Fig. 3J). They are a characteristic finding in melanoma and hepatocellular carcinoma. They are rare in non-neoplastic cells. Nuclear grooves are not unique to PTC. The grooves are similar to the grooves in granulosa cell tumor (below), Langerhans cell histiocytosis, and occasionally in other carcinomas. Moreover, nuclear grooves can be seen in a variety of physiologically “reactive” normal cells.
Due to the distinctive morphology of PTC, tiny tumors composed of relatively few cells can be discerned. PTC appears histologically to arise in one step since there is no recognized precursor. The discovery of translocations activating one of two tyrosine kinases (RET and TRK, giving rise to RET/PTC or TRK/PTC) in very small PTCs that lacked other mutations, led to the direct demonstration that RET/PTC or TRK PTC were sufficient to induce nuclear lamina irregularity (as well as heterochromatin dispersal) when expressed in normal thyroid epithelium [Fischer et al., 1998a; Fischer et al., 2000]. Rearrangement of RET or TRK are mutually exclusive, and together these genetic changes are only identified in about one-third of PTC samples. More recently, point mutations in B-RAF were identified in the majority of PTC (including small probably diploid tumors), and mutations are also essentially always mutually exclusive to the presence of RET/PTC or TRK/PTC [Nikiforov, 2008]. While a direct test of the ability of B-RAF to induce heterochromatin dispersal and nuclear lamina irregularity has not been done, a transgenic mouse model expressing B-RAF develops multifocal PTC with characteristic nuclear changes [Knauf et al., 2005], suggesting that B-RAF is sufficient to induce the same changes.
RET/PTC and TRK/PTC rearrangements are virtually restricted to PTC and are not found in other types of tumors. However, B-Raf activation is detected in a wide variety of tumors. For example, it is activated in a majority of melanomas as well as benign melanocytic nevi [Michaloglou et al., 2008]. It is interesting that benign nevi and melanomas characteristically show intranuclear cytoplasmic inclusions, like those of PTC. Other tumors of diverse histogenesis can show PTC-like nuclear morphology, but a directed effort has never identified RET/PTC or TRK/PTC rearrangements in these PTC-like tumors [Hameed et al., 2009]. One single case of a PTC-like breast cancer was not found to have a B-Raf mutation [Cameselle-Teijeiro et al., 2006].
Nuclear grooves in granulosa cell tumor of the ovary associated with FOXL2
Adult granulosa cell tumor of the ovary, a relatively uncommon tumor compared to ovarian carcinomas, commonly has a diploid DNA content [Evans et al., 1995] and it shows very characteristic long nuclear lamina “grooves” (Fig. 3K), similar to PTC. Normal granulosa cells have a spherical shape. Granulosa cell tumors do not tend to show intranuclear cytoplasmic inclusions.
The FOXL2 (forkhead-winged helix family of transcription factors) gene was recently shown to have a point mutation in 97% of granulosa cell tumors [Shah et al., 2009]. Mutations were not found in more than 350 unrelated tumor types [Shah et al., 2009]. Remarkably, the FOXL2 mutation was identified by sequencing the entire transcriptome of diploid granulosa cell tumors, and it was essentially the sole abnormality identified. Given the mutation-free background in granulosa cell tumors, FOLXL2 would therefore be a strong candidate to induce formation of diagnostic nuclear grooves in this tumor. However, a direct test of this hypothesis has not been conducted.
Acquired Pelger–Huet anomaly
When cells of the myeloid lineage mature, they develop a post-mitotic progressive lobulation of the nucleus starting with an indentation at the stage of a metamyelocyte. The circulating cells of the myeloid series are called neutrophils, and their nuclei normally have 3–5 lobes joined by thin nuclear lamina-bound chromatin bridges. Pelger–Huet change refers to a decreased lobation of neutrophils, typically to two symmetric lobes or even a non-lobated round neutrophil nucleus. The Pelger–Huet anomaly can be seen in several settings, importantly as a highly specific marker for an acquired pre-leukemic clonal disorder called myelodysplasia [Speeckaert et al., 2009]. A hereditary form of Pelger–Huet anomaly is due to mutations of the nuclear lamina protein Lamin B receptor (LBR) [Hoffmann et al., 2002]. Heterozygotes have decreased LBR and Pelger–Huet anomaly with few if any measurable effects on neutrophil function and no predilection for leukemia. It is not yet known whether the myelodysplasia-associated Pelger–Huet change is related to LBR [Speeckaert et al., 2009].
