Embryonic undifferentiated cells show scattering activity on a surface coated with immobilized E-cadherin
Abstract
Rearrangement of cell–cell adhesion is a critical event in embryonic development and tissue formation. We investigated the regulatory function of E-cadherin, a key adhesion protein, in the developmental process by using E-cadherin/IgG Fc fusion protein as an adhesion matrix in cell culture. F9 embryonal carcinoma cells usually form colonies when cultured on gelatin or fibronectin matrices. However, F9 cells cultured on the E-cadherin/IgG Fc fusion protein matrix formed a scattered distribution, with a different cytoskeletal organization and E-cadherin-rich protrusions that were regulated by Rac1 activity. The same scattering activity was observed in P19 embryonal carcinoma cells. In contrast, three types of differentiated cells, NMuMG mammary gland cells, MDCK kidney epithelial cells, and mouse primary isolated hepatocytes, did not show the scattering activity observed in F9 and P19 cells. These results suggest that migratory behavior on an E-cadherin-immobilized surface is only observed in embryonic cells, and that the regulatory mechanisms underlying E-cadherin-mediated cell adhesion vary with the state of differentiation. J. Cell. Biochem. 103: 296–310, 2008. © 2007 Wiley-Liss, Inc.
Cadherins are defined by the presence of five extracellular cadherin (EC) repeats that mediate calcium-dependent homophilic adhesion between cadherin molecules of the same type. More than 300 different cadherins have been reported [Nollet et al., 2000]. Tissue-specific expression of cadherin molecules is required for cell sorting and recognition during embryogenesis, and for preserving tissue architecture. E-Cadherin is one of the archetypal cadherin families essential for epithelialization of the early mouse embryo, cell rearrangement, tissue morphogenesis, establishment of cell polarity, and maintenance of tissue architecture [Takeichi, 1995; Gumbiner, 2000]. E-Cadherin-mediated adhesion is regulated by cytoplasmic proteins that are associated with the C-terminal domain of E-cadherin. β-Catenin and γ-catenin (plakoglobin) bind to the cytoplasmic domain of E-cadherin, and this molecular complex is linked to the actin cytoskeleton by α-catenin. p120ctn binds to the membrane-proximal region of E-cadherin, causing disruption of the homophilic interaction and loss of cell–cell contact [Lampugnani et al., 1997; Ohkubo and Ozawa, 1999; Thoreson et al., 2000]. This molecular complex regulates homophilic interaction and cis-dimerization of E-cadherin [Ozawa et al., 1990; Brieher et al., 1996].
Although many different cadherin molecules are expressed during developmental differentiation, E-cadherin is expressed not only by differentiated epithelial cells but also by the early embryo and by many stem cells [Larue et al., 1996]. The role of E-cadherin is not restricted to the maintenance of cell adhesion during embryogenesis. It is also critically related to compaction, cell rearrangement, and cell recognition for tissue organization during differentiation of stem cells. The E-cadherin-null mutant is embryonic lethal, because it lacks the ability to develop into a multicellular organism before implantation [Larue et al., 1994; Riethmacher et al., 1995]. Furthermore, the specificity of cadherin proteins plays a crucial role in cell sorting and cell rearrangement [Levine et al., 1994]. However, the functional regulation of E-cadherin in the developmental cell rearrangement process remains poorly understood, because of the complexity of cell–cell and cell–matrix interactions, and because of the expression of other cell–cell adhesion molecules during differentiation.
F9 cells are mouse teratocarcinoma-derived embryonal carcinoma cells that provide a useful model for the study of embryonic development [Hogan et al., 1983; Mohn et al., 2003]. Although F9 cells show very little spontaneous differentiation, they differentiate into two different extraembryonic endoderm-derived cells, visceral and parietal endoderm cells, when stimulated by retinoic acid [Strickland and Mahdavi, 1978; Strickland et al., 1980]. When F9 cells are cultured in bacteriological petri dishes to which they do not adhere, they form a compacted aggregate (similar to embryonal compaction), via E-cadherin-mediated cell–cell adhesion [Yoshida-Noro et al., 1984]. The aggregate differentiates into outer layers of visceral endoderm, surrounding a core of undifferentiated cells. Cell–cell adhesion plays a crucial role in this process [Grover et al., 1987]. These findings indicate that E-cadherin-mediated interactions and cell rearrangement processes are important during the differentiation of embryonic cells.
In this study, we used F9 cells as model embryonic cells, and cultured them on an E-cadherin-immobilized surface. Our aim was to determine the role of E-cadherin in regulating the rearrangement and sorting of embryonic cells.
MATERIALS AND METHODS
Expression of the Model Protein and Cell Culture
The expression and purification of immobilizable fusion protein (E-cad-Fc), which consists of the extracellular domain of mouse E-cadherin and the mouse IgG1 Fc domain, was as described previously [Nagaoka and Akaike, 2003]. To prepare the E-cad-Fc-coated surface, purified E-cad-Fc solution was directly added to untreated polystyrene plates. The plates were incubated for 2 h at 37°C, washed once with phosphate-buffered saline, and then seeded with cells. F9 mouse teratocarcinoma cells and NMuMG mouse mammary gland epithelial cells were obtained from the American Type Culture Collection. P19 cells and MDCK cells were kindly provided by the Cell Resource Center for Biomedical Research (Tohoku University) and the Riken Cell Bank (Tsukuba, Japan), respectively. Mouse primary hepatocytes were isolated from male ICR mice (7–9 weeks; SLC, Shizuoka, Japan) by the modified in situ collagenase perfusion method, as described previously [Ise et al., 2001]. F9, P19, MDCK, and NMuMG cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% (v/v) fetal bovine serum. Primary hepatocytes were maintained in Williams' E medium (Invitrogen), without serum. All media contained 50 µg/ml penicillin, 50 µg/ml streptomycin, and 100 µg/ml neomycin.
