Effect of UV irradiation on colorectal cancer cells with acquired TRAIL resistance
Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a member of the TNF superfamily. TRAIL shows strong cytotoxicity to many cancer cells but minimal cytotoxicity to most normal cells. Interestingly, our recent studies have demonstrated that pretreatment with TRAIL induces acquired resistance to TRAIL (Song et al. 2007 J Biol Chem 282: 319). Acquired TRAIL resistance develops within 1 day and gradually decays within 5 days after TRAIL treatment. In our current study, we examined whether human colorectal carcinoma CX-1 cells with acquired TRAIL resistance are resistant to UV irradiation as well. CX-1 cells were treated with 200 ng/ml TRAIL for 6 h and incubated various times (0.25–5 days) and then challenged to UV irradiation. Unexpectedly, we observed an increase in apoptosis in acquired TRAIL resistant cells after UVC as well as UVB exposure. This was due to an increase in caspase activation which was mediated through cytochrome c release. These results suggest that cells with acquired TRAIL resistance are sensitive to UV irradiation. J. Cell. Biochem. 104: 1172–1180, 2008. © 2008 Wiley-Liss, Inc.
Abbreviation used:
DTT, dithiothreitol; ERKs, extracellular signal-regulated kinases; FADD, Fas-associated death domain; FLICE, Fas-associated death domain-like interleukin-1β-converting enzyme; FLIP, FLICE inhibitory protein; IAP, inhibitor of apoptosis; JNKs, c-jun N-terminal protein kinases; MAPKs, mitogen-activated protein kinases; PAGE, polyacrylamide gel electrophoresis; PARP, poly(ADP-ribose) polymerase; PBS, phosphate-buffered saline; PI3K, phosphatidylinositol 3-kinase; SDS, sodium dodecy sulfate; TNF, tumor necrosis factor; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; UV, ultra violet.
Apoptosis, a controlled form of cell death, plays a central role in the development and homeostasis of multicellular organisms. In contrast to necrosis, a form of cell death that results from acute tissue injury and provokes an inflammatory response, apoptosis is carried out in a regulated way. Apoptotic cell death is associated with typical morphological features including cell shrinkage, chromatin condensation and cytoplasmic membrane blebbing. Deregulated apoptosis has been implicated in a variety of diseases. Too much apoptosis can cause cell-loss disorders, whereas too little apoptosis results in uncontrolled cell proliferation, leading to cancer [Falschelehner et al., 2007].
Tumor necrosis factor α-related apoptosis-inducing ligand (TRAIL), also known as Apo2L, is a member of the tumor necrosis factor (TNF) superfamily proteins. It is a membrane-bound apo-protein that can be cleaved to generate soluble TRAIL. TRAIL is being actively investigated as a cancer therapeutic agent, because various types of tumor cells are vulnerable to apoptotic death by soluble TRAIL, whereas normal cells are relatively insensitive to this effect [Koschny et al., 2007].
Although the unique feature of selectivity for cancer cells has drawn considerable attention to TRAIL as a potential therapeutic agent against human cancers, the physiological role of TRAIL is certainly more complex than merely activating the caspase-dependent apoptosis of cancer cells. Previous studies have shown that repeated application of TRAIL induces acquired resistance to TRAIL [Zhang et al., 2002a; Lee et al., 2006; Song et al., 2007]. This may be the result of decrease of caspase activation caused by changes in proteins which include the mutational inactivation of proapoptotic molecules (Bax, Bak, Bad, Bim, or Bid) or the overexpression of death inhibitors (FLIP, FAP-1, Bcl-2, Bcl-xL, or inhibitor of apoptosis (IAP)). These death inhibitors operate by several different mechanisms. The anti-apoptotic molecules of the Bcl-2 family (Bcl-2, Bcl-xL) heterodimerize with pro-apoptotic members of the Bcl-2 family (Bax, Bak) and interfere with release of cytochrome c by pore-forming proteins (Bid, Bik) [Gross et al., 1999]. Recently, we observed that dissociation of Bad from Bcl-xL, an increase in the intracellular level of Bcl-xL, and/or down-regulation of death receptors (DR4 and DR5) may be responsible for development of acquired TRAIL resistance [Song et al., 2007; unpublished data].