Nuclear molding in small cell carcinomas
Small cell carcinoma (see Fig. 3L) shows prominent nuclear “molding” in which the nuclei are so soft that they conform to each other, and they break open or “crush” easily in pathology samples. Small cell carcinomas are highly aggressive, with marked genetic instability, high proliferation, and early metastasis.
Other nuclear shape abnormalities
Many other tumors show stochastic irregularity that is difficult to classify and separate from underlying genetic instability. Pancreatic adenocarcinoma typically has marked polylobulation of the nuclei into “popcorn” like shapes. In general, many forms of cancer have a larger nucleus than the corresponding normal cells, with more lamina surface area. However, DNA content is commonly also increased in cancer cells, and it is not obvious how these two features related to each other.
A single recent report linked nuclear contour irregularity to loss of the transcription factor GATA6 and diminished expression of the nuclear lamina protein emerin in ovarian cancer [Capo-chichi et al., 2009]. The loss of emerin was at a post-transcriptional level. The authors showed that direct knock down of emerin (but not lamin B1) was sufficient to induce nuclear irregularity [Capo-chichi et al., 2009]. Further, the authors showed that loss of immunohistochemically detectable emerin was present in about a third of clinical ovarian cancer samples [Capo-chichi et al., 2009]. A general contribution of emerin to lamina irregularity in cancer is not clear, however, as discussed below.
Cell Biology basis of nuclear irregularity
The large-scale organization of the nuclear lamina defines nuclear shape (reviewed in [Gruenbaum et al., 2005]). The lamina is the proteinaceous layer associated with the inner surface of the inner nuclear membrane, and it comprises non-transmembrane lamins A/C, B1, and B2 as well as about 80 transmembrane proteins specifically localized at the lamina [Schirmer et al., 2003]. Many of them are still poorly characterized and the majority have >10-fold variation in expression amongst different cell types [Wilkie and Schirmer, 2006]. These proteins intersect a wide variety of nuclear physiologies [Gruenbaum et al., 2005], and it is tempting to relate changes in nuclear shape in tumor cells to alterations in physiologies associated with these proteins. Lamina proteins are mutated in a growing number of “laminopathies”—degenerative diseases involving principally muscle, nerves, and fat. For example, mutations in Lamin A/C or the transmembrane protein emerin is found in various neuromuscular degenerative diseases, and nuclear irregularity has been hypothesized to be important in the cellular degeneration characteristic of these diseases [Broers et al., 2004; Lammerding et al., 2004] (reviewed in [Dauer and Worman, 2009]).
However, the relation of lamina proteins to abnormal nuclear shape in cancer cells, or more broadly, a relation of nuclear lamina irregularity to an altered lamina physiology in tumor cells is far from clear. Most cells in patients with laminopathies have no discernable abnormality of shape [Lammerding et al., 2005], and conspicuously absent from the disease associations in the laminopathies is predisposition to cancer [Dauer and Worman, 2009]. While experimental mutations in lamins lead to abnormal nuclear shapes [Schirmer et al., 2001; Favreau et al., 2003], the mutations described so far induce aggregation of lamins. Aggregated lamina proteins have not, to the best of our knowledge, been observed in any tumor cells with nuclear irregularity. Lamina immunostaining shows a regular thickness of the lamina for lamin A/C, Lamin B1, emerin, and LAP 2 throughout the irregular folds and intranuclear inclusions of the nuclei of PTC, with a regular distribution of nuclear pores [Fischer et al., 2001]. Similar smooth distribution of lamina proteins has been shown in breast cancers with nuclear shape abnormality [Bussolati, 2008; Bussolati et al., 2008]. Overexpression of lamin B1 can cause expansion of the nuclear lamina with resulting redundant folds [Favreau et al., 2003]. However, normal amounts of lamin B1, have been detected in the irregular (diploid) nuclei of PTC with nuclear irregularity [Fischer et al., 2001]. Depletion of the newly discovered transmembrane nuclear lamina protein LEM2 in tissue culture cells leads to nuclear lamina irregularity, but with reduced cell survival [Ulbert et al., 2006]. A proteomic approach [Schirmer et al., 2003] to compare nuclear lamina composition between normal cells and genetically stable tumor cells with lamina irregularity (e.g., normal thyroid epithelium compared to PTC) would be a direct means to begin to test whether lamina proteins contribute to nuclear shape abnormalities.