Adhesion and Detachment Assays
Cells were seeded at a density of 3.0 × 104 cells/well on 96-well plates coated with various matrices. After 4 h in culture, the culture media and nonadhered cells were removed and washed with phosphate-buffered saline containing 0.9 mM Ca2+ and 0.33 mM Mg2+. To count the living cell number, the adhered cells were stained with resazurin (alamarBlue; Biosource International, Inc.); absorbance at 570 nm was measured using a microplate reader. For detachment assays, cells were incubated with 5 mM EDTA, 5 mM EGTA and/or 5 mM MgCl2 for 30 min, and the remaining cells were then counted by the same method as mentioned above.
Quantitative Analysis of Cell Scattering
To quantify the cell scattering, the Hopkins–Skellam index (HSI) was used [Hopkins and Skellam, 1954; Numahara et al., 2001]. If cells are distributed independently and at random, then HSI = 1; if they are aggregated, then HSI > 1; if they are regularly distributed, then HSI < 1. Cells were cultured for 3 days and then stained with Hoechst 33342 solution (1.0 µg/ml) for 30 min. Fluorescence images were obtained using a fluorescence microscope and cell distribution was mapped by Image J software. The Hopkins–Skellam Index (HSI) and intercellular distance were determined using the Mathematica program [Numahara et al., 2001].
Plasmids and Transfection
For the colony formation assay, pEGFP-N2 and pDsRed2-N1 (Clontech) were transfected using LipofectAMINE Plus reagent (Invitrogen). Stable clones were selected by addition of 800 µg/ml G418 (Invitrogen). Hemagglutinin (HA)-tagged RhoA, Rac1, and Cdc42 variants (kindly provided by Prof. Kozo Kaibuchi of Nagoya University) [Fukata et al., 2002] were also transfected using LipofectAMINE Plus reagent. After 8 h in culture, cells were detached using trypsin-EDTA and reseeded to gelatin- or E-cadherin-coated dishes. For construction of the expression vector for siRNA against mouse E-cadherin, the oligonucleotide pair containing the target sequence of E-cadherin (5′-GTA CAT CCT CTA TTC TCA T-3′) was inserted into the siRNA expression vector pSilencer™ 3.1-H1 neo (Ambion, Inc.). After transfection of the siRNA-expressing vector, five stable clones were selected by addition of 1,000 µg/ml G418.
Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA was isolated with Trizol reagent (Invitrogen). First strand cDNA was synthesized with Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen). Reverse transcription-polymerase chain reaction (RT-PCR) was carried out with rTaq polymerase (TOYOBO). The primers used were Ecad-S (5′-CAG ACG ATG ACG TCA ACA CC-3′) and E-cad-AS (5′-AGG ATG TAT TGC TGC TTG GC-3′) for E-cadherin, Ncad-S (5′-ACG GTG TAT GCT GTG AGA AGC-3′) and Ncad-AS (5′-GAC CGT CAT CAC ATA CGT CC-3′) for N-cadherin, and GAPDH-S (5′-GGA AGC TTG TCA TCA ACG G-3′) and GAPDH-AS (5′-CTC TTG CTC AGT GTC CTT GC-3′) for GAPDH. PCR products were resolved by 1% agarose gel electrophoresis.
Western Blot Analysis
Total protein was extracted with lysis buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Nonidet P40, 1% Triton X-100, 1 mM CaCl2, and proteinase inhibitors). Samples were separated by electrophoresis on 7.5% polyacrylamide gels and electrophoretically transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore). Blots were probed with anti-E-cadherin antibody (BD Transduction Laboratories), anti-β-catenin antibody (BD Transduction Laboratories), anti-α-catenin antibody (BD Transduction Laboratories), anti-N-cadherin antibody (Santa Cruz Biotechnology) or anti-β-actin antibody (Sigma), followed by horseradish peroxidase-conjugated secondary antibodies, and developed by ECL reagent (GE-Healthcare Biosciences).
Immunofluorescence Staining
Cells were fixed with 8% formaldehyde solution (pH 7.0–7.5; Wako Pure Chemical) and permeabilized with 0.2% Triton X-100. F-actin was stained with Alexa Fluor 488-conjugated phalloidin (Molecular Probes). Endogenous E-cadherin was detected with monoclonal antibodies against mouse E-cadherin intercellular domain antibodies (BD Transduction Laboratories), followed by Alexa Fluor 488- or 546-conjugated secondary antibodies. HA-tagged Rho family variants were stained with anti-HA tag antibody (Medical & Biological Laboratories) followed by Alexa Fluor 546-conjugated secondary antibody. Nuclei were counterstained with DAPI (Sigma).
Immunoprecipitation Analysis of Conditioned Media
Conditioned media were collected and the immunoprecipitation was performed with anti-mouse E-cadherin antibody (DECMA-1; Sigma) for 2 h at 4°C. Immune complexes were collected with protein G-sepharose. Samples were separated by electrophoresis on 7.5% polyacrylamide gels and electrophoretically transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore). Blots were probed with anti-E-cadherin antibody (DECMA-1) followed by horseradish peroxidase-conjugated anti-rat IgG antibody or horseradish peroxidase-conjugated anti-mouse IgG antibody, and developed using ECL reagent (GE-Healthcare Biosciences).