Radiation in the UVC (wavelength, 180–280 nm), UVB (280–320 nm), and UVA (UVA I, 340–400 nm; UVA II, 320–340 nm) regions shows strong carcinogenic activity with regard to the induction of squamous-type tumors in mouse skin [Ley, 1993]. In those experimental systems, UV light acts as both an initiator, presumably by causing DNA damage leading to gene mutations, and a tumor promoter [Setlow, 1974; Kusewitt et al., 1993]. Although all of the UVC and much of the UVB radiation are absorbed by the ozone layer of Earth's atmosphere [De Gruijl, 2000; De Gruijl et al., 2001], 1–10% of the total UV at Earth's surface is UVB [Matsui and DeLeo, 1995] which can be absorbed by the DNA and proteins of unprotected organisms. In addition, UVA makes up more than 90% of the solar UV at Earth's surface and is becoming more seriously recognized as a major contributor to carcinogenesis [Moan et al., 1999; Woodhead et al., 1999].
In addition to its carcinogenic activity, UV irradiation induces apoptosis in cultured cells and in vivo [Slominski and Pawelek, 1998]. Cell surface death receptors may be activated either directly at high doses of UV radiation or indirectly through induction of the secretion of latent ligands. In cells treated with moderate doses of UV radiation, caspase activation occurs mainly via the intrinsic, mitochondria-driven pathway downstream of cytochrome c release [Takahashi et al., 2001]. p53 activity is involved with the mitochondrion itself and with members of the Bcl-2 family of proteins [Oren, 2003]. UV light can damage nuclear DNA due to direct absorption, producing either cell cycle arrest or apoptosis depending on the extent of the damage.
In this study, we examined whether CX-1 cancer cells with acquired TRAIL resistance are resistant to UV irradiation. We observed that acquired TRAIL resistant cells are sensitive to UV irradiation. Our studies demonstrate that UV augments TRAIL-induced apoptosis by promoting the mitochondria-dependent apoptotic pathway through cytochrome c release and subsequently leads to an increase in caspase activation.
MATERIALS AND METHODS
Cell Culture and Survival Assay
Human colorectal carcinoma CX-1 cells were cultured in RPMI-1640 medium (Gibco BRL) containing 10% fetal bovine serum (HyClone, Logan, UT) and 26 mM sodium bicarbonate for monolayer cell culture. The dishes containing cells were kept in a 37°C humidified incubator with 5% CO2. One or 2 days prior to the experiment, cells were plated into 60-mm dishes. For trypan blue exclusion assay [Burow et al., 1998], trypsinized cells were pelleted and resuspended in 0.2 ml of medium, 0.5 ml of 0.4% trypan blue solution, and 0.3 ml of phosphate-buffered saline solution (PBS). The samples were mixed thoroughly, incubated at room temperature for 15 min, and examined under a light microscope. At least 300 cells were counted for each survival determination.
Production of Recombinant TRAIL
A human TRAIL cDNA fragment (amino acids 114–281) obtained by RT-PCR was cloned into a pET-23d (Novagen, Madison, WI) plasmid, and His-tagged TRAIL protein was purified using the Qiagen express protein purification system (Qiagen, Valencia, CA).
Morphological Evaluation
Approximately 5 × 105 cells were plated into 60-mm dishes overnight. Cells were treated with TRAIL and/or UVC irradiation and then analyzed by phase-contrast microscopy for signs of apoptosis.
Antibodies
Rabbit polyclonal anti-caspase-3 antibody, rabbit polyclonal anti-caspase-9, and mouse monoclonal anti-caspase-8 were purchased from Santa Cruz (Santa Cruz, CA). Anti-PARP antibody from Biomol Research Laboratory (Plymouth Meeting, PA), anti-cytochrome c from PharMingen (San Diego, CA), and anti-actin antibody from ICN (Costa Mesa, CA).