The irregularity of the lamina induced by RET/PTC develops during interphase following microinjection of RET/PTC, without an intervening post-mitotic nuclear lamina reassembly [Fischer et al., 2003]. These results point to the possibility of an interphase force exerted on the lamina from chromatin or the cytoskeleton (reviewed in [Fischer et al., 2004a]). One single study identified beta catenin accumulation in many of the intranuclear cytoplasmic inclusions of PTC [Rezk et al., 2004]. Electron microscopy studies have not shown consistently distinctive traits of the cytoplasm or nuclear lamina associated with the intranuclear cytoplasmic inclusions in PTC [Johannessen et al., 1983; Oyama, 1989]. Distortion of the interphase lamina by chromatin-based forces could arise from large-scale interphase chromatin repositioning [Chuang et al., 2006], or theoretically from interphase condensations and decondensations of chromatin.
The lobulated nuclei of cell lines from pancreatic cancer, melanoma, and HeLa cells were shown to be demarcated by bands of perinuclear intermediate filaments, suggesting that lamina indentations can sometimes be caused by disorganized bundles of intermediate filaments pushing on the lamina [Kamei, 1994].
It is important to note that dramatic nuclear shape changes are physiological in the differentiation of lymphoid cells.
The molecular basis of the lack of resiliency of the lamina of small cell carcinoma is not known. Small cell carcinomas have very scant cytoplasm with a dot-like aggregation of their scant keratin as a diagnostic trait (see above). The absence of a robust cytoplasm could contribute to a lack of rigidity of the nuclei in small cell carcinomas, but bare nuclei stripped of cytoplasm from other cell types do not appear to crush as easily. Small cell carcinomas lack lamins A and C [Broers et al., 1997]. However, other normal cells and tumors (including the histogenetically related low-grade neuroendocrine carcinomas) also lack lamin A and C and they do not show fragile nuclei. Small cell carcinomas also show a distinctive chromatin organization with respect to the lamina (see below), and this may also bear a relation to the fragility of the nucleus.
Chromatin Alterations
A wide variety of chromatin texture alterations have diagnostic value in specific contexts. Chromatin changes are difficult to quantify and we only describe a few relatively clear-cut examples. Some chromatin texture changes develop as a consequence of cancer gene signaling, but the actual structural basis of altered chromatin packaging in these examples is not understood. What follows is a morphologic description of a few criteria involving chromatin, with a description of the associated cancer genes and observations relevant to finding their precise structural basis.
Euchromatic appearance of papillary thyroid carcinoma
PTC shows a characteristic lack of heterochromatin aggregates in the central parts of the nucleus, and the heterochromatin associated with the lamina is thickened and smooth across the folds of the lamina (Fig. 3G,I). In comparison, follicular type thyroid tumors show distinct blocks of heterochromatin (Fig. 3H).
The tyrosine kinases RET/PTC and TRK/PTC and probably activated B-Raf induce the chromatin dispersal in thyroid follicular cells [Fischer et al., 1998a, 2000]. The chromatin dispersal is difficult to quantify (though it is one of the most important diagnostic traits of PTC?), and no data exists on the time course of the chromatin changes.
The large-scale packaging of chromatin is related to the patterns of histone modifications of nucleosomes—an apparent histone code for cytologists [Cremer et al., 2004; Zinner et al., 2006]. However, it is not clear how a histone code could dictate cell-type or tumor-type specific intranuclear patterns of heterochromatin formation. Three possibilities (not mutually exclusive) would be for global changes in histone modifications, redistribution of histone marks at different chromosomal loci, or reorganization of existing marks into different heterochromatic domains.