RESULTS
Adhesion of F9 Cells to an E-Cadherin-Immobilized Surface
We previously reported that primary-cultured hepatocytes adhere to E-cad-Fc-coated surfaces in an E-cadherin-dependent manner while maintaining differentiated phenotypes [Nagaoka et al., 2002]. F9 cells adhere to an E-cad-Fc-coated surface in the same ratio as on collagen-coated plates, and in a dose-dependent manner (Fig. 1A). The Ca2+ ion is essential for E-cadherin-mediated cell adhesion, whereas the adhesion of the integrins to extracellular matrices (ECMs) instead requires Mg2+ and Mn2+ ions [Staatz et al., 1989; Leitinger et al., 2000]. To confirm the mechanism of adhesion to E-cad-Fc, we investigated the effect of divalent cations on adhesion of the cells to several matrices (Fig. 1B). The removal of divalent cations by EDTA caused the detachment of F9 cells both from collagen-coated and E-cad-Fc-coated surfaces, while EGTA, which is a Ca2+ ion-specific chelating agent, did not disrupt integrin-collagen adhesion. Furthermore, the supplementation of Mg2+ ions rescued the adhesion of cells to the collagen-coated surfaces but not to the E-cad-Fc surface. These results indicated that the adhesion to E-cad-Fc required Ca2+ ions and was not mediated by a Mg2+-dependent integrin interaction.
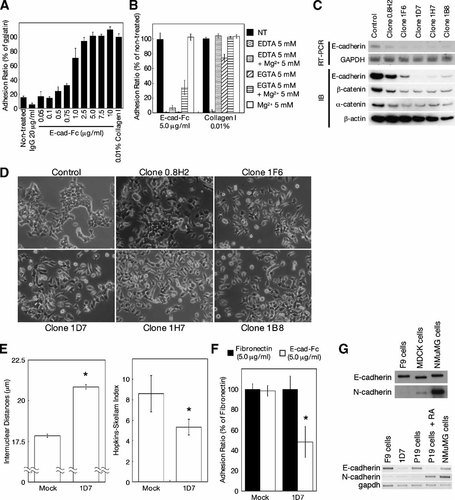
Adhesion of F9 cells to E-cad-Fc fusion protein. A: F9 teratocarcinoma cells adhered to E-cad-Fc-coated dishes as well as to collagen-coated surfaces, after 3 h of incubation. IgG was used as a negative control. The data indicate the mean ± SD of three experiments. B: Chelating reagents and/or Mg2+ ions (5 mM) were added to culture medium 3 h after seeding. Detached cells were removed and remaining cells were counted using resazurin. The data indicate the mean ± SD of three experiments. C: F9 cells were transfected with siRNA-expression vector against E-cadherin. The expression of E-cadherin and associated proteins was analyzed by RT-PCR (upper) or Western blotting (IB: lower). GAPDH and β-actin were used as internal controls. D: Morphological observation of five clones that continuously expressed E-cadherin-specific siRNA. Bar indicates 100 µm. E: Cell scattering was analyzed by measuring internuclear distance and HSI (Hopkins–Skellam Index). Cells were cultured for 3 days on gelatinized surfaces and analyzed as described in Materials and Methods. The data indicate the mean ± SEM of 24 different fields. *P < 0.01 versus mock cells. F: Adhesion to E-cad-Fc-coated surfaces was inhibited by expression of siRNA. Cells were seeded at a density of 3.0 × 104 cells/well on 96-well plate coated with 5.0 µg/ml fibronectin (closed bar), or 5.0 µg/ml E-cad-Fc (open bar) without serum. After 3 h incubation, the number of adherent cells was counted as described in Materials and Methods. The data indicate the mean ± SD of three experiments. *P < 0.01 versus fibronectin-coated dishes (closed bar). G: The expression of E-cadherin and N-cadherin was analyzed by Western blotting (upper) and RT-PCR analysis (lower).
We also examined the effect of E-cadherin-specific siRNA, to confirm the mechanism of adhesion of F9 cells onto the E-cad-Fc-coated surface. The expression vector for E-cadherin-specific siRNA was transfected to F9 cells and stably expressing clones were selected. We obtained five clones with different levels of E-cadherin expression, and confirmed that the inhibition of E-cadherin expression affected the levels of associated proteins (Fig. 1C). The reduction of E-cadherin expression disrupted cell–cell adhesion, which induced cell scattering and elongation of internuclear distances on gelatin-coated surfaces (Fig. 1D,E). Furthermore, HSI revealed that siRNA-treated cells proliferated randomly and did not form compacted colonies (Fig. 1E). The adhesion of siRNA-expressing cells onto E-cad-Fc-coated surfaces was inhibited (Fig. 1F).
Because it has been reported that a cadherin deficiency can be rescued by a cadherin of another type [Luo et al., 2001], and because N-cadherin can interact with E-cadherin in a heterophilic fashion [Niessen and Gumbiner, 2002; Prakasam et al., 2006], we analyzed the expression of N-cadherin (Fig. 1G). The expression level of N-cadherin was not high in F9 cells and was slightly enhanced by the E-cadherin-specific siRNA expression. In contrast, NMuMG cells and differentiated P19 cells expressed high levels of N-cadherin, indicating that N-cadherin might have a partial effect on the adhesion onto the E-cad-Fc-coated surface. MDCK cells expressed low levels of N-cadherin, which is same results as reported previously that indicates MDCK cells have a N-cadherin-rich subpopulation [Youn et al., 2005]. These results indicated that adhesion onto the E-cad-Fc-coated surface was mainly mediated by homophilic adhesion of E-cadherin.
