p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes
Abstract
Studying cartilage differentiation, we observed the emergence of inflammation-related proteins suggesting that a common pathway was activated in cartilage differentiation and inflammation. In the present paper, we investigated the expression pathway of the inflammation-related enzyme Cyclooxygenase-2 (COX-2) during differentiation and inflammatory response of the chondrocytic cell line MC615. Cells were cultured either as (i) proliferating prechondrogenic cells expressing type I collagen or (ii) differentiated hyperconfluent cells expressing Sox9 and type II collagen. The p38 and the NF-kB pathways were investigated in standard conditions and after inflammatory agents treatment. NF-kB was constitutively activated in differentiated cells. The activation level of NF-kB in differentiated cells was comparable to the level in proliferating cells treated with the inflammatory agent LPS. In both cases, p65 was bound to the NF-kB consensus sequence of COX-2 promoter. p38, constitutively activated in differentiated cells, was activated in proliferating cells by treatment with LPS or IL-1α. In stimulated proliferating cells the two pathways are connected since addition of the p38-specific inhibitor SB203580 inhibited p38 activation, significantly reduced NF-kB activation and repressed COX-2 synthesis indicating that p38 is upstream NF-kB activation and COX-2 synthesis. In differentiated cells, the treatment with the inflammatory agent neither enhance NF-kB activation, nor synthesis of COX-2 while the addition of SB203580 neither repressed activation of p38, nor COX-2 synthesis, suggesting a constitutive activation of a p38/NF-kB/COX2 pathway. Our data indicate that in chondrocytes, COX-2 is expressed via p38 activation/NF-kB recruitment during both differentiation and inflammatory response. J. Cell. Biochem. 104: 1393–1406, 2008. © 2008 Wiley-Liss, Inc.
In previous publications on avian growth plate cartilage differentiation we described the expression of several proteins related to stress conditions in fully differentiated hyperthrophic cells. In particular we reported that Ex-FABP, an extracellular lipocalin that specifically binds unsaturated long chain fatty acids, avidin, a biotin binding protein, and ovotransferrin, an iron binding protein, were constitutively (“physiologically”) expressed in differentiated chondrocytes, and strongly overinduced by stimulation of the same cells with inflammatory agents such as LPS and inflammatory cytokines [Cancedda et al., 1990; Gentili et al., 1994; Cancedda et al., 1996; Zerega et al., 2001]. Serum Amyloid A mRNA was also highly expressed in hyperthrophic cells. The expression of the protein, involved in cholesterol transport in inflammatory conditions, was induced by treatment with LPS [Zerega et al., 2004]. Based on these data we suggested that (a) pathway(s) active in chondrocyte inflammation was “physiologically” activated also in chondrocyte differentiation.
More recently, we reported in fully differentiated cells of mouse cartilage the presence of the lipocalin SIP24, an acute phase antibacterial protein able to bind iron and playing a role in the differentiation of several tissues [Mallbris et al., 2002; Flo et al., 2004; Schmidt-Ott et al., 2006]. In the same manuscript we provided evidence that a common pathway leading to the expression of SIP24 and involving p38 activation and NF-kB recruitment, was active in both mouse cartilage differentiation and inflammation [Ulivi et al., 2006].
In the present paper, we investigated the signaling pathway activated for the expression of the Cyclooxygenase-2 (COX-2), an enzyme playing a key role in the inflammatory cascade leading to the acute phase response, during differentiation and inflammatory response of the chondrocytic cell line MC615. Expression of COX-2 was observed in fracture callus formation and COX-2 function was described as essential for bone fracture healing [Simon et al., 2002; Zhang et al., 2002; Arasapam et al., 2006]. In physiological conditions, the expression of COX-2 was reported in differentiating growth plates as well as the presence of the receptors for the PGE2, the COX-2 enzymatic product [Brochhausen et al., 2006]. For investigating the signaling pathway leading to the expression of COX-2 in chondrocyte differentiation and inflammatory response we took advantage of the MC615 chondrogenic cell line able to differentiate in culture, a cell system already used to study the signaling pathway activated for the expression of SIP24 [Ulivi et al., 2006]. MC615 cells show a polygonal morphology during the proliferation phase. After they have reached hyperconfluence, the cells differentiate and can be maintained in culture as such for several days. Hyperconfluent differentiated cells are surrounded by a highly refractile matrix and form nodules that stain with alcian blue at pH1 due to the presence of sulfated glycosaminoglycans and synthesize molecules characteristic of matrix cartilage such as aggrecan, link protein, biglycan, decorin and type II collagen [Mallein-Gerin and Olsen, 1993].
In the present study, we observed that COX-2, barely expressed in proliferating cells, is highly expressed in hyperconfluent cultures and is induced in proliferating cells in inflammatory conditions. These observations suggest that a common pathway leading to the expression of COX-2 could be activated in differentiated cells and in proliferating cells treated with inflammatory agents. p38 and NF-kB activation pathways have been investigated and a possible interconnection was established.
MATERIALS AND METHODS
Materials
Bacterial endotoxin lipopolysaccaride (LPS), BAY117082, TLCK were obtained from Sigma (St. Louis, MO); IL-1α and TNF-α were from Peprotech (London, UK); IL-6 was from Genzyme (Cambridge, MA).
SB203580 was purchased from Calbiochem (Merck KgaA, Darmstadt). COX-2 polyclonal antiserum was from Cayman (Ann Arbor, MI).