Protein Extracts and Polyacrylamide Gel Electrophoresis (PAGE)
Cells were lysed with 1× Laemmli lysis buffer (2.4 M glycerol, 0.14 M Tris, pH 6.8, 0.21 M sodium dodecyl sulfate (SDS), 0.3 mM bromophenol blue) and boiled for 10 min. Protein content was measured with BCA Protein Assay Reagent (Pierce, Rockford, IL). The samples were diluted with 1× lysis buffer containing 1.28 M β-mercaptoethanol, and equal amounts of protein were loaded on 8–12% SDS–polyacrylamide gels. SDS–PAGE analysis was performed according to Laemmli [1970] using a Hoefer gel apparatus.
Immunoblot Analysis
Proteins were separated by SDS–PAGE and electrophoretically transferred to nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% non-fat dry milk in PBS-Tween-20 (0.1%, v/v) at 4°C overnight. The membrane was incubated with primary antibody (diluted according to the manufacturer's instructions) for 2 h. Horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG was used as the secondary antibody. Immunoreactive protein was visualized by the chemiluminescence protocol (ECL, Amersham, Arlington Heights, IL).
UV Source and Exposure
For UV irradiation experiments, cells were exposed to UVB/UVC in a UV cross-linker (Spectronics Corporation, Westbury, NY).
Preparation of Cytosolic Fraction
Mitochondrial cytochrome c release assay was performed according to the method as described by Mitochondrial Fractionation Kit (Active Motif: Cat. No.:40015). Human colorectal carcinoma CX-1 cells were either left untreated or TRAIL-treated, and incubated for 1 day before UVC-irradiation, and then harvested by centrifugation at 600g at 4°C for 5 min. After washing the cells three times with ice-cold PBS, supernatant was removed. The pellet was added to 1 ml ice-cold 1× cytosolic buffer. On ice, samples were homogenized five times with a syringe. Lysates were spinned and transferred to fresh, pre-chilled microcentrifuge tubes. The supernatants were again centrifuged at 16,000g for at least 20 min at 4°C to remove any residual mitochondria. Lysates were spinned and transferred to fresh, pre-chilled microcentrifuge tubes. Samples were measured for the protein concentration of each fraction using a Bio-Rad assay with a BSA standard curve.
RESULTS
Development of Acquired TRAIL Resistance
We investigated whether TRAIL resistance develops after TRAIL treatment in CX-1 cancer cells. CX-1 cells were exposed to 200 ng/ml TRAIL for 6 h, detached cells (>90% dead cells) were washed out, and then attached cells were incubated for various times before being challenged to 200 ng/ml TRAIL for 6 h. First, we examined the time course of TRAIL-induced cytotoxicity during the first TRAIL exposure. TRAIL treatment led to apoptosis, as shown by cell surface blebbing and the formation of apoptotic bodies (Fig. 1A). These observations were consistent with poly(ADP-ribose) polymerase cleavage (PARP) (Fig. 1B), which is the hallmark feature of apoptosis. Figure 2B shows that ∼30% of the cells died and were lysed within 6 h of treatment with 200 ng/ml TRAIL. Interestingly, repopulation occurred among the remaining cells. In a following experiment, CX-1 cells were exposed to 200 ng/ml TRAIL for 6 h (lane 2 in Fig. 2), detached cells (>90% dead cells) were washed out, and then attached cells were incubated for various times (0–5 days) before being challenged to 200 ng/ml TRAIL for 6 h (lanes 3–8 in Fig. 2). Data from survival assay and Western blot analysis show that acquired TRAIL resistance developed immediately, was sustained for 3 days, and then gradually decayed within 5 days.