Scattering Activity of F9 Cells Cultured on an E-cad-Fc-Coated Surface
We next observed the morphological changes of F9 cells cultured on an E-cad-Fc-coated surface. F9 cells formed tight colonies when they were cultured on ECM-coated surfaces such as gelatin, collagen, fibronectin, or vitronectin (Fig. 2A, and data not shown). Laminin I induced a different morphology from the other ECMs, but colony formation was still observed. In contrast, F9 cells showed spindle-like morphology and were scattered (without colony formation) on the E-cad-Fc-coated surfaces. Since F9 cells differentiate into two different endoderm derivatives by addition of all-trans-retinoic acid [Strickland and Mahdavi, 1978; Strickland et al., 1980], and parietal endoderm derivatives also exhibit scattering morphology, we checked the differentiation status of F9 cells cultured on E-cad-Fc. Expression of differentiation marker genes, however, could not be detected by RT-PCR analysis of cells cultured on E-cad-Fc-coated dishes (data not shown), suggesting that the cell scattering activity was independent of differentiation. The proliferative activity of F9 cells was the same on either gelatin or on E-cad-Fc (data not shown). These results suggested that F9 cells scattered on the E-cad-Fc-coated surface without any spontaneous differentiations or changes in cell characteristics. Time-lapse images indicating the motile activity of F9 cells on the E-cadherin model protein are shown in Figure 2B (see also Movies 1–2, Supplementary Materials). The HS index was much lower on the E-cad-Fc-coated surface than on the gelatinized surface, and the distance between neighboring cells was greater, indicating a random distribution and a higher scattering capability (Fig. 2C).
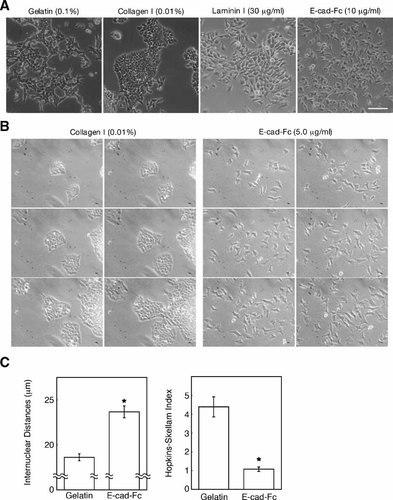
Morphological changes of F9 cells cultured on different matrices. A: Morphological characteristics were observed as phase contrast images 2 days after seeding F9 cells onto polystyrene surfaces coated with 0.1% (w/v) gelatin, 0.01% (w/v) collagen, 30 µg/ml laminin I, or 10 µg/ml E-cad-Fc. Scale bar indicates 100 µm. B: Time-lapse observation of F9 cells that were cultured on polystyrene surfaces coated with 0.01% collagen or 5.0 µg/ml E-cad-Fc. Images were recorded every 200 min. C: Cell scattering activity was analyzed by HSI and by measuring the internuclear distances. The data indicate the mean ± SEM of 28 different fields. *P < 0.01 versus gelatinized surface.
We next investigated the scattering activity of F9 cells by using a colony-formation assay. Stable F9 cell clones expressing GFP or DsRed2 were established, and then GFP-labeled or DsRed-labeled cells were mixed and cocultured on collagen-coated or E-cad-Fc-coated surfaces (Fig. 3A). If cell migration was activated, the two different colors would be blended. If cell migration was instead limited, colonies consisting of cells labeled with the same color would be formed. On collagen-coated plates, F9 cells formed tight colonies consisting of cells with the same fluorescent label. On E-cad-Fc surfaces, the differently labeled cells were mixed, and few numbers of colonies containing cells with the same fluorescent label were observed. This result also indicated the propensity of F9 cells to scatter on E-cad-Fc-coated surfaces.
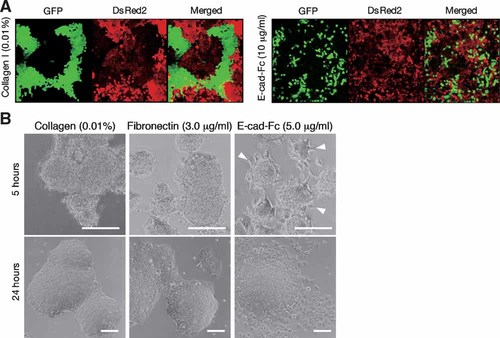
Cell scattering activity of E-cad-Fc. A: F9 cells were transfected with EGFP or DsRed2, then cocultured on polystyrene surfaces coated with 0.01% collagen or 10 µg/ml E-cad-Fc until confluent. Cell distribution was observed with a confocal laser microscope. B: Scattering capability from multilayer cell aggregates was also observed. Multilayer cell aggregates formed from a bovine serum albumin-coated dish were plated onto a polystyrene surface coated with 0.01% collagen type I, 3.0 µg/ml fibronectin, or 5.0 µg/ml E-cad-Fc. Morphological changes were monitored using a phase contrast microscope at 5 and 24 h. Bar indicates 100 µm.