Immunohistochemistry
For immunohistochemical localization of COX-2 protein in the tissues, biopsies were embedded in paraffin. Serial sections (5 µm) were made, dewaxed and dipped in methanol/H2O2 (49/1) for 30 min to inhibit endogenous peroxidases. After treatment with 1 mg/ml hyaluronidase in Phosphate buffered saline (PBS) for 20 min at 37°C sections were washed with PBS before incubation with goat serum for 20 min to reduce non-specific binding of the secondary antibody. The specific antibody was added for 1 h at room temperature. After washing several times with PBS, sections were challenged with biotinylated goat anti-rabbit IgG (Jackson Laboratory Inc., West Grove, PA) and peroxidase- conjugated egg-white avidin (Jackson Laboratory Inc.). After additional washing with PBS and 50 mM sodium acetate, pH 5, the peroxidase activity was visualized by enzymatic modification of the 3-amino-9-ethylcarbazole substratum (3-amino-9-ethylcarbazole 0.4% in dimethylformamide: 50 mM sodium acetate, pH 5: 30% H2O2; 100:900:1) during 15 min incubation in the dark at room temperature. Sections were then counterstained with Harri's hematoxylin and mounted with Gel mount from Biomeda Corp. (Foster City, CA). Slides were observed and photographed with a Zeiss Axiophot.
Cell Culture
MC615 cells were cultured on tissue colture dishes in 1:1 high glucose DMEM/Ham's F-12 supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin as previously described [Mallein-Gerin and Olsen, 1993]. Medium was renovated every 2 days. During culture expansion cells never exceeded 60–80% confluency. Experiments were performed on proliferating cells and on hyperconfluent cells. Proliferating cells refer to sparse prechondrogenic cells (2 days after plating), hyperconfluent cells refer to differentiated cells kept in culture 7 days after confluence (12 days after plating), organizing a highly refractile extracellular matrix. Cell density of proliferating cells and hyperconfluent cells was respectively 40 × 103+/−2 × 103/cm2 and 830 × 103+/−15 × 103/cm2.
Immunoblot Analysis
MC615 cells harvested with a cell scraper in an ice cold buffer containing 20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1 mM β-glycerophosphate, 2.5 mM sodium pyrophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, were lysed by incubating the suspension on ice for 5 min. Protein concentration of lysate was measured using the Bradford method.
Lysate protein (50–100 µg) were boiled in a Laemmli buffer, separated on a 10% SDS–polyacrylamide gel, and transferred to nitrocellulose. The blot was blocked with 5% non-fat milk in TTBS (20 mM Tris-HCl, pH 7.5, 500 mM NaCl, and 0.1% Tween 20) for 1 h and then incubated in a cold room overnight with antibodies specific to COX-2, p38, phosphorylated p38, p65 NF-kB, SIP24. Antibodies were obtained from various sources; antibodies to p38 and p65 NF-kB were purchased from Santa Cruz Biotechnology. All phosphorylation-specific antibodies were from Cell Signaling Technology. Antibody directed against mouse SIP24 was kindly supplied by Dr. M. Nielsen-Hamilton. The nitrocellulose paper was subsequently washed with TTBS and incubated with a peroxidase-labeled goat anti-rabbit IgG antibody preparation (1:5000 dilution, Amersham) for 1 h at room temperature. The blot was washed with TTBS and incubated with an enhanced chemiluminescence substrate mixture (Amersham, UK). The blot was then exposed on an X-ray film (Amersham, UK) to obtain the image.
p38 MAP Kinase Assay
The level of active p38 MAPK was analyzed with the non-radioactive p38 MAPK assay (Cell Signaling Technology), according to the manufacturer's instructions.
Briefly, treated cells were lysed in the lysis buffer provided (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% TritonX-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 µg/ml leupeptin) and supplemented with 1 mM phenylmethylsulfonylfluoride. p38 MAPK was immunoprecipitated from 200 µg of whole cell lysate using beads coated with phospho-p38 MAPK (Thr-180/Tyr-182) monoclonal antibody overnight at 4°C with constant agitation. The immunoprecipitates were washed twice with lysis buffer and twice with the kinase buffer (25 mM Tris pH 7.5, 5 mM β-glycerolphosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2) provided with the kit. The immunoprecipitated p38 MAPK was incubated for 30 min at 30°C in kinase buffer containing 200 µM ATP and 2 µg of GST-ATF-2 fusion protein as a substrate. The reaction was terminated by adding 2 × Laemmli buffer and heating the samples to 95°C for 5 min. The samples were then separated on a 12% SDS–PAGE, immunoblotted and tested for the presence of phosphorylated ATF-2.
Nuclear Extracts
MC615 proliferating cells were stimulated for 2 h with LPS, IL-1α or TNF-α. Cells were scraped into ice cold PBS and transferred to a microfuge tube, sedimented by centrifugation for 5 min and resuspended in Sucrose Buffer (0.32 M Sucrose, 10 mM Tris-HCl pH 8.0, 3 mM CaCl2, 2 mM MgOAc, 0.1 mM EDTA, 0.5% NP-40, 1 mM DTT, 0.5 mM PMSF). Samples were re-centrifuged for 5 min and the supernatant fraction (cytoplasmic extract) was transferred into a new tube. The pellet was washed in Sucrose Buffer and resuspended in Low Salt Buffer (20 mM HEPES pH 7.9, 1.5 mM MgCl2, 20 mM KCl, 0.2 mM EDTA, 25% glycerol, 0.5 mM DTT, 0.5 mM PMSF). After the addition of an equal volume of High Salt Buffer (20 mM HEPES pH 7.9, 1.5 MgCl2, 800 mM KCl, 0.2 mM EDTA, 25% glycerol, 1% NP-40, 0.5 mM DTT, 0.5 mM PMSF, 4 µg/ml protease inhibitors) the samples were incubated for 30–45 min at 4°C for extraction. Samples were then centrifuged for 15 min and the supernatant fraction (nuclear extract) was stored at −70°C.
