Mechanical control of cAMP signaling through integrins is mediated by the heterotrimeric Gαs protein†
Francis J. Alenghat and Jessica D. Tytell contributed equally to this work.
Abstract
Mechanical stresses that are preferentially transmitted across the cell surface via transmembrane integrin receptors activate gene transcription by triggering production of intracellular chemical second messengers, such as cAMP. Here we show that the sensitivity of the cAMP signaling pathway to mechanical stresses transferred across β1 integrins is mediated by force-dependent activation of the heterotrimeric G protein subunit Gαs within focal adhesions at the site of stress application. Gαs is recruited to focal adhesions that form within minutes following clustering of β1 integrins induced by cell binding to magnetic microbeads coated with activating integrin ligands, and β1 integrin and Gαs co-precipitate when analyzed biochemically. Stress application to activated β1 integrins using magnetic twisting cytometry increases Gαs recruitment and activates these large G proteins within focal adhesions, as measured by binding of biotinylated azido-anilido-GTP, whereas application of similar stresses to inactivated integrins or control histocompatibility antigens has little effect. This response is relevant physiologically as application of mechanical strain to cells bound to flexible extracellular matrix-coated substrates induce translocation of phospho-CREB to the nucleus, which can be attenuated by inhibiting Gαs activity, either using the inhibitor melittin or suppressing its expression using siRNA. Although integrins are not typical G protein-coupled receptors, these results show that integrins focus mechanical stresses locally on heterotrimeric G proteins within focal adhesions at the site of force application, and transduce mechanical stimuli into an intracellular cAMP signaling response by activating Gαs at these membrane signaling sites. J. Cell. Biochem. 106: 529–538, 2009. © 2009 Wiley-Liss, Inc.
Mechanotransduction—the process by which cells sense a mechanical stimulus and convert it into an intracellular biochemical response—is critical for normal tissue development, and abnormal mechanosensitivity contributes to the development of a wide variety of diseases, including atherosclerosis, hypertension, osteoporosis, and cardiac arrhythmias [Ingber, 2003]. All cells within solid tissues experience mechanical stress through distortion of the extracellular matrix (ECM) due to both large-scale forces (e.g., hemodynamic stresses, lung ventilation, bone compression) and microscale forces arising from cell-generated contractility [Davies and Tripathi, 1993; Gimbrone et al., 2000; Ingber, 2006]. These mechanical forces impact tissue development and homeostasis by altering cell behaviors, including growth, differentiation, migration, gene expression, protein synthesis, and apoptosis [Ingber, 2006]. Yet, little is known about the molecular mechanisms by which individual cells within these tissues sense mechanical stresses and alter their intracellular signaling activities so that they can rapidly respond and adapt to their dynamic physical environment.
The cAMP (adenosine 3′,5′-cyclic monophosphate) cascade was the first intracellular signaling pathway found to be sensitive to mechanical stress in living tissues [Rodan et al., 1975]. This pathway is activated by mechanical stress in endothelial cells [Manolopoulos et al., 1995], and it is important for control of vascular permeability [Moore et al., 1994]. The effects of mechanical force on cAMP signaling have been shown to be mediated by transmembrane integrin receptors that anchor adherent cells to ECM [Meyer et al., 2000]. Integrins mediate adhesion by binding ECM ligands and promoting formation of a focal adhesion anchoring complex that serves as a molecular bridge between integrins and the internal actin cytoskeleton [Romer et al., 2006]. These connections physically link the ECM to the cytoskeleton, and thereby provide a specific path for mechanical force transfer across the cell surface [Wang et al., 1993]. These structural linkages also provide mechanical continuity from the molecular to the cell and tissue levels, a hierarchical property that is critical for mechanical regulation of cell function in the context of the whole organism [Ingber, 2006].
Because integrins also recruit components of many signal transduction pathways to the focal adhesion [Plopper et al., 1995; Miyamoto et al., 1995b], they represent potential points of convergence for mechanical and chemical signals, and thus they are ideally suited for mediating mechanotransduction [Ingber, 1991; Geiger and Bershadsky, 2001; Alenghat and Ingber, 2002]. Indeed, many studies have demonstrated that integrins mediate stress-dependent activation of downstream signaling components in various transduction cascades, including Src, p130Cas, MAP kinase, and Rho, as well as cAMP [Ishida et al., 1996; Riveline et al., 2001; Tzima et al., 2001; Wang et al., 2005; Sawada et al., 2006]. In addition, focal adhesion assembly itself is sensitive to control by mechanical forces transmitted across integrins [Riveline et al., 2001]. However, the early immediate mechanisms by which mechanical stress on transmembrane integrin receptors trigger intracellular mechanosignaling cascades that regulate cell-wide functions, such as the cAMP pathway, have yet to be clarified.
cAMP signaling is usually stimulated at the cell surface by heterotrimeric G protein-coupled receptors (GPCRs), which typically contain seven membrane-spanning domains that physically associate with the Gαs subunit. Upon ligand binding, conformational changes of the GPCR allow GTP to displace GDP on Gαs, which can then dissociate from its Gβγ binding partner and activate the plasma membrane enzyme, adenylyl cyclase. Activated adenylyl cyclase catalyzes conversion of ATP to cAMP, which acts as a second messenger and relays signals to protein kinase A (PKA), directing its catalytic subunit to translocate into the nucleus. This primary cascade, along with collateral pathways, results in phosphorylation of downstream effector proteins, such as the transcription factor CREB and induction of gene transcription. Although Gαs is the primary mediator of cAMP signaling, other G proteins, such as Gαi, also can contribute to this signaling mechanism in certain cells [Gronroos et al., 1998]. We have previously shown that cAMP signaling activated by mechanical stresses transferred across integrins can be blocked by the G protein inhibitor, GDP-β-S [Meyer et al., 2000]. Activation of this pathway was mediated by cytoskeletal linkages within the focal adhesions. These data indicate that the mechanism of activation differs from other studies which suggest that large G proteins can be activated directly by shear stress application to the lipid bilayer [Chachisvilis et al., 2006]. Gα proteins can interact indirectly with integrins through intermediary proteins, including caveolin-1, CD47, urokinase receptor (uPAR), and the EGF receptor [Chapman et al., 1999; Green et al., 1999; Poppleton et al., 2000; Short et al., 2000; Wei et al., 2001; Moro et al., 2002] indicating that Gαs may function at focal adhesions. Because integrins are not conventional GPCRs, they may interact with heterotrimeric G proteins to activate cAMP signaling via non-canonical pathways.