When there is no suitable substrate for adhesion, F9 cells form compacted aggregates mediated by E-cadherin interactions [Yoshida-Noro et al., 1984]. To assess the effect of immobilized E-cad-Fc on the rearrangement of cell–cell adhesion, cell migration activity from compacted aggregates was examined on several matrices (Fig. 3B). Compacted cell aggregates, which were formed on surfaces coated with bovine serum albumin, were seeded onto collagen-, fibronectin-, or E-cad-Fc-coated surfaces. Although the cell aggregates adhered to surfaces coated with collagen or fibronectin, at 5 h after seeding there was no dispersion of aggregates or cell migration. On E-cad-Fc-coated surfaces, cell motile activity was markedly induced, and scattering of cells from the aggregates was observed within 5 h after seeding (Fig. 3B, arrowheads). After 24 h, the cell aggregates on E-cad-Fc were disrupted, while those on collagen or fibronectin were unaffected. This suggested that immobilized E-cad-Fc induced the rearrangement of cell–cell adhesion between F9 cells and promoted their migration.
Motility of F9 Cells was Induced by Immobilized E-cad-Fc
Previous studies have reported that 80-kDa fragments of the E-cadherin extracellular domain induce scattering of murine mammary tumor epithelial cells [Wheelock et al., 1987] and have inhibitory effects on cell compaction [Herrenknecht and Kemler, 1993]. The polypeptide containing His-Ala-Val (HAV) sequence which is included in the extracellular domain of E-cadherin has also been reported to inhibit the homophilic adhesion of E-cadherin [Vleminckx et al., 1991; Noë et al., 1999]. We examined the effect of 80-kDa fragments on the scattering activity of F9 cells. F9 cells were cultured on collagen-, gelatin-, or E-cad-Fc-coated dishes for 2 days, and conditioned media were collected. The 80-kDa fragments of E-cadherin in the conditioned media were immunoprecipitated with anti-E-cadherin extracellular domain antibody (DECMA-1). Large amounts of 80-kDa fragments and dissociated E-cad-Fc from the plates were obtained from conditioned media from E-cad-Fc-coated surfaces, but only small quantities of 80-kDa fragments were obtained from the conditioned media from collagen- or gelatin-coated dishes (Fig. 4A). To ascertain the effect of these fragments and soluble E-cad-Fc on cell scattering, E-cad-Fc or conditioned media from cells cultured on E-cad-Fc-coated surfaces were added to F9 cells cultured on collagen-coated dishes. Cell scattering was not induced in the presence of conditioned media containing 80-kDa E-cadherin fragments or high concentrations of soluble E-cad-Fc (Fig. 4B), suggesting that soluble E-cadherin fragments and detached E-cad-Fc did not initiate cell scattering on a gelatinized surface at the concentration used. While DECMA-1 and chelating reagents inhibit the homophilic interaction of E-cadherin molecules that cause the detachment of cell–cell adhesion and cell scattering, soluble E-cad-Fc had no effect on the stability of cell–cell adhesion (Fig. 4C,D). F9 cells, however, did not scatter on the surface coated with DECMA-1 (data not shown). Since the concentration of supplemental E-cad-Fc (10 µg/ml) was less than that reported previously [Vleminckx et al., 1991; Noë et al., 1999] and the local concentration would be higher on immobilized surface, these results suggested that the scattering activity of F9 cells was induced by immobilized E-cad-Fc, not by soluble E-cad-Fc or E-cadherin fragments in the culture media.
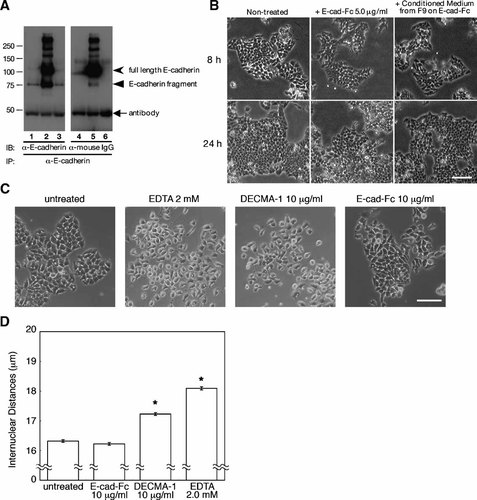
The effect of E-cadherin fragments on F9 cells. A: Immunoprecipitation and Western blot analysis of E-cadherin fragments in the conditioned media from F9 cells that were cultured on 0.01% collagen (lanes 1 and 4), 5.0 µg/ml E-cad-Fc (lanes 2 and 5), or 0.1% gelatin (lanes 3 and 6). Conditioned media from F9 cells that were cultured on three different substrates were immunoprecipitated with anti-E-cadherin antibody (DECMA-1). The 80-kDa E-cadherin fragments and detached E-cad-Fc were analyzed by Western blotting with anti-E-cadherin antibody (lanes 1–3) or anti-mouse IgG antibody (lanes 4–6). B: The effect of soluble E-cad-Fc on the scattering activity of F9 cells. Conditioned media from F9 cells cultured on E-cad-Fc for 2 days and soluble E-cad-Fc (final concentration 5.0 µg/ml) were added to F9 cells cultured on a collagen-coated surface. Cell scattering was monitored at 8 and 24 h by phase contrast microscopy. Bar indicates 100 µm. C: Cells were cultured on collagen-coated surfaces for 2 days and treated with EDTA (2.0 mM), DECMA-1 (5.0 µg/ml), or E-cad-Fc (10.0 µg/ml). Seven hours later, disruption of E-cadherin-mediated cell adhesion in the presence of reagents was observed by phase contrast microscopy. Bar: 100 µm. D: Disruption of intercellular adhesion was analyzed by measuring internuclear distances. The data indicate the mean ± SEM of four different fields. *P < 0.01 versus untreated cells.