NF-kB Activity Assay
Binding of NF-kB p65 subunit to the NF-kB binding consensus sequence 5′-GGGACTTTCC-3′ was measured with the ELISA-based Trans Am NF-kB kit (Active Motif, CA) according to manufacturer's instruction. Briefly, whole cell lysates were prepared from MC615 proliferating and hyperconfluent cultures. The Trans-Am kit employs 96-well microtiter plates coated with an oligonucleotide containing the NF-kB binding consensus sequence. The active form of p65 subunit was detected using Abs specific for an epitope that is accessible only when the subunit is activated and bound to its target DNA. Specificity was checked by measuring the ability of soluble wild-type or mutated oligonucleotides to inhibit binding. Results are expressed as specific binding, i.e., by subtracting the absorbance values observed in the presence of the wild-type oligonucleotide to the one observed in the presence of the mutated oligonucleotide. All measures were performed in triplicate.
Chromatin Immunoprecipitation Assay
Proliferating MC615 cells cultured for two days in DMEM:F12 (1:1 v/v) medium supplemented with 10% FCS or hyperconfluent cell maintained for 7 days after confluence in the same medium were rinsed with PBS and transferred to a serum-free (SF) medium for 20 h. When indicated, LPS (1.0 µg/mL) was added to the SF medium for 20 h. In all experiments, assays were performed on duplicate plates for each cell treatment condition. Cells were washed twice in PBS buffer and fixed in paraformaldehyde (1% in PBS) for 15 min at room temperature. Cells were scraped, resuspended in collection buffer (100 µM Tris-HCl, pH 9.4; 10 mM DTT) and incubated for 15 min at 30°C. Pellets were obtained by centrifugation at 2,000g for 5 min and washed in sequence with ice-cold PBS, buffer I (0.25% Triton X-100; 10 mM EDTA; 0.5 mM EGTA; 10 mM HEPES, pH 6.5) and buffer II (200 mM NaCl, 1 mM EDTA; 0.5 mM EGTA; 10 mM HEPES, pH 6.5). Samples were then centrifuged for 5 min at 2000g. Pellets were resuspended in lysis buffer (1% SDS; 10 mM EDTA; 50 mM Tris-HCl, pH 8.1; 1 × protease inhibitor cocktail (Sigma) and sonicated six times with a 10-s burst.
Lysates were diluted 1:10 with dilution buffer (1% Triton X-100; 2 mM EDTA; 150 mM NaCl; 20 mM Tris-HCl, pH 8.1; 1 × protease inhibitor cocktail) and the total proteins concentration assessed on the soluble chromatin using the standard Bradford method. Volumes with an equivalent amount of protein were immunoprecipitated overnight at 4°C with the anti-NF-kB p65 (C-20) antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA; 2 µg/mg soluble chromatin) and protein A-Sepharose beads (Amersham Biosciences, Uppsala, Sweden). Preclearing of the protein A resin was performed, prior to the immunoprecipitation, by incubation with sonicated salmon sperm DNA and Rabbit IgG (Sigma) (4 µg/mg soluble chromatin and 40 µl/mg soluble chromatin, respectively) for 2 h at 4°C. When indicated an anti-phospho (S10)-acetyl (K14)-Histone H3 antibody (Upstate, Lake Placid, NY) was used as unrelated control.
Immunoprecipitated beads were pelleted by centrifugation at 1,000g for 10 min the supernatant was collected and used as input. The beads were washed sequentially with TSE I buffer (0.1% SDS; 1% Triton X-100; 2 mM EDTA; 20 mM Tris-HCl, pH 8.1; 150 mM NaCl), TSE II buffer (0.1% SDS; 1% Triton X-100; 2 mM EDTA; 20 mM Tris-HCl, pH 8.1; 500 mM NaCl) and buffer III (0.5 M LiCl; 1% NP40; 1% deoxycolate; 1 mM EDTA; 10 mM Tris-HCl, pH 8.1). Additional washes with standard TE buffer were also performed. The immunocomplexes were eluted from the beads by three subsequent washes with 1% SDS, 0.1 M NaHCO3. Crosslinks reversion was performed overnight at 65°C on the eluted samples; additional proteinase K treatment was performed at 50°C for 2 h on the recovered volumes. Immunoprecipitated DNA was eluted through the SK-400 Microspin colums (Amersham BioSciences, Uppsala, Sweden) and its concentration determined by spectrophotometry.
Each PCR reaction amplified 500 ng of immunoprecipitated or input DNA as template, using the following primer set to amplify the region encompassing the putative NF-kB binding element on the mouse COX-2 promoter (GeneBank ref. NC_000067.4): 5′-CACCAGTACAGATGTGGAC-3′ and 5′-GCTCTCTTGGCACCACCT-3′. Amplified fragments were resolved on 1% agarose gels and visualized with ethidium bromide.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNAs were extracted from proliferating prechondrogenic cells and hyperconfluent MC615 cells in TRIzol reagent (Invitrogen, San Diego, CA) as recommended by the manufacturer.
For RT-PCR, a 20 µl reaction contained 3 µg of total RNA, 0.5 µg/ul oligo(dT) primers, 500 µM each dNTP, 10 mM DTT, 5 mM MgCl2, 40 U of Rnase inhibitor and 40 U of Super ScriptII in RT buffer as recommended in SuperScript First Strand Synthesis System for RT-PCR. Reactions were carried out at 42°C for 50 min, followed by an inactivation of the enzyme at 70°C for 15 min. The cDNAs were incubated with 2 U/ml of Rnase H (Invitrogen) at 37°C for 20 min.
For PCR amplifications each 25 µl reaction contained 1 µl of RT aliquot, 200 µM each dNTP, 0.5 µM each primer, 1.5 mM MgCl2, and 3 U/µl Taq DNA Polymerase in buffer. Following an initial denaturation step of 2 min at 96°C, amplification consisted of 25–35 cycles of 30 s at 96°C, 30 s at optimal temperature, 30 s at 72°C, followed by a final extension step of 5 min at 37°C.