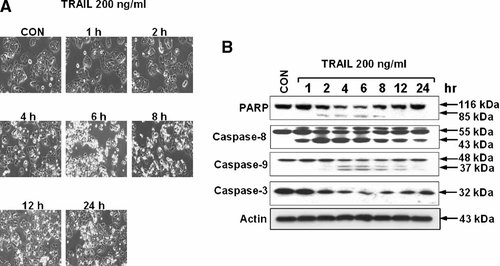
TRAIL-induced cytotoxicity in human colorectal cancer CX-1 cells. Cells were treated with TRAIL (200 ng/ml) for various times (0–24 h). A: Morphological features of each cell were analyzed with a phase-contrast inverted microscope. B: Cell lysates were subjected to immunoblotting for PARP, caspase-8, caspase-9, caspse-3, or actin. Immunoblots of PARP show the 116 kDa and the 85 kDa apoptosis related cleavage fragments. Antibody against caspase-8 detects inactive form (55 kDa) and cleaved intermediate (43 kDa). Anti-caspse-9 antibody detects both inactive form (48 kDa) and cleaved intermediate (37 kDa). Anti-caspase-3 antibody detects inactive form (32 kDa). Actin was used to confirm the equal amounts of protein loaded in each lane.
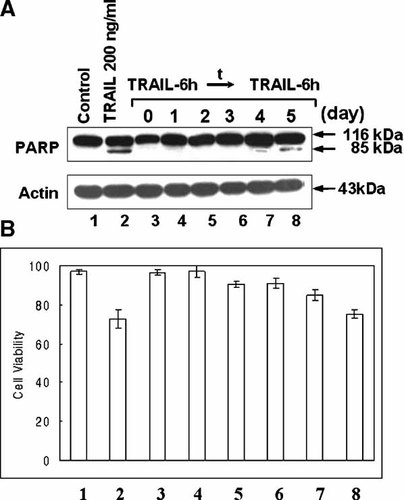
Acquisition of TRAIL resistance in TRAIL treated CX-1 cells. Cells were treated with TRAIL (200 ng/ml) for 6 h, and then detached cells were removed by washing with PBS. After removal of the detached cells, fresh media was added to the remaining attached cells and cells were incubated for the time indicated (1–5 days) and then treated a second time with TRAIL (200 ng/ml) for 6 h. A: Cell lysates were subjected to immunoblotting for PARP or actin. B: Cell survival was determined by trypan blue exclusion assay. Error bars represent the SE from three experiments. t, time.
Effect of UVC Irradiation on Colorectal Cancer Cells With Acquired TRAIL Resistance
It is well known that UVC irradiation is more genotoxic than UVB or UVA. CX-1 cancer cells were exposed to 500 J/m2 of UVC irradiation and incubated for 1–4 days (Fig. 3). PARP cleavage was observed at 2 days after UVC exposure. In a following experiment, CX-1 cells were treated or not treated with TRAIL (200 ng/ml) for 6 h (lane 2 in Fig. 4A,C,E) and then detached cells were removed by washing with PBS. After removal of the detached cells, fresh media was added onto the remaining attached cells and incubated for 0 day (Fig. 4A,B), 1 day (Fig. 4C,D), or 2 days (Fig. 4E,F). At this point in the experiment, we examined whether human colorectal carcinoma CX-1 cells with acquired TRAIL resistance are resistant to UVC irradiation as well. Cells were exposed to 500 J/m2 of UVC irradiation and incubation for 6, 24, or 48 h (lanes 7–9 pretreated with TRAIL and lanes 4–6 without TRAIL treatment in Fig. 4A,C,E). We observed an increase in apoptosis in TRAIL pretreated cells after 500 J/m2 UVC exposure. These results suggest that cells with acquired TRAIL resistance are sensitive to UVC irradiation. Data from morphological feature analysis were consistent with these observations (Fig. 4G). As shown in Panels g–o from Figure 4G, apoptotic cell death, which is associated with typical morphological features like cell shrinkage and cytoplasmic membrane blebbing, was observed in TRAIL pretreated cells after UVC exposure.