Actin Reorganization During Cell Scattering
Reorganization of the actin cytoskeleton is important for cell movement, so we next examined the distribution of the actin cytoskeleton, along with E-cadherin. E-cadherin was localized at cell–cell contact sites on ECM-coated dishes. Polymerized F-actin also accumulated at the cell–cell contact sites (Fig. 5A, arrow). When F9 cells were cultured on E-cad-Fc-coated dishes, cell–cell adhesion was disrupted and the localization of E-cadherin at cell–cell contact sites was lost (Fig. 5A). Staining of F-actin in these cultures revealed intense localization at the peripheral cell membranes, and formation of actin microspikes extending from the cell periphery (Fig. 5B, arrowheads). Moreover, small cell protrusions intensely stained for E-cadherin were observed when cells were cultured on E-cad-Fc-coated dishes (Fig. 5A, arrowheads), and actin microspikes were also formed from these protrusions. Figure 5C shows that DECMA-1-treated F9 cells displayed the disruption of cell–cell contact, and the delocalization of actin filaments and E-cadherin. In contrast, soluble E-cad-Fc had no effect on the localization of actin or E-cadherin. These results indicate that the cell motility induced by the E-cadherin-model protein was regulated by the protrusion of parts of the cell that were rich in E-cadherin and by rearrangement of the actin cytoskeleton.
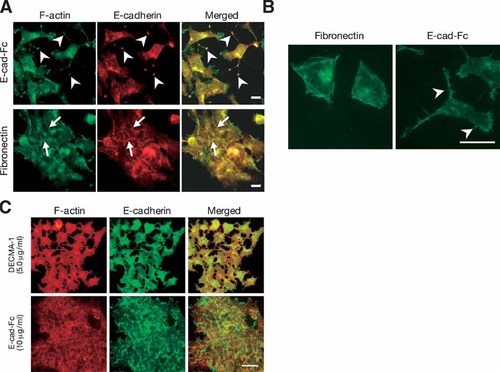
Distribution of polymerized actin, E-cadherin in F9 cells. A, B: F9 cells were plated onto 3.0 µg/ml fibronectin-coated or 5.0 µg/ml E-cad-Fc-coated surfaces and fixed with formaldehyde after 6 h (A) or 1 day (B), respectively. Polymerized actin filament was visualized with Alexa Fluor 488-conjugated phalloidin, and E-cadherin was stained with Alexa Fluor 546-labeled antibody. Their distributions were observed by fluorescence microscopy. Bar indicates 25 µm. C: Fluorescence microcopy images showing disruption of cell adhesion and changes in distribution of polymerized actin and E-cadherin in F9 cells. F9 cells were cultured on collagen-coated dishes and treated with DECMA-1 or E-cad-Fc. Six hours after treatment, the cells were fixed and stained with Alexa Fluor 488-conjugated phalloidin and anti-E-cadherin antibody (DECMA-1) followed by Alexa Fluor 546-labeled antibody. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Scattering Activity was Specific to Undifferentiated Embryonic Cells
We previously reported that fully differentiated primary hepatocytes form aggregates when cultured on E-cad-Fc-coated dishes [Nagaoka et al., 2002], in contrast to the scattering activity of the undifferentiated F9 cells. To clarify whether these differences in cell behavior are related to their differentiation status, we examined the responses of other differentiated and embryonic cell lines to an E-cad-Fc-coated surface. The NMuMG mouse mammary gland cell line and the MDCK kidney epithelial cell line were used as examples of differentiated cells. They adhered to an E-cad-Fc-coated surface by a E-cadherin-mediated interaction (data not shown) and formed aggregated colonies (Fig. 6A,B). Analysis of HSI and internuclear distances indicated that the MDCK cells formed compacted cell–cell interactions rather than scattering on the E-cad-Fc-coated surface (Fig. 6C). Rearrangement of the actin cytoskeleton also differed between differentiated cells and embryonic cells. F9 cells displayed E-cadherin-rich pseudopodial extrusions from the cell periphery containing many actin microspikes (Fig. 5B), while the differentiated NMuMG cells, MDCK cells and primary hepatocytes displayed branched lamellipodial extrusions (Fig. 6D,E). Similar pseudopodial formations were reported by Kovacs et al. [2002a,b] when Chinese hamster ovary (CHO) cells were used. P19 embryonal carcinoma cells, used as another example of an embryonic cell line, also showed scattering activity on an E-cad-Fc-coated surface (Fig. 6F). Furthermore, we reported that undifferentiated embryonic stem cells demonstrated the scattering activity on an E-cad-Fc surface [Nagaoka et al., 2006]. Thus, among these cell types, only the undifferentiated embryonic cells displayed scattering activity, and their actin rearrangements were different from those of differentiated cells. These findings suggest that the responses to E-cadherin-based homophilic interactions are different between embryonic cells and differentiated cells.