Amplification was performed in an Eppendorf MasterCycler; specific primers were designed from sequences available in the data banks (see Table I).
| Gene | Primers | Strand | Prod. size (bp) | T (°C) | PCR cycles | Reference sequence |
|---|---|---|---|---|---|---|
| Col Ia1 | GTTCAGCTTTGTGGACCTCC | F | 274 | 59 | 33 | MMU08020 |
| TGATACGTATTCTTCCGGGC | R | |||||
| Col II | CCAGACTGCCTCAACCCCGAGAT | F | 509 | 66 | 35 | NM 031163 |
| AGAGACACCAGGCTCGCCAGGT | R | |||||
| Coll X | TGGGTAGGCCTGTATAAGAACGG | F | 210 | 65 | 35 | MMCOL10A |
| CATGGGAGCCACTAGGAATCCTGAGA | R | |||||
| Sox9 | TGGCAGACCAGTACCCGCATAC | F | 136 | 65 | 35 | |
| TCTTTCTTGTGCTGCACGCGC | R | |||||
| GAPDH | ACCACAGTCCATGCCATCAC | F | 412 | 58 | 25 | MUSGAPDH |
| TCCACCACCCTGTTGCTGTA | R |
No PCR product was observed when DNA was replaced with water in the PCR reaction (data not shown).
Message for the glyceraldehyde-3-phosphate dehydrogenase gene was used to ascertain that an equivalent amount of cDNA was synthesized from the different samples.
RESULTS
COX-2 Expression in Mouse Embryo Cartilage
Localization of COX-2 protein in histological sections of 14.5 days old mouse embryos was performed by immunohistochemistry with a specific antibody against COX-2 (Fig. 1A). COX-2 was observed in the bone growth plate cartilage, and was particularly evident in the region of prehypertrophic/hypertrophic chondrocytes.
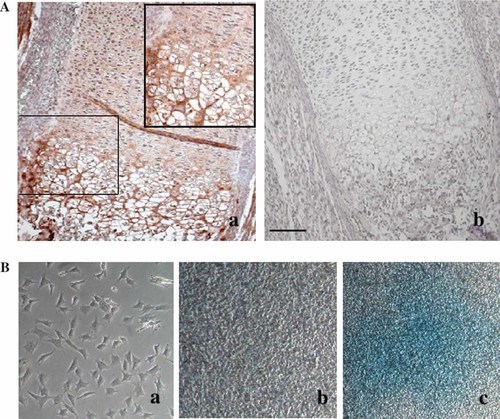
Immunolocalization of COX-2 protein in a 14.5 days old mouse embyo. A: The protein was localized in sections of tibia, using a specific COX-2 polyclonal antibody (a). Negative control was performed with rabbit serum IgG (b). B: Cell morphology and Alcian Blue staining of proliferating (a) and hyperconfluent cells (b, c). Bar: A = 60 µm, enlargement in Aa = 30 µm, Ba,b = 60 µm, and Bc = 120 µm.
Correlation Between the Level of COX-2 Expression and the Differentiation Stages of MC615 Cells
The MC615 cell line, an established, immortalized, clonal chondrocyte cell line obtained by infection of primary embryonic limb mouse chondrocytes with a retrovirus carrying SV40 early regions, was used for our experiments. In culture, these cells show an undifferentiated phenotype during the proliferation phase, but form cartilage nodules after they have reached hyperconfluence and stopped to grow [Mallein-Gerin and Olsen, 1993]. Morphological observation of cultures of proliferating and hyperconfluent cells clearly shows cartilage nodules stained with alcian blue in hyperconfluent cultures (Fig. 1B), confirming the differentiation capability of the cell line. Additional evidence of the chondrogenic differentiation of MC615 cells when they go by a proliferation to a hyperconfluent stage is presented in Fig. 2A. The mRNA for SOX9, the master gene of the chondrogenic lineage, starts to be synthesized, the cartilage-specific type II collagen mRNA becomes the predominant collagen mRNA and a small but detectable amount of mRNA coding for type X collagen, expressed by chondrocytes at a late differentiation stage is also observed. The same cell line was previously used for our study on the signaling pathway leading to the expression of the lipocalin SIP24 [Ulivi et al., 2006].
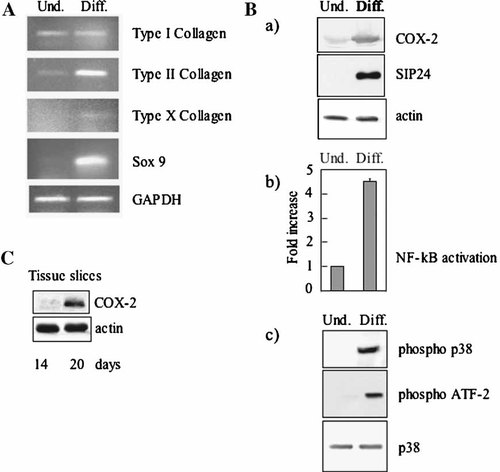
A: RT-PCR analysis of mRNA extracted from undifferentiated (Und) proliferating and differentiated (Diff) hyperconfluent MC615 cultures. Proliferanting cells refer to sparse prechondrogenic cells (2 days after plating), hyperconfluent cells refer to differentiated cells kept in culture 7 days after confluence (12 days after plating). B: COX2 and SIP24 expression; activation of p65 NF-kB, p38 MAPK, in proliferating prechondrogenic cells and hyperconfluent differentiated MC615 cells. a: Western blot analysis of MC615 conditioned media with SIP24 antibody, of cell lysates with COX-2 antibody. Normalization with actin was performed. b: Activation of NF-kB in proliferating and hyperconfluent cells. Five micrograms of proliferating and hyperconfluent cell lysates were tested for binding of the activated p65 NF-kB subunit to an NF-kB consensus sequence using the Trans-Am NF-kB ELISA kit. Average of four experiments is shown with standard deviation. The values are normalized to the value of the control proliferating cells. c: p38 phosphorylation is shown by Western blot with antibodies anti-phospho-p38. p38 activity: cell lysates were incubated with an anti-phospho-p38 antibody; immune complexes were collected and kinase assay for p38 MAPK activity was performed using ATF-2 fusion protein as phosphorylation substrate. Western blot with antibody anti-phospho ATF-2 is shown. Total p38 is also shown. C: Western blot of mouse embryo tibia cartilage at 14 and 20 days with COX-2 antibody. Normalization with actin was performed.