UVC-induced apoptosis in CX-1 cells. Cells were exposed to 500 J/m2 of UVC (254 nm) and incubated for 1–4 days. Cell lysates containing equal amounts of protein were separated by SDS/PAGE and immunoblotted with anti-PARP antibody. Actin was shown as an internal standard.
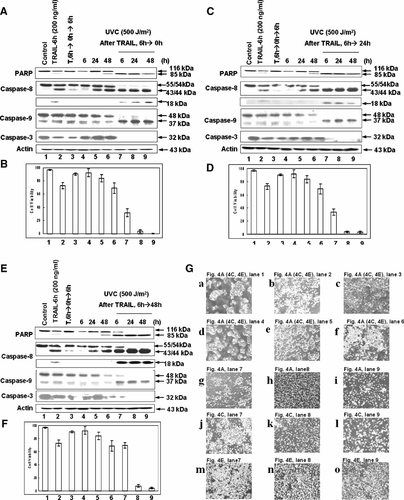
Effect of UVC 500 J/m2 on CX-1 cells with acquired TRAIL resistance. Cells were treated or not treated with TRAIL (200 ng/ml) for 6 h, and then detached cells were removed by washing with PBS. After removal of the detached cells, fresh media were added onto the remaining attached cells and incubated for 0, 24, and 48 h and then were exposed to 500 J/m2 of UVC (254 nm) and incubated for 6 h, 1, and 2 days. A,C,E, Cell lysates were subjected to immunoblotting for PARP, caspase-8, caspase-9, caspase-3, or actin as described in Figure 1. B,D,F, Cell survival was determined by trypan blue exclusion assay. Error bars represent the SE from three experiments. G: Morphological features of cells were analyzed with a phase-contrast inverted microscope.
Effect of UVB and Low Dose UVC on Colorectal Cancer Cells With Acquired TRAIL Resistance
We also examined whether human colorectal carcinoma CX-1 cells with acquired TRAIL resistance are also sensitive to low dose UVC (Fig. 5A,B) and UVB (Fig. 5C,D) irradiation. CX-1 cells were treated or not treated with 200 ng/ml TRAIL for 6 h and incubated for 1 day. After incubation, cells were challenged to UVB/UVC irradiation and incubated for 6 h (0.25 day), 1 day, or 2 days. We observed an increase in apoptosis in TRAIL pretreated cells after 100 J/m2 UVC exposure (Fig. 5A) and 500 J/m2 UVB exposure (Fig. 5B). These results also suggest that cells with acquired TRAIL resistance are sensitive to UVB as well as UVC.
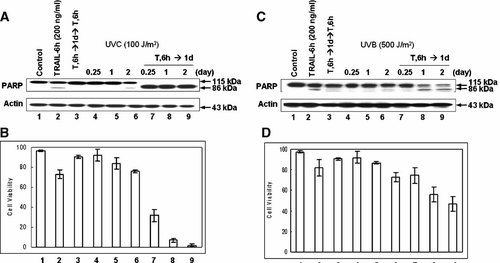
Effect of 100 J/m2 UVC and 500 J/m2 UVB on CX-1 cells with acquired TRAIL resistance. CX-1 cells were treated or not treated with TRAIL (200 ng/ml) for 6 h, and then detached cells were removed by washing with PBS. After removal of the detached cells, fresh media was added onto the remaining attached cells. Cells were incubated for 1 day and then exposed to 100 J/m2 of UVC or 500 J/m2 of UVB. After UV irradiation, cells were incubated for various times (0.25–2 days). A,C: Cell lysates were subjected to immunoblotting for PARP or actin. B,D: Cell survival was determined by trypan blue exclusion assay. Error bars represent the SE from three experiments.