In the present study, we set out to determine whether mechanical stresses applied to cell surface integrin receptors stimulate cAMP signaling by activating Gαs locally within focal adhesions at the site of force application. We find that Gαs is recruited to focal adhesions and activated in response to stress, and thereby mediates force-induced cAMP signaling through integrins.
MATERIALS AND METHODS
Cell Culture
Human aorta endothelial (HAE) cells (Cascade Biologics) were grown through passage 4 in M200 media with Low Serum Growth Supplement (LSGS, final concentration 2% fetal bovine serum (FBS), 10 µg/ml Heparin, 1 µg/ml hydrocortisone, 10 ng/ml EGF, 3 ng/ml bFGF) in 5% CO2 at 37°C. Cells were trypsinized with Trypsin/EDTA (TE) buffer, quenched with Trypsin Neutralizer (TN) buffer (all reagents are supplied by Cascade Biologics) and cultured in fresh medium on glass coverslips, coverslip-bottomed 35 mm dishes (Mattek), or bacteriological 35 mm dishes (Falcon) pre-coated with 500 ng/cm2 fibronectin (FN). Cells were serum-deprived in M200 medium with 1% BSA for 2–3 h prior to the experiment. Bovine capillary endothelial cells were grown and plated in experimental dishes as previously described [Plopper et al., 1995]. Human umbilical vein endothelial cells (HUVECs) (Lonza) were grown through passage 7 in EBM2 medium with EGM2 Bullet supplements (Lonza) 5% FBS in 5% CO2 at 37°C. Cells were passaged in 10 or 15 cm tissue culture treated dishes (Falcon) and plated into 35 mm Bioflex plates (Flex Cell International) precoated with 5 µg/ml FN. Cells were serum-deprived in 0.3% FBS in EBM2 for 24 h prior to experiments. NIH 3T3 cells were cultured in DMEM high glucose (Invitrogen) supplemented with 10% FBS at 37°C 10% CO2. The B9 clone of NIH3T3 cells stably expressing the KID/KIX FRET constructs were cultured as the 3T3 cells except the cells were cultured with 1.5 mg/ml of G418 to maintain selection.
Magnetic Twisting Cytometry
To ligate and apply controlled mechanical stresses to specific cell surface receptors, we used 4.5 µm diameter carboxylated ferromagnetic beads (Spherotech) that were pre-coated with RGD (Peptite-2000, 0.25 mg/ml, Integra Life Sciences), acetylated LDL (AcLDL, 0.25 mg/ml, Biomedical Technologies), mouse anti-β1 integrin (K20, 0.1 mg/ml, Beckmann Coulter or BD15, 0.1 mg/ml, Biosource) or goat anti-mouse Fc (0.5 mg/ml, Sigma) as described previously [Wang et al., 1993; Meyer et al., 2000]. For anti-HLA coating, anti-mouse Fc beads were incubated with affinity-purified, mouse anti-HLA antibody (W6/32HL clone, 0.1 mg/ml, Chemicon). After adding blocking medium containing BSA, the beads were added to the cells (∼20/cell) for 15–20 min (RGD-coated beads) or 30 min (all antibody-coated beads) prior to analysis. In some experiments, 300 µM soluble RGD peptide (GRGDSP, American Peptide) was added to K20-bound beads to induce integrin activation. When PKA-c translocation was measured, cells were pretreated with 100 µM 3-isobutyl-1-methylxanthine during bead binding. After bead binding, cells were washed 3 times in PBS (containing Ca2+ and Mg2+) to remove unbound beads, and then re-fed with experimental medium. To apply a torque to beads bound to cell surface receptors, cells were exposed to a brief (10 µs) but strong (1,000 G) horizontal magnetic field to align the magnetic dipoles of the beads. They were then exposed to a prolonged (10 min) but weaker (30 G) vertical field that twists the beads to create shear stress (15.6 dynes/cm2) at the bead-cell surface interface [Wang et al., 1993].
Immunofluorescence Staining
Cells were fixed in 4% paraformaldehyde/4% sucrose in PBS, permeabilized in PBS with 0.1% Triton-X, blocked in PBS with 1% BSA, and stained sequentially with primary antibody (1:100 to 1:1,000 dilution) and secondary antibody (1:1,000, Alexa Fluor 488 or 594, Molecular Probes) in PBS with 0.5% BSA. Antibodies used for staining were rabbit anti-Gαs (K-20, Santa Cruz), mouse anti-paxillin (349, BD Transduction Labs.), mouse anti-human vinculin (hVin1, Sigma), rabbit mAb anti-phosphoCREB (Ser133) (87G3) (Cell Signaling Technologies).
Computerized Image Analysis
To quantify protein staining intensity around surface beads, 1 micron thick image slices recorded at 60× magnification (1.4 NA) using a Nikon Diaphot 300 microscope, CCD camera (Hamamatsu, Japan), and IPLab acquisition software (Scanalytics), were digitally deconvolved using Power Microtome software (Vaytek). Bead outlines were traced on the fluorescent image slice where the bead diameter was largest. Matched images in phase contrast were used if the bead was not discernable in the fluorescent image. Fluorescence intensity with a 1.5 µm wide annulus immediately surrounding the bead was measured and compared to background staining using IPLab software.