Here we investigated the expression of COX-2 as an enzyme central in the inflammatory cascade leading to the acute phase response. COX-2, like the SIP24, was barely expressed in proliferating cells, but was strongly increased in hyperconfluent cultures (Fig. 2B). In the same hyperconfluent cultures, activation of p65 NF-kB was about 4.5 times higher than in proliferating cells. In addition p38 protein, although present in both proliferating and hyperconfluent cultures, was phosphorylated only in the hyperconfluent cultures where p38 is activated because it is able to phosphorylate the GST-ATF-2 fusion protein in an activity assay (Fig. 2Bc).
In order to confirm the data obtained with the cell line, we investigated the physiological expression of the COX-2 protein, in differentiating cartilage by Western blot of the protein in tibial cartilage slices of mouse embryos at different stages (Fig. 2C). COX-2 was barely expressed at an early stage of differentiation (14 days), when cells are proliferating while it was strongly represented at late stage of differentiation (20 days) validating the data obtained with the cell line.
NF-kB is Constitutively Activated in Hyperconfluent Cells and Induced by LPS in Proliferating Cells. Correlation to the COX-2 Expression
Treatment with LPS of proliferating MC615 cells resulted in a substantial increase in the COX-2 synthesis (Fig. 3Aa). A comparable result was obtained with a primary culture of 14 day mouse chondrocytes at different passages. At the second cell culture passage a low amount of protein was detectable that was completely absent in the sample from the fifth cell culture passage, while a strong induction of the protein was observed in both cultures following treatment with LPS (Fig. 3Ab).
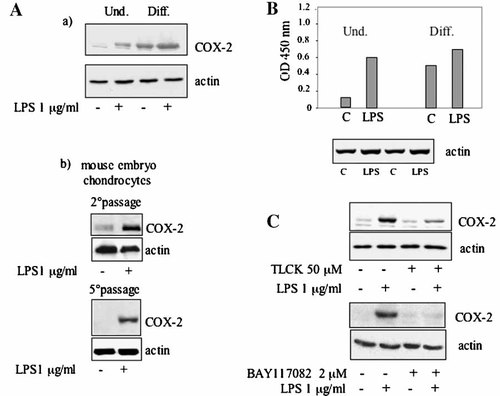
NF-kB is activated in proliferating cells induced by LPS and in hyperconfluent cells and is involved in COX-2 expression. Cells were stimulated overnight in SF condition with 1 µg/ml LPS. A(a): Western blot analysis of proteins of cell lysate from proliferating and hyperconfluent MC615 was performed using a COX-2 polyclonal antibody. Normalization with actin was performed. A(b): Western blot analysis of proteins of cell lysate from proliferating mouse embryo chondrocytes was performed using a COX-2 polyclonal antibody. Cells at the second passage and at the fifth passage were used. Normalization with actin was performed. B: Five micrograms of cell lysates from proliferating and hyperconfluent MC615 were tested using the Trans-Am NF-kB ELISA kit. Results are expressed as specific binding, (i.e., as the absorbance values observed in the presence of the mutated oligonucleotide minus those observed in the presence of the wild-type oligonucleotide. The mesurements were performed in triplicate). Data refer to a typical experiment carried out more than three times with similar results. Normalization with actin was performed. C: Upper panel: MC615 cells were treated with 50 µM TLCK for 4 h and then supplemented with 1 µg/ml LPS for 20 h. COX-2 synthesis was determined by Western blot analysis of 20 µg of cell lysates. Normalization with actin was performed. Lower panel: MC615 cells were treated with 2 µM BAY117082 for 2.5 h and then supplemented with 1 µg/ml LPS for 20 h. COX-2 synthesis was determined by Western blot analysis of 20 µg of cell lysates. Normalization with actin was performed.
In differentiated hyperconfluent cultures of MC615 cells, treatment of cells with LPS did not significantly increase the expression of COX-2 already expressed to a large extent in these culture conditions (Fig. 3Aa). Determination of NF-kB activity showed a much higher activity in hyperconfluent cells compared to proliferating cells (Figs. 2Bb and 3B). LPS treatment only slightly raised the level of NF-kB activity in hyperconfluent cells, but drastically increased NF-kB activity in proliferating cells to a level comparable to the one constitutively present in hyperconfluent cells. The concurrent responses to LPS stimulation of NF-kB activation and COX-2 increased synthesis suggested a correlation between the two events. Noticeably the NF-kB inhibitor TLCK inhibited also the expression of COX-2 induced by LPS (Fig. 3C). Treatment with TLCK diminished the expressed protein level to less than 40% of the normal LPS-induced amount of COX-2. Indeed, in the assessed membranes, the image analysis indicated a TLCK+LPS/LPS ratio of 0.36 with a SD value of 0.06 (n = 8; P < 0.05). In addition the specific NF-kB inhibitor BAY117082 [Pierce et al., 1997] repressed the expression of COX-2 induced by LPS (Fig. 3C).
Chromatin immunoprecipitation with anti-p65 NF-kB antibody of nuclear extracts from LPS-treated proliferating cells allowed the amplification of the COX-2 promoter region that includes the NF-kB responsive element (Fig. 4). Thus p65 NF-kB binds to the COX-2 promoter in response to LPS stimulation. Interestingly, the same results could be obtained in the templates derived from not stimulated hyperconfluent cells, where the amplicon fragments could be detected both in the input and in the immunoprecipitated DNA. Chromatin immunoprecipitation failed to identify NF-kB on the mouse COX-2 promoter in proliferating cells not stimulated with LPS. In fact no amplified fragment was detected in the immunoprecipitated DNA template sample, whereas, as expected, the proper amplicon was present in the input DNA. This last finding is in agreement with the low level of expression of COX-2 in the same cells (Fig. 3A) and with the low/basal level of NF-kB activity in whole cell extracts from cells cultured in the same experimental conditions (Fig. 3B). On the overall the chromatin immunoprecipitation experiments confirmed the role played by NF-kB in the control of COX-2 expression in the experimental system.