Global increases in histone acetylation, induced by trichostatin, causes a large-scale global euchromatinization in vitro [Toth et al., 2004; Bartova et al., 2005]. However, by electron microscopy, the total volume of PTC heterochromatin is the same, but dispersed into smaller aggregates [Johannessen et al., 1978, 1983]. Accordingly, global differences have not been found to distinguish PTC from normal thyroid epithelium for histone H3K4 mono-, di-, and tri-methylation, H3K9mono and tri methylation, H3K9/14 acetylation, H3K27mono and tri methylation, or H2AX phosphorylation (A.H. Fischer and S. Schattgen, unpublished work). If histone marks were shifted to new positions in PTC, one would expect relatively major changes in gene expression. The interphase organization of transcription in PTC (distribution of nuclease hypersensitive sites, phosphorylated RNA Pol II, and splicing factors) are not distinctively different between follicular type thyroid tumors and PTC (A.H. Fischer and J. Nickerson, unpublished work). Relatively few changes in gene expression distinguish PTC from follicular neoplasm or normal thyroid cells, and consistent differences are limited to an even smaller handful of genes [Huang et al., 2001; Borrello et al., 2005]. It is therefore not obvious that the dramatically altered chromatin morphology of PTC relates to alteration in transcription. It seems unlikely that the altered chromatin of PTC relates to cell cycle kinetics or apoptosis resistance since PTC and follicular neoplasms both show a very low and overlapping proliferation rate and apoptotic rate [Yoshida et al., 1999; Biesterfeld et al., 2003].
Heterochromatin reorganization can have surprising and unexpected functional significance, apparently independent of transcription, cell cycle signaling, or susceptibility to apoptosis: In one well-characterized dramatic example, heterochromatin distribution is shifted to the center of the nuclei of the rod cells of the retina of nocturnal mammals, apparently in order to function as a lens to focus light on the underlying photoreceptors [Solovei et al., 2009]?
Compared to normal thyroid, PTC shows lower average global DNA methylation levels by immunohistochemistry. However, the levels vary in different PTC cases and are not different on average from follicular-type carcinomas that have a different chromatin morphology [de Capoa et al., 2004; Galusca et al., 2005]. A general contribution of DNA methylation to large-scale chromatin organization is not clear. While pericentric heterochromatin is highly methylated, pericentric heterochromatin does not appear to disperse upon 5-azacytidine treatment [de Capoa et al., 1998]. Demethylation of DNA with 5-azacytosine was not observed to grossly alter heterochromatin organization in two cell lines with different baseline heterochromatin textures (A.H. Fischer, unpublished work).
Coarse chromatin texture
Various tumors commonly show coarser, more angular heterochromatin than normal cells (Fig. 2A). The heterochromatin in resting lymphocytes or maturing nucleated red blood cell precursors is more rounded. The coarsening appears distinct from the hyperchromasia due to hyperdiploidy.
H-(V-12)-Ras activation is common in follicular thyroid neoplasms (but not PTC), and expression of H-(V-12)-Ras in normal thyroid epithelial cells in vitro induces coarsening of heterochromatin similar to what is sometimes seen in follicular neoplasms [Fischer et al., 1998a]. When expressed in rodent fibroblasts, activating mutations in H-RAS, SRC, C-RAF, and v-Mos induce a similar angular coarsening of heterochromatin [Mello and Chambers, 1994; Fischer et al., 1998b]. In one study, the rank order of increasing coarsening of the chromatin by two independent pathologists was virtually identical, and the rank-order correlated nearly perfectly with the rank-order of increasing metastatic potential of the mouse fibroblast cell lines [Fischer et al., 1998b]. An interesting observation was that the extent of coarsening did not correlate with growth fraction, suggesting that the coarsening has a biological association independent of cell cycle progression signaling [Fischer et al., 1998b]. Accordingly, no change in heterochromatin aggregation is induced by re-feeding serum-starved fibroblasts [Fischer et al., 2004a], and coarsening of chromatin is not induced by a constitutively active MAPKK or Myc [Fischer et al., 1998b]. A morphologically similar coarsening of chromatin is induced in fibroblasts expressing the superoxide producing transforming protein Mox1 [Suh et al., 1999] (A.H. Fischer, unpublished work). It is not known if the coarsening is related to the formation of senescence-associated heterochromatin that can be induced by some of these oncogenes in certain cellular contexts [Narita et al., 2003]. Senescence-associated heterochromatin appears to have a more smoothly rounded appearance.