Comparison of the Effect of UVC Irradiation on Colorectal Cancer Cells With Various Incubation Times of Acquired TRAIL Resistance
We examined how long human colorectal CX-1 cells with acquired TRAIL resistance are sensitive to UVC irradiation after TRAIL treatment. CX-1 cells were incubated for 0–5 days after TRAIL treatment and exposed to 500 J/m2 of UVC and then incubated for 1 and 2 days (Fig. 6). As in previous studies, acquired TRAIL resistance develops within 1 day and gradually decays within 5 days after TRAIL treatment. On the basis of this, both PARP cleavage and caspase activation appeared for only on day 1 through day 4 after TRAIL treatment. These results suggest that sensitivity to UV radiation is inversely related to acquired TRAIL resistance. Similar results were observed in cell survival and morphological feature analysis (Fig. 6B,C). We observed an increase in cell death by UVC irradiation within 4 days after TRAIL treatment (Fig. 6B). As shown in Panels e–g from Figure 6C, apoptotic cell death, which is associated with typical morphological features like cell shrinkage and cytoplasmic membrane blebbing, was observed when cells were exposed to UVC within 4 days after TRAIL treatment.
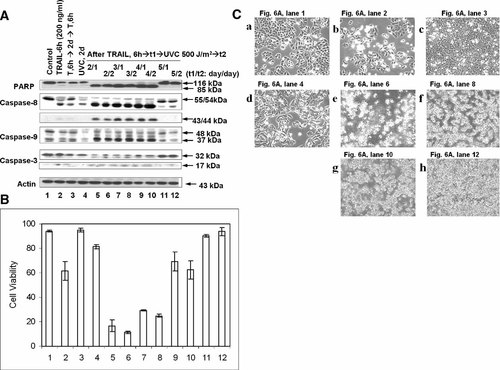
Effect of various pre- and post-incubation periods on UVC-induced apoptotic death in TRAIL-pretreated CX-1 cells. A: CX-1 cells were treated with TRAIL (200 ng/ml) for 6 h, and then detached cells were removed by washing with PBS. After removal of the detached cells, fresh media were added onto the remaining attached cells and incubated for various times (2–5 days: t1) and then exposed to 500 J/m2 of UVC. After UVC exposure, cells were incubated for 1 or 2 days (t2). Cell lysates were subjected to immunoblotting for PARP, caspase-8, caspase-9, caspase-3, or actin as described in Figure 1. Control, untreated cells. TRAIL-6 h (200 ng/ml), Cells were treated 200 ng/ml for 6 h. T, 6 h → 2d → T, 6 h, Cells were treated 200 ng/ml TRAIL for 6 h and incubated for 2 days before challenged to 200 ng/ml TRAIL for 6 h. UVC, 2d, Cells were exposed to UVC (500 J/m2) and incubated for 2 days. B: Survival was determined by trypan blue exclusion assay. Error bars represent the SE from three experiments. C: Morphological features of cells were analyzed with a phase-contrast inverted microscope.
Cytoplasmic Release of Cytochrome c Caused by UVC Irradiation on Colorectal Cancer Cells With Acquired TRAIL Resistance
We measured cytochrome c release to determine whether UVC irradiation-promoted TRAIL cytotoxicity is mediated through the mitochondria-dependent apoptotic pathway in acquired TRAIL resistant cells. CX-1 cells were treated or not treated with TRAIL (200 ng/ml) for 6 h, and then detached cells were removed by washing with PBS. After removal of the detached cells, fresh media was added onto the remaining attached cells and incubated for 1 day. After incubation, cells were exposed to 500 J/m2 of UVC and incubated for 3, 6, 12, or 24 h (Fig. 7). UVC-exposed CX-1 cells with acquired TRAIL resistance exhibited a significant release of cytochrome c in comparison to UVC-exposed CX-1 cells without acquired TRAIL resistance.
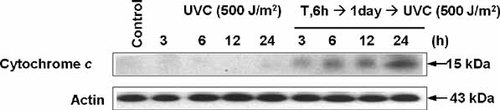
UVC-induced cytochrome c release in acquired TRAIL resistant CX-1 cells. Cells were treated or not treated with TRAIL (200 ng/ml) for 6 h, and then detached cells were removed by washing with PBS. After removal of the detached cells, fresh media were added onto the remaining attached cells and incubated for 1 day. After incubation, cells were exposed to 500 J/m2 of UVC and incubated for 3, 6, and 24 h. Cytosolic and mitochondrial fractions were separated. Proteins from cytosol aliquots (20 µg) were resolved by 15% SDS–PAGE and then immunoblotted using anti-cytochrome c antibody.