Nuclear phospho-CREB staining was quantified by tracing the outline of nucleus on the fluorescent image slice and fluorescence intensity within the nucleus were measured and compared to background cell staining using the public domain NIH ImageJ program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). In some experiments, the number of cells that are positive for nuclear phospho-CREB were counted and presented as percentage of positive cells.
Isolation of Bead-Associated Protein Complexes
Protein complexes associated with surface-bound magnetic beads were isolated with using a modification of previously published techniques [Plopper and Ingber, 1993; Plopper et al., 1995; Green et al., 1999]. In brief, cells with bound beads were washed with PBS, and cold cytoskeleton stabilizing buffer (300 mM sucrose, 80 mM NaCl, 3 mM MgCl2, 10 mM PIPES pH 6.8, with Roche Protease Inhibitor tablet; CSB), before being extracted in the same CSB with 0.5% Triton-X (CSB+) for 1 min. This buffer was collected as the “soluble” protein fraction of the cells. After a second 1 min wash with CSB+, the remaining cytoskeletons were scraped from the substrate, collected in CSB+ and then passed through a 22 gauge needle for 10 strokes. Beads and associated proteins were pulled down with a neodymium magnet and the supernatant was collected as the “cytoskeletal” fraction. Beads were then resuspended in CSB+, passed through a fresh needle for five additional strokes, pulled down magnetically, and washed in CSB+ before the bead pellet was solubilized in boiling sample buffer (1% SDS, 50 mM Tris–HCl, pH 7.6), placed at 95°C for 5 min, and passed through the needle 20 more times. The sample was centrifuged at 10,000 rpm for 1 min and the supernatant containing the “bead-associated protein” fraction was collected; the undissolved bead pellet was discarded.
Analysis of G Protein Activation
In HAE cells, after bead binding as described, cells were washed 3 times with Hank's Balanced Salt Solution (HBSS) and incubated for 3 min with 25 ng/ml saponin, 2 mM ATP, and 5 µM GDP in HBSS to permeabilize the membrane. This was then replaced by fresh HBSS containing 1.25 µM GTP [γ] 4-Azidoanilide 2′,3′-Biotin (AAGTP, Affinity Labeling Technologies), 500 µM ATP, and 5 µM GDP for 3 min prior to magnetically twisting the surface-bound beads for 6 min at 15.6 dynes/cm2. Dishes were exposed to 254 nm UV light for last 3 min of the twist or control trials. Dishes were immediately transferred to ice, rinsed with cold PBS (with Ca2+ and Mg2+) containing 2 mM DTT for 20 s, extracted with CSB+ for 1 min, rinsed twice with PBS and then either prepared for fluorescence staining or isolation of bead-associated proteins, as described above.
BCE cells were trypsinized and incubated in HBSS containing 0.5% HSA incubated 30 min at RT. Cells were then aliquoted at 1,106 cell/ml in HBSS containing 0.1% HSA and 0.2 mM MnCl2 and incubated 20 min at 37°C with 10 µl of anti-β1integrin mAbs (MAB2000, Chemicon) coated spherotech beads. Cells were then centrifuged 3 min at 3,000 rpm and incubated in 100 ml of TEMN buffer with or without 0.2% saponin at 22°C. After 2 min incubation, 350 µl of TEMN containing 10 µM AA-GTP-biotin was added and incubated 3 min at RT.
At the end of stimulation, tubes were placed on magnet, supernatant removed, and pellets were resuspended in 200 µl HBSS buffer containing 0.1% HSA and 0.1 mM MnCl2 and exposed to UV for 2 min on ice. Beads were concentrated on magnet and resuspended in 50 ml of Laemmli 4X. After 5 min at 95°C, proteins were separated on 4–12% acrylamide gel and transferred to PVDF membrane for Western blotting as described above. Membrane was blotted first with HRP streptavidin followed by sequential blotting with anti-Gαs (Santa Cruz).
Western Blot Analysis
Equal amounts of protein were separated through a Tris-HCl gel by electrophoresis, transferred to nitrocellulose, and blocked in 3% milk, 3% BSA, 0.1% Tween-20 in PBS for 1 h. Incubation with primary antibody (between 1:100 and 1:1,000 dilution) was carried out in blocking buffer for 1 h at 22°C or overnight at 4°C. After washing 4 times in 0.5% milk, 0.5% BSA, 0.1% Tween-20 in PBS, the membrane was placed in a 1:10,000 dilution of HRP-conjugated secondary antibody (Santa Cruz) in blocking buffer for 1 h at 22°C, then again washed 4 times before incubating in chemiluminescent substrate (SuperSignal Dura, Pierce) and imaging with Kodak Biomax Light Film. To probe for biotinylated AAGTP, the nitrocellulose membrane was blotted with 4 µg/ml HRP-avidin (Pierce) in blocking buffer overnight at 4°C. Additional antibodies used in Western blots were rabbit anti-PKA-c (C-20, Santa Cruz) and rabbit anti-β1 integrin (M-106, Santa Cruz).
In Western analysis for HUVEC RNAi experiment equal amounts of total protein were loaded onto a Novex 4–12% Bis-Tris gel (Invitrogen) by SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membrane, blocked in 5% BSA in 1% Tween-20 in TBS for 1 h at 22°C, incubated with1:500 Gαs primary antibody (Upstate Biotechnology/Millipore) in blocking buffer 1 h at 22°C or overnight at 4°C. After washing 3 times in 1% Tween in TBS (TBST), the membrane was incubated for 1 h at 22°C with 1:7,500 HRP-conjugated anti-rabbit antibody (Jackson Immunologicals or Vector Biologicals), washed 3 more times with TBST before incubating in Lumiglo chemiluminescent substrate (KPL laboratories) and developed with Kodak-AR X-ray film.