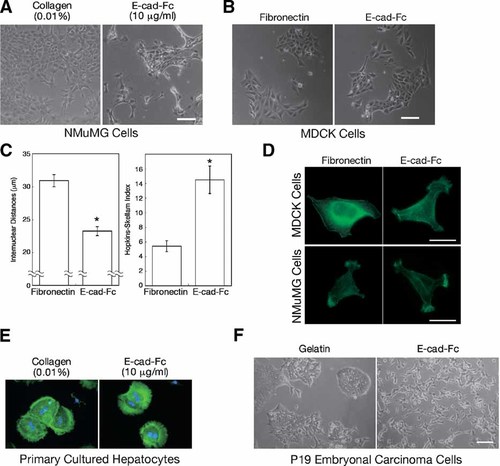
Responses of differentiated or undifferentiated cells to an E-cadherin-immobilized surface. A: Morphological characteristics of NMuMG mammary epithelial cells cultured on polystyrene surfaces coated with 0.01% collagen or 30 µg/ml α-mouse IgG Fcγ antibody followed by 10 µg/ml E-cad-Fc were observed as phase contrast images at 4 h and 2 days after seeding. Scale bar indicates 100 µm. B: MDCK cells were seeded on fibronectin-coated or E-cad-Fc-coated surfaces. Morphological characteristics were observed 2 days later. C: Cell scattering activity of MDCK cells was analyzed by HSI and by measuring internuclear distances. The data indicate the mean ± SEM of 28 different fields. *P < 0.01 versus fibronectin-coated surface. D,E: Reorganization of actin cytoskeleton. NMuMG cells and MDCK cells were cultured for 1 day on 5.0 µg/ml fibronectin or 5.0 µg/ml E-cad-Fc (D). Primary hepatocytes were also cultured on 0.01% collagen- or 5.0 µg/ml E-cad-Fc-coated plates for 4 h (E). Cells were fixed by formaldehyde and polymerized actin was stained with Alexa Fluor 488-conjugated phalloidin. Nuclei were counterstained with DAPI. Bar indicates 25 µm. F: P19 embryonal carcinoma cells were cultured on gelatin-coated or E-cad-Fc-coated dishes and morphological characteristics were observed as phase contrast images, 2 days after seeding. Scale bar indicates 100 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Rac1 is a Candidate Regulatory Factor for Cell Movement on an E-Cadherin Surface
The Rho family small G proteins regulate actin cytoskeletal rearrangement and epithelial morphogenesis [Hall, 1998; Fukata and Kaibuchi, 2001; Van Aelst and Symons, 2002]. Previous reports have suggested that filopodial protrusion is required for the interactions of E-cadherin [Vasioukhin et al., 2000], and that homophilic interactions of E-cadherin activate Rac1 and Cdc42, leading to the formation of tight adhesive complexes [Kim et al., 2000; Nakagawa et al., 2001]. On E-cad-Fc-coated surfaces, F9 cells displayed spindled morphology and E-cadherin-rich protrusions. We examined the association between the activation of Rho family small G proteins and cell scattering activity.
F9 cells were transfected with HA-tagged Rho family small G protein variants (RhoA, Rac1, and Cdc42) and seeded onto E-cad-Fc-coated or gelatin-coated dishes. Reconstruction of actin filaments and the distribution of transfected proteins were then observed (Fig. 7A,B). RhoA and Cdc42 had no effect on the cell scattering or the reorganization of actin filaments, as assessed by the expression of constitutively active mutants (RhoAV14 and Cdc42V12) or dominant-negative mutants (RhoN19 and Cdc42N17) (Fig. 7A and data not shown). However, F9 cells transfected with wild-type or constitutively active Rac1 (Rac1V12) and cultured on an E-cad-Fc-coated surface showed many branched neurite-like filamentous protrusions and spotted cell fragments. These contained the transfected Rac1 variants. Dominant-negative Rac1 (Rac1N17) induced fewer filamentous Rac1-positive cell fragments, indicating that the activated Rac1 contributed to the formation of the neurite-like filaments and pseudopodial protrusions. Formation of Rac1-rich filaments was also observed when F9 cells were cultured on gelatin-coated surfaces, and spotted cell fragments were observed around cell–cell contact sites (Fig. 7B, arrowheads). Differentiated NMuMG cells and MDCK cells did not exhibit such filamentous protrusions even when the constitutively active Rac1 (Rac1V12) was transfected (Fig. 7C). These results suggest that Rac1 is a candidate factor for the regulation of F9 cell scattering, and that the effect of Rac1 on the scattering activity was restricted to embryonic cells.
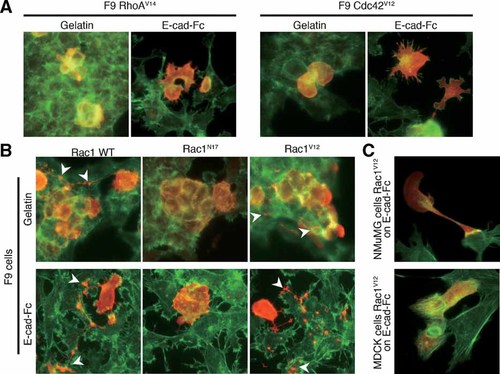
Contribution of Rho family small G proteins to scattering activity of F9 cells. A: F9 cells were transfected with HA-tagged constitutively active mutants of Rho family small G proteins (RhoAV14, Cdc42V12). Eight hours after transfection, cells were detached and plated onto E-cad-Fc-coated or gelatin-coated dishes, and then stained with anti-HA antibody (red), and phalloidin (green). B: F9 cells were transfected with HA-tagged wild-type (WT), dominant negative (Rac1N17), or constitutively active (Rac1V12) mutants of Rac1. Eight hours after transfection, the cells were detached and plated onto E-cad-Fc-coated or gelatin-coated dishes, and then stained with anti-HA antibody (red), and phalloidin (green). C: NMuMG cells and MDCK cells were transfected with constitutively active Rac1 mutant (Rac1V12). Eight hours after transfection, the cells were detached and plated onto E-cad-Fc-coated dishes, and then stained with anti-HA antibody (red) and phalloidin (green). Differentiated cells did not show the pseudopodial protrusions observed in F9 cells. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The adhesion molecule E-cadherin plays keys roles in compaction and cell rearrangement during embryogenesis. Although the expression of other cadherin family molecules increases during cell differentiation and their expression patterns are tissue-specific, E-cadherin is not restricted to differentiated epithelial cells, being also present in embryo-derived stem cells. Thus, an understanding of E-cadherin-mediated interactions is crucial for analysis of developmental morphogenesis, including the processes of compaction, migration and cell differentiation. In differentiated cells, cadherin is a component of the cell–cell junctional complex, and it plays an important role in the maintenance of tissues and in the specific functions of these cells. In this study, we focused on the functional regulation of E-cadherin in embryonic undifferentiated cells by using embryonal carcinoma cells as a model of early embryonic cells. When cultured on E-cadherin-coated surfaces, they displayed different responses from epithelial cells, including high motile activity, formation of E-cadherin-rich protrusions and actin microspikes, and also pseudopodial formations induced by activated Rac1. We recently reported that undifferentiated embryonic stem cells also display scattering activity as F9 cells and P19 cells on an E-cad-Fc surface, whereas differentiated embryonic stem cells form colonies like NMuMG cells and MDCK cells [Nagaoka et al., 2006]. These results indicate the possibility of differences between differentiated cells and embryonic undifferentiated cells in the regulatory mechanisms of E-cadherin-mediated cell–cell adhesion.