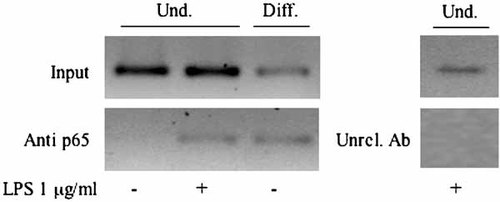
Chromatin immunoprecipitation assay for localization of NF-kB on the mouse COX-2 promoter. Fragments (325 bps) were amplified from input (Input) and immunoprecipitated (anti-p65) DNA samples. Template specimens were prepared from proliferating undifferentiated (und) or hyperconfluent differentiated (diff) MC615 cells cultured for 24 h in SF medium. Results obtained after LPS treatment of the proliferating cells are also depicted (LPS). No fragment amplification was detected from DNA templates derived from LPS-treated proliferating MC615 and immunoprecipitated with control anti-phospho (S10)-acetyl (K14)-Histone H3 antibody (Ab). Depicted images are representative of results obtained in triplicate sets of experiments.
Induction of COX-2 Expression by Inflammatory Agents in Proliferating MC615 Cells Occurs Via a p38-Dependent Pathway
Proliferating MC615 cells were treated at established times with either 1 µg/ml LPS or 100 U/ml IL-1α or 200 U/ml TNFα. When indicated the p38 inhibitor SB203580 was added at a 5 µM final concentration.
Figure 5A shows that in a time course experiment LPS and IL-1α, but not TNFα, induced p38 activation, measured by the capability of p38 to phosphorylate the GST-ATF-2 fusion protein in an activity assay. Similarly, COX-2 was induced by LPS and IL-1α, but not by TNFα (Fig. 5B). Treatment with the p38 inhibitor SB203580 resulted in an inhibition of the LPS-induced p38 activation and in a substantial, although not complete, inhibition of the LPS-induced COX-2 expression (Fig. 6), indicating that in proliferating cells the p38 pathway is involved in the COX-2 induction.
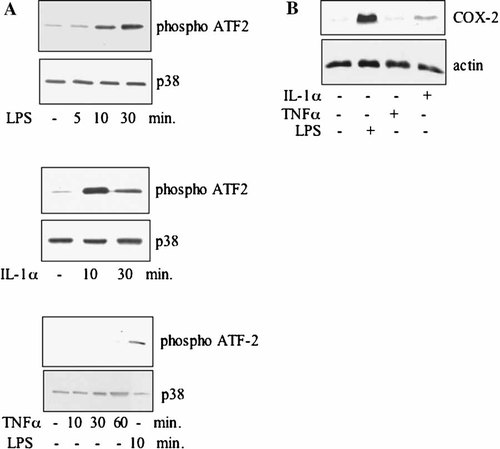
p38 is activated in proliferating cells treated with LPS and IL-1α but not with TNFα⋅ A: Time-dependent induction of p38 MAPK activity. Proliferating MC615 cells were incubated with LPS 1 µg/ml (A), or 100 U/ml IL-1α or TNFα 200 U/ml in SF conditions for the indicated time periods. Cell lysates were incubated with an anti-phospho-p38 antibody; immune complexes were collected and kinase assay for p38 MAPK activity was performed using ATF-2 fusion protein as phosphorylation substrate. Expression of p38 was determined by Western blotting. Data refer to a typical experiment. Similar experiments have been performed more than three times with similar results. B: COX-2 induction by inflammatory stimuli. Proliferating MC615 cells were supplemented with inflammatory cytokines for 20 h. Cell lysates were subjected to immunoblot analysis using specific COX-2 polyclonal antibody. Lane 1: control SF; lane 2: LPS 1 µg/ml; lane 3: TNF-α 200 U/ml; lane 4: IL-1 α 100 U/ml. Normalization with actin was performed.
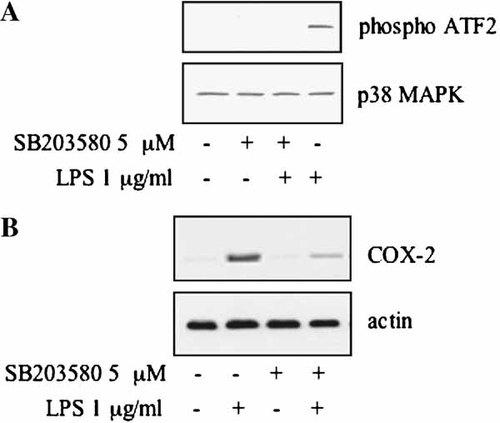
p38-specific inhibitor SB203580 inhibits LPS-mediated induction of p38 MAPK activity and COX-2 expression in proliferating MC615 cells. A: Cells were incubated with 5 µM SB203580 for 2 h plus 1 µg/ml LPS for additional 10 min. Cell lysates were incubated with an anti-phospho-p38 antibody; immune complexes were collected and p38 MAP kinase assay of immune complexes was performed using ATF-2 fusion protein as phosphorylation substrate. Total p38 protein level was determined by Western blotting. Data refer to a typical experiment. Similar experiments have been performed more than three times with similar results. B: Cells were treated with 5 µM SB203580 for 2 h then supplemented with LPS 1 µg/ml for 20 h. Western blot analysis of cell layer proteins was performed using a COX-2 polyclonal antibody. Normalization with actin was performed.