KID/KIX Assay
We developed NIH-3T3 fibroblast stable cell lines (clone B9) that express a FRET probe responsive to cAMP activation (KID-KIX) (Montoya-Zavala and Ingber, Manuscript Submitted, see Overby 2004 for similar method) and force was applied through mechanical stretch. The cells were cultured on collagen-I (10 µg/ml) coated BioFlex plates, then treated with melittin (1 µM) or left untreated for 2 h. After loading with 1 µM of CCF2-AM, the cells were subjected to mechanical stretch (15% elongation, 20 cycles/min) for 5 min. Cells on the same plate that were not subjected to mechanical stretch served as control. The cells were examined under a Leica confocal microscope and images were acquired (excitation: 405 nm; emission: 460 ± 20 nm for blue and 530 ± 15 nm for green and the increase in blue/green ratio was used as a measure of cAMP activation.
Cell Stretching
Cells were cultured on fibronectin coated 35 mm BioFlex dishes and cultured in full medium. HUVECs were serum-deprived as described above 24 h prior to stretching experiments. Cells were stretched at 15% strain, 20 cycles/min for 5 min. Unstretched cells on the same plate served as negative controls. Cells were then fixed and immunostained for phospho-CREB as described above.
siRNA Knockdown of Gαs
For RNAi experiments, negative control RNAi (Negative Control #1, Invitrogen) or Gαs RNAi [sequence as described in Zheng et al. 2004, Dharmacon] were introduced into cells using SilentFect (BioRad). Media was replaced after 12–24 h and cells were used for stretch experiments 72 h after transfection with RNAi as described above.
Statistics
All error bars shown indicate standard error of the mean (SEM). Significance was determined using the Student's t-test.
RESULTS
cAMP Signaling Is Activated by Mechanically Stressing Integrins
In a previous study, we demonstrated that intracellular cAMP, PKA activation, CREB phosphorylation, and cAMP-dependent gene transcription all increase in a stress-dependent manner when mechanical shear stresses are applied directly to cell surface β1 integrin receptors on bovine capillary endothelial (BCE) cells or NIH 3T3 fibroblasts using magnetic twisting cytometry [Meyer et al., 2000]. In this technique, controlled mechanical forces are applied to receptors on the surface of living cells through bound ferromagnetic microbeads (4.5 µm diameter) coated with specific ligands, and twisting forces (shear stresses) are applied through these bound ligand-coated beads (Fig. 1A), as previously described [Wang et al., 1993; Wang and Ingber, 1994; Lele et al., 2007]. First, to determine whether this response occurs in human endothelial cells, we carried out twisting experiments in human aortic endothelial (HAE) cells. These studies confirmed that HAE cells, like BCEs, rapidly (within 10 min) respond to mechanical strain (15.6 dynes/cm2) applied to integrins bound to RGD-coated magnetic microbeads by activating the cAMP signaling cascade, as measured by quantitation of nuclear localization of the catalytic subunit of PKA, whereas magnetic field application in the absence of bound beads had no effect (Fig. 1B). To determine if physiological mechanical strain (stretching forces applied via the basement membrane of cells) could also activate the cAMP pathway, another cell type—human umbilical vein endothelial (HUVE) cells—were cultured on fibronectin-coated flexible substrates and subjected to biaxial mechanical strain through their integrin-mediated ECM adhesions (15% strain; 20 cycles/min). We observed a significant increase in cAMP signaling indicated by nuclear CREB phosphorylation, as measured by increases in the intensity of nuclear phospho-CREB staining using immunofluorescence, which was accompanied by a fourfold increase in the percentage of cells with labeled nuclei (89% vs. 21% in stretched and control cells, respectively; Fig. 1C). Hence, activation of the cAMP pathway in response to force appears to be a general and physiological mechanism for signal transduction in a range of cell types.
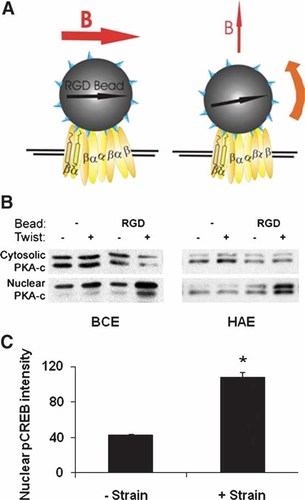
Experimental System. A: In magnetic twisting cytometry, ferromagnetic microbeads coated with specific ligands, such as RGD peptide (small triangles) or antibodies, are allowed to bind to cell surface receptors. A strong, brief magnetic field pulse (horizontal arrow B) is applied to align the magnetic dipoles of the beads horizontally (left panel). A subsequent prolonged, weaker field directed upward (vertical arrow B) causes the beads to twist, resulting in a shear stress at the bead-cell membrane interface via the ligated receptors (e.g., integrin heterodimers) (right panel). B: Cytosolic and nuclear catalytic subunit of protein kinase A (PKA-c) quantitated in Western blots of proteins from bovine capillary endothelial (BCE) cells or human aortic endothelial (HAE) cells cultured with no beads (−) or RGD-coated beads (RGD), in the absence (−) or presence (+) of 10 min applied stress (twist). C: Quantitation of nuclear intensity of nuclear phospho-CREB in Human Umbilical Vein Endothelial Cells (HUVEC) bound to fibronectin-coated flexible membrane exposed to no strain or 5 min of strain at 15%, 20 cycles/min, as applied using a Flex Cell System and fixed and stained for phospho-CREB by immunofluorescence (*P < 0.001).