Chromatin of small cell carcinoma
Small cell carcinomas have no large internal areas of euchromatin, and the lamina does not have the same predictable aggregation of heterochromatin seen in normal cells or other types of tumors. Angular blocks of heterochromatin seem dispersed throughout the nucleus without accentuation of heterochromatin at the lamina (Fig. 3L). Small cell carcinomas also characteristically do not show prominent nucleoli. The fragility of the lamina, and scant cytoskeleton with dot-like keratin aggregates complete the morphologic description of this aggressive tumor.
Abnormalities of Nucleoli or Ribosomes
A common important criterion is nucleolar prominence in the absence of an apparent increase in the number of ribosomes in the cytoplasm [Frost, 1986] (Fig. 2D). A probably related criterion is “high nuclear to cytoplasmic ratio.” In physiologically activated cells (e.g., in epithelial cells proliferating to cover an ulceration), nucleolar prominence is matched by an increase in the amount of cytoplasm and cytoplasmic protein synthesis. The quantity of ribosomes and mRNA can apparently be roughly estimated by the amount of hematoxylin staining in the cytoplasm. Histochemical studies from the 1940s documented a disproportionate amount of nucleolar RNA to cytoplasmic RNA and protein as a specific feature of cancers [Caspersson and Santesson, 1943]. Explaining this finding, Caspersson et al. wrote: “A cell can be diagnosed as a malignant tumour cell…when external factors are so unfavorable for growth that no cytoplasmic increase takes place although the system of protein formation gives evidence of intense stimulation” [Caspersson and Santesson, 1943]. The molecular mechanism and genetic underpinning of the disproportionate increase in nucleolar size compared to cytoplasmic protein production or accumulation remains obscure [Fischer et al., 2004a], but the cross-talk between the protein synthetic machinery and other cell physiologies, including cell cycle progression, is extensive [Young et al., 2007] (reviewed in [Ruggero and Pandolfi, 2003; Lempiainen and Shore, 2009]). The effects of increased nucleolar size are not likely to be trivial to the cell, given the high energy requirement for producing the protein synthetic machinery [Warner, 1999].
It is important to note that inconspicuous nucleoli are actually characteristic of some cancers and pre-malignant neoplastic processes. Examples include small cell carcinoma (Fig. 3L) and intraepithelial squamous dysplasia (Fig. 2A), respectively. Both are genetically unstable and have a high growth fraction, yet throughout the clonal proliferation there is a uniform absence of nucleolar prominence.
Generally, cellular level cytologic features do not predict whether a cancer cell is invasive or whether it is still growing in situ. A major exception is invasive squamous cell carcinoma (e.g., the most common form of cervical cancer). While the pre-invasive intraepithelial neoplasias characteristically lack nucleoli, invasive squamous cell carcinomas often acquire an enlarged nucleolus along with more euchromatin, and increased amounts of cytoplasm (Fig. 1H) [Cibas and Ducatman, 2009].
CONCLUDING REMARKS
The reciprocal relation of structure and function at all levels of biology suggest that the criteria of malignancy relate in an essential manner to the altered physiologies of cancer cells. Several distinct criteria are directly induced by specific cancer genes, but for many criteria there are no known relationships to particular cancer genes. More attempts to relate specific genetic events with particular cellular level structural changes would likely expose other cancer genes that function like “nuclear morphogens” in cancer. Even for criteria linked to particular cancer genes, there is still a lack of understanding of the precise molecular basis of the structural changes. Deciphering the molecular basis of any of the criteria will undoubtedly lead to improved cancer diagnosis, and provide insights into the mechanisms by which cancer genes contribute to clonal evolution.
The ASC, through the Cell Biology Liaison Working Group is reaching out to cancer researchers. At the ASC website (www.Cytopathology.org) protocols for Cell Biologists can be found. Researchers can also send their model systems coded for cytologic assessment, using the Papanicolaou stain to cast a broad net for cell structural correlates of experimental manipulations. A future goal is to catalog the Papanicolaou stained appearance of different model systems. It is through collaboration between Cell Biologists and Cytologist that we can extract the full meaning of the criteria of malignancy.