NF-kB is Activated in Proliferating Cells Treated With LPS, IL-1α, TNFα
In proliferating cells, NF-kB was activated by LPS, IL-1α, TNFα, that promote p65 NF-kB nuclear traslocation, as shown in Figure 7 where p65 is clearly enriched in nuclear extracts of treated cells. It must be noted that TNFα did not induce COX-2 expression (Fig. 5B), indicating that NF-kB activation is not sufficient to induce COX-2 expression.
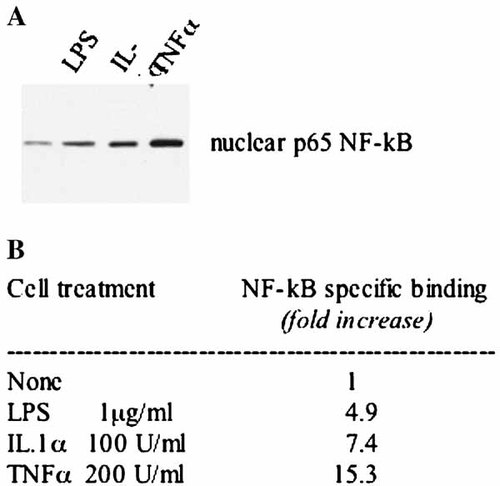
NF-kB activation by inflammatory agents in proliferating cells. A: Western blot analysis of 20 µg of nuclear proteins using a p65 NF-kB polyclonal antibody. B: MC615 proliferating cells were stimulated with 1 µg/ml LPS, or 100 U/ml IL-1α, or 200 U/ml TNF-α in SF conditions overnight. Five micrograms of cell lysates were tested for binding of the activated p65 NF-kB subunit to an NF-kB consensus sequence using the Trans-Am NF-kB ELISA kit. Results are expressed as specific binding, (i.e., as the absorbance values observed in the presence of the mutated oligonucleotide minus those observed in the presence of the wild-type oligonucleotide, in triplicate performed measurements) and reported as fold increase with respect of untreated cells.
COX-2 Expression is Correlated to the Activation of NF-kB by a p38-Dependent Pathway
COX-2 expression induced by LPS was triggered by NF-kB activation (Fig. 3C and Fig. 4) and inhibited by the p38-specific inhibitor SB203580 (Fig. 6), indicating that both the p38 and the NF-kB pathways are involved in COX-2 expression. To demonstrate a possible link between the two pathways we measured in undifferentiated proliferating cells the NF-kB activation by LPS in the presence of the p38-specific inhibitor. The activity induced by LPS in the control cells was 4.6-fold (statistically highly significant by t-test analysis: P < 0.01). But decreased to 2.7-fold (statistically highly significant by t-test analysis: P < 0.01) in the presence of the inhibitor SB203580 indicating a 41% repression of the LPS induction (statistically highly significant by t-test analysis: P < 0.01) (Fig. 8). The results indicate that p38 is at least partially involved in the activation of NF-kB leading to the expression of COX-2 in response to an inflammatory stimulus. Interestingly, the low NF-kB activity constitutively expressed in undifferentiated proliferating cells, was not repressed by the SB203580 treatment suggesting that p38 is not involved in NF-kB constitutive activation in this culture condition.
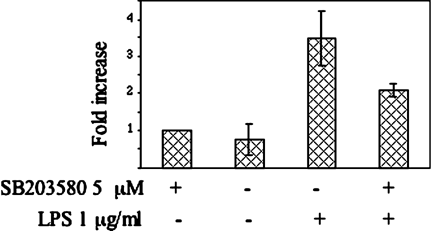
p38 is involved in NF-kB activation by LPS in proliferating cells. Proliferating MC615 cells were stimulated overnight with 1 µg/ml LPS in the presence or absence of the p38 MAPK inhibitor 5 µM SB203580; 10 µg of cell lysates were tested for binding of the activated p65 NF-kB subunit to an NF-kB consensus sequence using the Trans-Am NF-kB ELISA kit. Results are expressed as specific binding. Average of four experiments is shown with standard deviation. All the values are normalized to the value of the sample treated with SB203580.
Because the p38 pathway is constitutively activated in differentiated hyperconfluent cells and COX-2 is constitutively expressed (Fig. 2B), cells were treated with the p38-specific inhibitor SB203580 at a concentration up to 20 µM. The compound did not cause a decrease in the COX-2 expression (Fig. 9A). Interestingly also p38, constitutively activated (as shown by the capability of p38 to phosphorylate the GST-ATF-2 fusion protein in an activity assay) was clearly not inactivated by the inhibitor (Fig. 9B). This result is in agreement with the finding that the activation state of p38 determines the efficiency of ATP competition for SB203580. Indeed ATP cannot compete with the inhibitor for the ATP binding site of the inactive p38 while effectively competes with the inhibitor when p38 is activated [Frantz et al., 1998]. From these data, we cannot exclude that the constitutively expressed COX-2, mediated by p65 NF-kB (Fig. 4), is also depending on p38 pathway.
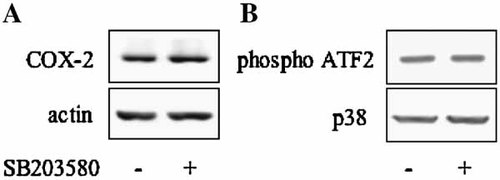
A: COX2 is constitutively expressed in hyperconfluent cells. Hyperconfluent cells were treated with 20 µM SB203580 for 24 h. Cell lysates were collected and analyzed by Western blot using a specific COX-2 polyclonal antibody. Normalization with actin was performed. B: p38 is constitutively activated in hyperconfluent cells: cell lysates were incubated with an anti-phospho-p38 antibody; immune complexes were collected and p38 MAP kinase assay of immune complexes was performed using ATF-2 fusion protein as phosphorylation substrate. Total p38 protein level was determined by Western blotting.