Gαs Is Recruited to Bead-Associated Focal Adhesions
We have previously shown cAMP signaling induced by mechanical force application to integrins can be inhibited using the general G protein inhibitor GDP-β-S, thus suggesting that this mechanotransduction cascade may be mediated by heterotrimeric G proteins [Meyer et al., 2000]. Because increases in cAMP produced by canonical GPCR signaling are usually mediated by the Gαs subunit of heterotrimeric G proteins, we explored whether Gαs mediates this integrin-dependent mechanical signaling response. We and others have shown that focal adhesion-like complexes form beneath magnetic beads coated with ECM proteins or anti-integrin antibodies when they bind cell surface integrin receptors, and that rapid protein recruitment to these ligand-coated beads facilitates analysis of early steps in integrin activation and focal adhesion formation [Plopper et al., 1995; Miyamoto et al., 1995b; Meyer et al., 2000; Matthews et al., 2006].
Immunofluorescence microscopic analysis of cells bound to RGD-coated magnetic microbeads was performed to determine whether large G proteins are recruited to focal adhesions. These experiments confirmed that focal adhesions containing vinculin and paxillin form at the bead-membrane interface within 20 min after bead binding, as previously described (Fig. 2A) [Plopper et al., 1995; Matthews et al., 2004]. Importantly, Gαs codistributed with vinculin and paxillin in these nascent focal adhesions (Fig. 2A). Virtually every bound RGD-bead that recruited vinculin or paxillin also displayed Gαs staining, and this distribution is consistent with the past finding that bead binding alone is sufficient to induce low baseline levels of cAMP signaling in endothelial cells [Meyer et al., 2000].
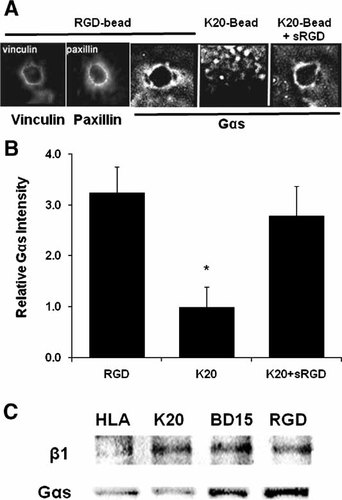
Gαs is recruited to activated integrins. A: Fluorescent images of vinculin, paxillin, and Gαs recruitment to the cell membrane region bound to RGD-coated bead (4.5 µm diameter) or K20-beads in the presence or absence of soluble RGD peptide (sRGD) that activates the K20-bound integrin receptors. B: Intensity of Gαs recruitment from studies shown in (A) was quantitated by measuring the average pixel intensity within a 1.5 µm wide annulus at the bead periphery relative to local background, and normalized against the K20 condition for each G protein subunit [average of 35 beads per condition; *P < 0.005]. C: Bead-associated fraction purification with beads coated with HLA, K20, BD15, or RGD probed by Western blot for β1 integrin and Gαs.
Integrin activation through occupancy of its RGD-ligand binding site is required for the recruitment of many structural proteins and signaling molecules to focal adhesions [Miyamoto et al., 1995a; Meyer et al., 2000], as well as for stress-dependent activation of downstream signaling events in the cAMP pathway [Meyer et al., 2000]. To explore whether integrin activation is required for Gαs recruitment to bead-associated focal adhesions, cells were bound to either RGD-beads or similar microbeads coated with a non-activating anti-β1 integrin antibody (K20) that produces only receptor clustering [Miyamoto et al., 1995a]. Additional controls included use of beads coated with an antibody directed against human histocompatibility antigen (HLA) that does not effectively mediate mechanical force transfer across the surface, although it spans the plasma membrane [Yoshida et al., 1996; Chicurel et al., 1998]. We found that while RGD-beads that are activating ligands for β1 integrins induced Gαs to organize in a linear pattern in the focal adhesion outlining the surface of the beads, Gαs was not concentrated around the surfaces of bound beads coated with non-activating K20 anti-β1 antibody (Fig. 2A,B) or with anti-HLA (not shown). Moreover, the linear focal adhesion staining pattern for Gαs could be restored by adding soluble RGD peptide (sRGD) to cells already bound to K20-beads (Fig. 2A,B). These results indicate that integrin activation through ligand binding and associated focal adhesion formation is essential in Gαs recruitment to the bead-binding site.
Independent evidence of recruitment of Gαs to the site of integrin binding was obtained through biochemical analysis of bead-associated supramolecular complexes, isolated using a modification of previously published magnetic techniques for isolation of bead-associated focal adhesions and membrane signaling complexes [Plopper and Ingber, 1993; Green et al., 1999]. The beads and tightly associated proteins were magnetically isolated from detergent-extracted cells after using mechanical shearing to physically separate the beads from the remaining cytoskeleton. This yields a bead-associated protein fraction that is enriched for β1 integrin, when the magnetic beads were coated with RGD peptide, non-activating anti-β1 integrin antibody (K20), or an activating anti-β1 integrin antibody (BD15) relative to antibodies against HLA (Fig. 2C). Western blot analysis of this fraction once again demonstrated that Gαs protein was preferentially recruited to the RGD- and BD15-beads, compared to the beads coated with K20 or anti-HLA antibodies, under conditions in which integrin recruitment levels were similar (Fig. 2C). This pattern of recruitment is similar to that of the focal adhesion protein vinculin, which is also preferentially recruited to RGD beads but not to K20 or control antibody coated beads [Miyamoto et al., 1995a]. Taken together, these data indicate that Gαs is preferentially recruited to focal adhesions formed at sites of membrane binding to beads coated with activating β1 integrin ligands.