DISCUSSION
In this study, we observed that acquired TRAIL resistant cells are sensitive to UV irradiation. Interestingly, sensitivity to UV radiation is inversely related to acquired TRAIL resistance (Fig. 6A). Our studies demonstrate that UV irradiation promotes the mitochondria-dependent apoptotic pathway by releasing cytochrome c (Fig. 7) and subsequently leads to an increase in caspase activation (Figs. 4 and 4, 6).
UV radiation induces a number of protein kinase and transcription factors, including the three mitogen-activated protein kinase (MAPK) pathways. The MAPKs are serine–threonine kinases that transmit signaling cascades from extracellular stimuli into cells; examples of MAPKs include extracellular signal-regulated kinases (ERKs), c-jun N-terminal protein kinases (JNKs), and p38 MAP kinases. Like NF-κB, MAPKs participate in a wide variety of cellular processes, including immunoregulation, inflammation, cell growth, cell differentiation, and cell death. Responses downstream of MAPK activation vary according to the dose of radiation or cell type but in many cases, JNK and p38 MAPK signals lead to apoptosis while ERK pathways attenuate cell death. JNK and p38 MAPK contribute to apoptotic cell death through the mitochondria-dependent pathway [Kabuyama et al., 2001; Kyriakis and Avruch, 2001]. In addition, activated MAPKs induce the transcription of COX-2 and also phosphorylate and activate a number of transcription factors (c-Jun and ATF family) leading to increased transcriptional activity of AP-1 [Bachelor et al., 2002]. At this time only speculations can be made concerning the role of MAPK in the UV radiation-promoted apoptotic death in acquired TRAIL resistant cells. One possibility is that differential activation of MAPKs occurs in acquired TRAIL resistant cells after UV exposure. Decrease in ERK activation and increase in JNK and p38 MAPK may be responsible for facilitation of apoptotic death. Obviously, this possibility needs to be investigated in the near future.
Understanding how apoptosis can be triggered and how that process goes awry may contribute to a better understanding of why UV exposure preferentially stimulates apoptosis in acquired TRAIL resistant cells. Two proteins which become more active in response to UVA exposure are sphingomyelinase and ataxia telangiectasia mutated (ATM). The acid spingomyelinase (Smase) signaling pathway may activate JNKs to induce apoptosis [Zhang et al., 2001], perhaps through hydrolysis of ceramide. Ceramide, which is possibly increased by UV exposure [Okazaki et al., 1998], can act as a modulator of various stress-related responses which can stimulate phosphorylation and activation of JNKs, including cell cycle arrest, cell senescence, and apoptosis [Hannun, 1996]. ATM, the kinase responsible for ataxia telangiectasia, a genetic disorder associated with a high incidence of cancer [Barlow et al., 1996; Khanna, 2000], is a member of the PI3K family [Rotman and Shiloh, 1999] and is a serine threonine protein kinase with a COOH-terminal domain. ATM is activated by UVA exposure and is involved in the cellular decision to trigger p53- and JNK-dependent apoptosis [Zhang et al., 2002b]. Interestingly, UVC exposure appears to stimulate ATR, a kinase that is structurally and functionally similar to ATM, and likewise triggers p53- and JNK-dependent apoptosis. ATR functions as a sensor of DNA damage in response to UVC [Unsal-Kacmaz et al., 2002]. Others have also shown that ATM is activated in response to ionizing radiation [Banin et al., 1998; Canman et al., 1998]. Possibly, ATR is selectively activated in acquired TRAIL resistant cells after UVB/C exposure. Although many critical questions still remain to be answered in order to understand the mechanisms of UV radiation response in acquired TRAIL resistant cells, this discussion will help provide a starting point for future studies.