DISCUSSION
Several proteins, usually connected to stress conditions and overinduced by treatment with inflammatory agents, were observed also in fully mature chondrocytes, during the late differentiation stages of the avian growth plate cartilage. This event led us to consider the hypertrophic cartilage as a site where a “physiological inflammatory status” exists. The COX-2 is an enzyme that has a main function in the cell acute phase response following an inflammatory stimulus. In the skeletal system, the expression of COX-2 was observed in fracture callus formation and its function was reported as essential for bone fracture healing [Simon et al., 2002; Zhang et al., 2002; Arasapam et al., 2006]. The expression of COX-2 was recently described in the differentiating growth plate as well as the synthesis of prostaglandins and PGE2 receptors [Brochhausen et al., 2006] In order to find a possible common pathway involved in COX-2 expression, in the present work we investigated the pathway active during cartilage differentiation and the one induced by inflammatory agents. For this study, we took advantage of the MC615 mouse cell line, a prechondrogenic cell line capable of forming cartilage nodules in hyperconfluent cultures. Similarly to the stress-related lipocalin SIP24, COX-2 barely detectable in undifferentiated proliferating cells, was clearly expressed in differentiated hyperconfluent cells. COX-2 expression was matching p65 NF-kB activation and p38 phosphorylation and activation. Interestingly, in differentiated hyperconfluent cells, the p38-specific inhibitor SB203580 was unable to inhibit p38 activation as well as COX-2 expression suggesting that the expression of COX-2 in differentiated cells was a consequence of a constitutive activation of the p38/NF-kB pathways.
Prechondrogenic proliferating cells expressed only a very low level of COX-2 and a low level of p65 NF-kB, nor they contained p38 in the phosphorylated active form. The treatment of these cells with the bacterial toxin LPS or the inflammatory cytokine IL-1α, led to the increased expression of COX-2 and the contemporary activation of p65 NF-kB. The involvement of NF-kB in the induction of COX-2 expression was proven by the fact that the NF-kB inhibitor TLCK repressed also the synthesis of COX-2. The control of the COX-2 expression by p65 NF-kB was confirmed by chromatin immunoprecipitation with antibodies against p65. These experiments showed an association of p65 with the COX-2 promoter both in LPS-stimulated proliferating cells and in differentiated hyperconfluent cells. The control of the COX-2 expression involves also p38 activation. The inhibition by SB203580 of the p38 activation observed after LPS or IL-1α treatment had as a consequence the inhibition of COX-2 synthesis. We suggest that also the two pathways of p38 and NF-kB are linked. Indeed the inhibition of the p38 activation resulted also in a significant inhibition of the p65 NF-kB activation. The fact that TNFα, a cytokine inducing NF-kB activation and nuclear translocation but not p38 activation, did not induce COX-2 is invigorating our hypothesis. Differences in the inactivation of p38 by SB203580 observed in differentiated hyperconfluent cells and in undifferentiated proliferating cells stimulated by inflammatory agents could be related to the constitutive activation of p38 and therefore to a non-availability of the p38 active site for the inhibitor.
p38 activation has been described in differentiation and inflammation of several tissues [Zarubin and Han, 2005] including cartilage [Oh et al., 2000; Zhen et al., 2001; Ulivi et al., 2006]. NF-kB activation has been extensively described in inflammation [for a review Natoli, 2006] while regulation of NF-kB activation in differentiation has been shown for several cells and tissues [Baeza-Raja and Munoz-Canoves, 2004; Mattson, 2005; Bottero et al., 2006; Wang et al., 2007]. In cartilage, the physiological activation of NF-kB in MC615 cells differentiated to the stage of type II collagen expressing cells has been recognized in a previous publication of ours [Ulivi et al., 2006]. This finding is in agreement with a recent literature report showing maximal NF-kB activation in chondrocytes expressing type II collagen and enhancement of viability of the same cells by the activated NF-kB [Park et al., 2007].
Expression of COX-2 in several differentiated tissues, including kidney, central nervous system, reproduction, stomach [Morham et al., 1995; Langenbach et al., 1999; Hoffmann, 2000; Reese et al., 2001; Akunda et al., 2004; Qi et al., 2006] in addition to cartilage [Arasapam et al., 2006] has been also reported, but the pathway leading to the physiological expression of COX-2 remains mostly unknown.
On the contrary, COX-2 expression in inflammation has been widely investigated and the signaling pathway leading to its expression studied [Herschman, 1994; Dubois et al., 1998; Sakamoto, 1998]. COX-2 induction was related to NF-kB activation [Wu, 2005; Deng et al., 2006] and to p38 activation [N'Guessan et al., 2006]. Our study shows that COX-2 is expressed in cartilage both during differentiation and in response to an inflammatory stimulus, that the same p38/NF-kB pathway is activated in both conditions and that this pathway is involved in COX-2 expression. This finding is in agreement with recent reports indicating that p38 activation participates in NF-kB-induced COX-2 expression in infection induced inflammation of lung epithelium and in IL-1α stimulated carcinoma cells [Duque et al., 2006; N'Guessan et al., 2007]. We plan to extend, the study to human articular cartilage in order to establish if the same pathways that are activated in growth plate cartilage are activated in differentiated cells or in pathology. Indeed the findings of this paper and in particular the NF-kB constitutive activation and the lack of effect of p38 inhibitor on the constitutive activation of p38 and COX-2 production could be relevant for articular cartilage pathology treatment.
Acknowledgements
Partially supported by funds from Ministero Universita' e Ricerca (PRIN) e from Centro Studi Termali Veneto, Pietro D'Abano di Abano Terme e Montegrotto Terme. We thank Marit Nilsen-Hamilton, Iowa State University, Ames, Iowa, USA for the generous supply of rabbit antiserum against SIP24. We thank Frederic Mallein- Gerin, Universitè Claude Bernard Lyon, Lyon, France for kindly supplying the MC615 cell line.