Effects of Mechanical Stress on Gαs Recruitment and Activation
Because stress application to integrins stimulates cAMP-mediated signal transduction, we explored whether mechanical force increases Gαs protein recruitment to focal adhesions. When surface-bound RGD-beads were stressed using magnetic twisting cytometry in HAE cells, Gαs intensity increased by almost 50% relative to control untwisted RGD-beads (Fig. 3A). While integrin clustering and activation cause Gαs recruitment to focal adhesions, and mechanical stress can further increase recruitment of Gαs, such targeted localization alone is not sufficient to explain mechanical stimulation of the cAMP pathway because to induce cAMP production, Gαs must be chemically activated. G protein activation occurs when GTP displaces GDP at the Gα subunit's nucleotide binding site. To determine directly the spatial distribution of G protein activation across the cell we measured binding of a biotin-labeled azido-anilido form of GTP (AAGTP) that irreversibly cross-links to the activated G protein when bound and exposed to ultraviolet light [Rasenick et al., 1994].
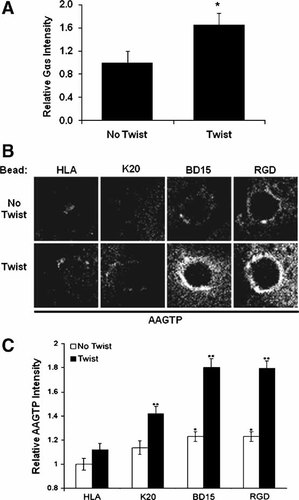
Twisting leads to increased Gαs and AAGTP recruitment to beads. A: Twisting of RGD-coated beads for 10 min (15.6 dynes/cm2) yielded significant increases in local recruitment of Gαs subunits in cells fixed and stained immediately after stress application. Bead staining intensity was quantitated and normalized to no twist levels [average 93 beads per condition; *P < 0.005]. B: Cells bound to RGD-beads and briefly incubated with biotin-labeled AAGTP were exposed to control or twisting conditions and subsequent UV light (3 min) to induce AAGTP cross-linking to activated G proteins. After fixation, cells were stained with fluorescent avidin and imaged using fluorescence microscopy; beads appear as dark holes in the fluorescent cytoplasm. Digitally deconvoluted images of representative surface-bound beads, with or without twist, stained for AAGTP. C: Bead staining intensity of AAGTP measured at the periphery of surface-bound beads and normalized against the “no twist HLA” condition [average of 143 beads per condition; *P < 0.005 compared to untwisted HLA; **P < 0.001 compared to corresponding untwisted condition].
Staining bead-bound cells for biotinylated AAGTP revealed that the amount of GTP binding around each RGD-coated bead, and the percentage of beads that exhibited AAGTP staining, increased with mechanical stress (Fig. 3B). A low baseline level of activation of G proteins could be detected under non-stressed conditions around beads coated with RGD or activating BD15-antibodies; however, this activity increased greatly when mechanical stress was applied to those beads compared to control beads coated with K20 or anti-HLA antibodies. Quantitation of average bead staining intensity revealed that both integrin activation and stress application independently increased AAGTP binding, and hence G protein activation, within bead-associated focal adhesions in living cells (Fig. 3C). Although a small, but significant, increase in G protein activation could be detected when stress was applied through K20-beads, the most striking and significant population differences arose when stress was applied via ligand-activated integrins bound to beads coated with RGD or BD15 antibodies (Fig. 3C). These results indicate that GTP-binding proteins that are recruited to these integrins upon ligand activation can be functionally activated within the focal adhesion in response to mechanical stress application.
To ensure that G protein activation in response to twisting is integrin-specific, we compared AAGTP recruitment of bead-associated fractions in HAE cells bound to beads coated with anti-HLA antibody or RGD, in the presence or absence of mechanical twisting forces. These studies confirmed that G protein activation measured by AAGTP staining in Western blots was minimal in bead fractions formed with HLA ligands relative to activating integrin ligands, and that application of shear stress only increased AAGTP staining (and hence G protein activation) in the bead complexes that formed around the RGD-beads (Fig. 4A).
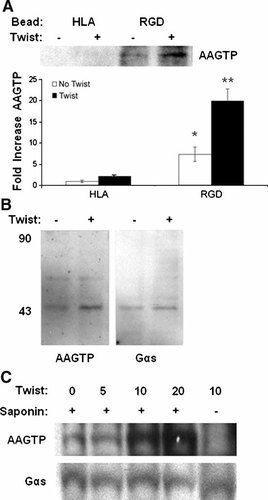
Measurement of bead-associated AAGTP at isolated focal adhesion complexes. A: Increase in AAGTP incorporation into bead-associated protein fractions with RGD and control HLA beads after biotin-AAGTP incubation and mechanical stress as probed by Western blotting with HRP-avidin (*P < 0.005 compared to HLA;**P < 0.005 compared to RGD no twist). B: After AAGTP incubation, mechanical stress and UV exposure, isolated proteins associated with cell-bound RGD-beads were subjected to gel electrophoresis and Western blotting with HRP-avidin to identify proteins bound to biotinylated-AAGTP (left panel). In the bead fraction, the primary GTP-binding species migrated at 45 kDa, exactly like Gαs, as determined upon reprobing with anti-Gαs (right panel). C: Integrin-binding beads were bound to bovine capillary endothelial cells and incubated for 0, 5, 10, or 20 min in the presence of saponin or 10 min in the absence of saponin to exclude AAGTP entrance into cells as a negative control. Lysates were prepared, normalized for protein levels, subjected to gel electrophoresis and probed with HRP-strepavidin to identify AAGTP-biotin incorporation or anti-Gαs antibody to determine Gαs recruitment. The same 45 kDa molecular weight region is shown in both blots.
To confirm that the biotinylated AAGTP was bound to Gαs, HAE cells were fractionated into cytosolic, cytoskeletal, and bead-associated protein fractions after exposure to ultraviolet light. These fractions were then subjected to Western blotting and probed with avidin-HRP to analyze the distribution of biotinylated-AAGTP. With a mass of 45 kDa, the primary AAGTP bound species in the bead-associated protein fraction co-migrated precisely with Gαs, as determined by subsequent immunoblotting (Fig. 4B). Moreover, the only detectable increase in AAGTP binding of Gαs occurred in the bead fraction (not shown).
Because endogenous Gαs levels are extremely low in HAE cells, we repeated these experiments in BCE cells. When BCE cells bound to β1 integrin antibody-coated beads were subjected to twisting forces for different durations (0, 5, 10, or 20 min), Gαs activation increased in parallel, as measured by quantitating AAGTP levels in isolated bead complexes using Western blotting (Fig. 4C). This increase was not observed in control cells that were cultured under identical conditions in the absence of saponin, which is required to permeabilize cell membranes and facilitate AAGTP entry into the cytoplasm (Fig. 4C). We also observed similar results in experiments with 293T cells that over express Gαs (not shown). These data indicate that Gαs localized to bead-associated focal adhesions is specifically activated by local force application to integrins but not unrelated control transmembrane receptors.
Gαs Mediates Mechanical Force-Induced cAMP Signaling
To explore whether Gαs mediates cAMP-dependent mechanotransduction through integrins, we exposed NIH-3T3 cells, which were previously shown to activate the cAMP pathway in response to force [Meyer et al., 2000], adherent to fibronectin-coated substrates to cyclical mechanical strain (15% elongation, 20 cycles/min for 5 min) in the presence or absence of the Gαs inhibitor, mellitin (1 µM); cAMP signaling was measured by quantitating nuclear phospho-CREB staining. Stretching these cells through their integrin-mediated basal adhesions to the flexible ECM substrate resulted in a significant increase in cAMP levels, and treatment of these cells with melittin almost completely inhibited this response relative to control unstretched cells (Fig. 5A). To further confirm these results, we used NIH-3T3 cells that stably express a KID/KIX-based fluorescent (FRET-based) cAMP reporter, which reads out cAMP signaling within minutes after cell activation [Overby et al., 2004; Spotts et al., 2002]. We similarly found that mechanical strain significantly increased cAMP activity measured by KID/KIX activation, and this response was again inhibited by melittin(Fig. 5B).
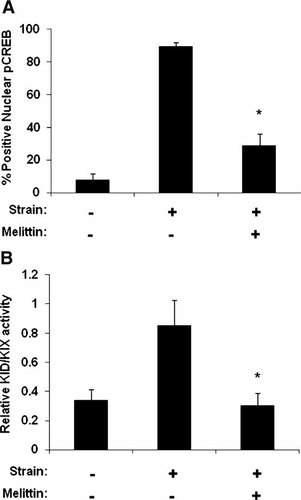
Inhibition of Gαs with melittin inhibits the cAMP response to stress. A: Percentage of nuclear phospho-CREB positive cells in NIH3T3 cells or (B) KID/KIX activity as measured in B9 cells (*P < 0.001) subjected to no strain or 5 min of mechanical strain (15% strain; 0.3 Hz) in the presence or absence of melittin (*P < 0.001).
These results suggest that Gαs is required for stress-induced activation of cAMP signaling through integrins; however, melittin can activate Gαi in addition to inhibiting Gαs [Fukushima et al., 1998]. Therefore, to confirm that Gαs mediates this mechanotransduction response, we utilized siRNA to specifically suppress Gαs expression. Using previously published RNAi sequences that specifically target Gαs [Zheng et al., 2004], we knocked down Gαs expression by more than 70%, as quantitated by RT-PCR (Fig. 6A) and Western blots in HUVE cells (Fig. 6B). Cells depleted of Gαs in this manner were plated on fibronectin-coated flexible substrates, exposed to mechanical strain, and cAMP signaling was measured by analyzing nuclear CREB phosphorylation (as described in Fig. 1C). Nuclear phospho-CREB staining increased significantly in control cells relative to non-stimulated cells within 5 min after strain application, as observed previously; however, this response was significantly (P < 0.001) inhibited in cells treated with Gαs siRNA (Fig. 6C). Overall we saw a 45% decrease in phospho-CREB levels in the Gαs RNAi-treated cells compared to cells treated with control siRNA. Taken together, these results confirm that Gαs is required for efficient cAMP signaling induced by mechanical forces applied to integrins.
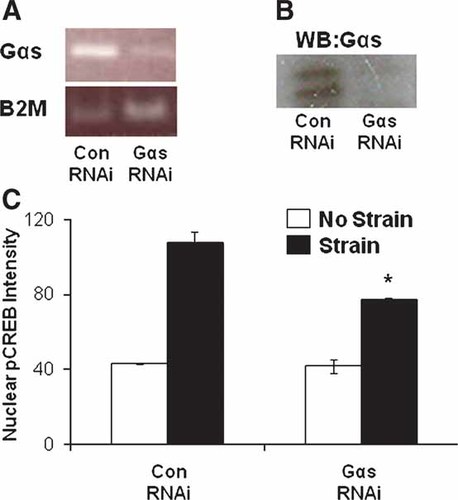
Depletion of Gαs using RNAi significantly diminishes phospho-CREB nuclear translocation in response to mechanical strain. A: Quantitative PCR analysis showing decrease in Gαs transcript in cells treated with Gαs RNAi as compared to the Control (Con RNAi). B2 M is shown as a control. B: Western blot analysis showing downregulation of Gαs protein levels in Gαs RNAi transfected cells compared to Control RNAi (Con RNAi) treated cells. C: Quantitative analysis of nuclear phospho-CREB intensity showing inhibition of phospho-CREB nuclear translocation in Gαs RNAi knockdown cells compared to Control RNAi (Con RNAi) treated cells (*P < 0.001). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
We previously showed that integrins mediate shear stress-induced cAMP signaling through a heterotrimeric G-protein dependent pathway [Meyer et al., 2000]. Here, we demonstrate that application of mechanical forces, including shear stress and physiological mechanical strain, through bound integrins activates cAMP signaling through the recruitment and activation of Gαs protein at focal adhesions.
The G protein subunit Gαs is preferentially recruited to nascent bead-associated focal adhesions formed by activated and clustered integrins, which closely matches the requirements for mechanical induction of cAMP signaling in endothelial cells and fibroblasts described previously [Meyer et al., 2000]. Gαs protein recruitment to focal adhesions is rapid (within 20 min of ligand binding) and physiologically relevant since force application to other transmembrane proteins (e.g., HLA antigen) that do not form focal adhesions neither recruit this G protein nor activate the cAMP signaling pathway. When integrins are mechanically stressed, additional Gαs proteins are recruited to these sites, and this is accompanied by increased local activation of G proteins at the site of force application within the focal adhesion. At present it remains unclear whether force application activates existing Gαs in the focal adhesion, or if it specifically induces recruitment of already activated Gαs subunits. However, when the purification of beads subjected to mechanical shear stress were normalized for protein, we observed an increase in AAGTP incorporation despite similar levels of Gαs (Fig. 4C). This finding suggests that force application to integrins likely both recruits and activates Gαs within the focal adhesion, which apparently is necessary to stimulate adenylyl cyclase at the cell membrane to produce cAMP.
While both clustering and activation of integrins were required for eliciting optimal Gαs activation in response to mechanical stress, a low level of AAGTP binding (and hence, G protein activation) was observed with integrin clustering alone produced by cell binding to non-activating K20-beads to β1 integrin receptors. Although binding of K20-beads does not promote formation of well-developed focal adhesions, it has been reported to induce recruitment of a subset of signaling proteins [Miyamoto et al., 1995b], and this could promote a low level of Gαs recruitment. Additionally, the slightly increased levels of GTP binding in response to integrin ligation and clustering in the absence of applied force agree well with earlier findings that bead binding alone produces a low level of activation of the cAMP pathway [Meyer et al., 2000]. When these bead-associated focal adhesions are then mechanically stressed, cAMP signaling increases further in a stress-dependent manner [Meyer et al., 2000]. This signal amplification may be aided in part by the force-induced recruitment of additional G proteins, similar to force-induced growth of focal adhesion complexes [Riveline et al., 2001; Galbraith et al., 2002], which may be mediated by alterations in molecular binding kinetics [Lele et al., 2006]. Alternatively, mechanical loads focused over integrins and the cytoskeletal backbone of the focal adhesions may alter Gαs activity by promoting mechanical distortion of the molecule itself, as observed with other focal adhesion signaling proteins (e.g., p130Cas) [Sawada et al., 2006].
Our studies indicate that the focal adhesion that forms when integrins are activated and clustered creates a signaling microcompartment at the plasma membrane where Gαs subunits concentrate inside the cell. Moreover, stress application to integrins and the focal adhesion results in rapid recruitment of additional Gαs subunits that exhibit enhanced activity at these sites, and that produce activation of adenylyl cyclase leading to increased production of cAMP and downstream signal transduction ending in changes in gene transcription. Stress-dependent activation of Gαs activity in our cells requires that these forces be transmitted into the cell over activated integrins that cluster within focal adhesions: mechanical stresses applied to the membrane via control antibodies that bound to either other transmembrane molecules (HLA antigen) or to non-activated integrins (K20 antibody) that do not form mature focal adhesions did not produce efficient G protein activation. Although Gαs is preferentially recruited to focal adhesions formed around β1-antibody or RGD-coated beads, we do not yet know the specific focal adhesion molecules that mediate this response. Future studies will therefore be required to determine the molecular linkage between the integrins and Gαs as well as the molecular mechanism of its force-induced activation.
The findings of this study that force transfer through integrins is required for activation of G-proteins is distinct from past studies that suggested fluid shear stresses may activate other G proteins in endothelial cells through generalized distortion of the lipid bilayer [Chachisvilis et al., 2006]. Importantly, our findings reveal that the Gαs subunit that has been previously shown to mediate activation of cAMP production by G protein-coupled receptors, also mediates integrin- and mechanical stress-dependent activation of this pathway. Suppression of Gαs activity, either by pharmacological agents or RNAi, inhibited the downstream cAMP signaling cascade in response to stress when measured through two independent assays of cAMP signaling (a cytoplasmic KID/KIX reporter and nuclear phospho-CREB). Although Gαs was found to be required for optimal mechanical signaling through cAMP, down regulation of Gαs using siRNA did not completely abolish force-induced nuclear phosphorylation of CREB, suggesting that other signaling mechanisms might also involved in relaying force to cAMP signaling. For example, CREB is substrate of multiple kinases and its phosphorylation could be mediated by other G proteins or other signaling molecules such as protein kinase C [Paruchuri et al., 2002]. Interestingly, we and others have found additional large heterotrimeric G proteins directly or indirectly associating with integrins [Brown and Frazier, 2001, and A. Derrien, unpublished work] supporting such a possibility. Further investigation will be necessary to determine the complete spectrum of G proteins at focal adhesions and how these additional players function in mechanotransduction. Nevertheless, these data show that Gαs contributes significantly to the mechanism by which stress application to integrins stimulates the cAMP signaling.
In summary, although heterotrimeric G proteins have been previously shown to mediate signaling downstream from integrin binding [Brown and Frazier, 2001], this study is the first to show a local mechanosensitive interaction between Gαs and activated β1 integrins within focal adhesions, and to demonstrate that Gαs mediates integrin-dependent mechanotransduction through the cAMP signaling pathway.
Acknowledgements
We thank M. Rasenick, P. Rim, and A. Greene for guidance with AAGTP experimental design. This work was supported by NASA (NN A04CC96G to DEI), AHA (0635095N to CKT), Howard Hughes Predoctoral Fellowship (to F.A.), and an NIH Postdoctoral Fellowship (J.D.T.; F32 HL086172). Dr. Ingber is a recipient of a DoD Breast Cancer Innovator Award.