During embryogenesis, cell adhesions must be reassembled for the sorting of cells into appropriate positions, a process in which cadherin plays a crucial part [Nose et al., 1988; Steinberg and Takeichi, 1994; Uemura et al., 1996; Niewiadomska et al., 1999; Babb and Marrs, 2004]. Stem and progenitor cells are also sorted into appropriate places by continuous rearrangements of E-cadherin-mediated interactions during their differentiation. Cell sorting and cell rearrangement processes have also been observed during the differentiation of F9 cells into endodermal lineages from embryoid body-like cell aggregates [Grabel and Watts, 1987]. However, to our knowledge, there have been no previous demonstrations of motile activity via the rearrangement of E-cadherin-mediated cell–cell adhesion in these embryonic cells. In this study, the movement of embryonic cells induced by rearrangement of cell–cell adhesion on an E-cadherin-model surface was assessed by cell scattering behavior. Our results indicated that the motile activity of embryonic cells was induced by continuous rearrangement of E-cadherin-mediated homophilic interactions (see Fig. 2 and Movies 1–2), and that the regulatory system for cell–cell adhesion differed according to differentiation status (Figs. 6 and 7). These observations should contribute to an understanding of the role of E-cadherin in cell sorting and cell rearrangement processes.
Recently, many reports have indicated that the small G proteins, Rac1 and Cdc42, regulate E-cadherin-mediated cell–cell interactions. In the cell adhesion process, Cdc42 and Rac1 regulate the reorganization of actin cytoskeleton. Cytoskeletal reorganization plays a key role in the maintenance of cell–cell adhesion and also cell-matrix adhesion, resulting in establishment of cell polarity. Kovacs et al. [2002a,b] showed that CHO cells expressing high levels of E-cadherin adhered to an E-cadherin-coated surface and displayed branched lamellipodial extrusions. The same response was shown by NMuMG cells, primary cultured hepatocytes and MDCK cells in this study (Fig. 6). Again, this suggests that Rac1 plays an important role in E-cadherin-mediated cell–cell adhesion and in cytoskeletal reorganization of differentiated cells, but not in cell mobility or the rearrangement of homophilic interactions. Ehrlich et al. [2002] analyzed the dynamics of Rac1 localization and E-cadherin-mediated cell–cell adhesion in MDCK cells. They showed that active Rac1 accelerated E-cadherin-mediated cell–cell adhesion (rather than stabilizing adhesion) and that it enhanced lamellipodia formation. In a study of Drosophila development, inactivation of Rac1 inhibited dynamic cell rearrangement, even though activated Rac1 alternatively caused loss of cell adhesion [Chihara et al., 2003]. These reports suggested that Rac1 is a key molecule in the regulation of E-cadherin-mediated cell rearrangement and tissue formation in embryogenesis. We found that activated Rac1 enhanced the protrusion of pseudopodia and increased the number of cell fragments containing activated Rac1 (Fig. 7). These findings also suggested that Rac1 may regulate the E-cadherin-mediated cell rearrangement and motile activity of embryonic cells on an E-cadherin-coated surface. Movies 1 and 2 (Supplementary Materials) show that F9 cells scatter by making repeated telescopic motions of E-cadherin-rich pseudopodia, and that the tips of pseudopodia were severed and incorporated into other cells. Vasioukhin et al. [2000] reported that filopodial protrusion was observed in the initial stages of E-cadherin-mediated adhesion. The E-cadherin-rich fragments and filopodia-like protrusions observed in Figures 2 and 5 are consistent with their findings. Furthermore, the incorporation of E-cadherin by endocytosis [Le et al., 1999; Palacios et al., 2002] is regulated by Rac1 [Akhtar and Hotchin, 2001], and claudins (a family of intercellular adhesion molecules) are incorporated into adjacent cells by endocytosis [Matsuda et al., 2003]. Thus, from our results we propose that E-cadherin-rich cell fragments could have been incorporated by Rac1-mediated endocytosis, resulting in the carriage of several signal proteins or low molecular weight compounds into neighboring cells via endocytosis of these small cell fragments.
In this study, we found that the regulatory system of E-cadherin-mediated cell–cell adhesion varied with the state of differentiation, and that the motile activity of embryonic cells on E-cad-Fc-coated surfaces was induced by the rearrangement of E-cadherin-mediated cell adhesion. Our findings support an important role for Rac1 in the induction of motility in these cells, but the mechanisms regulating the scattering activity remain unknown. One hypothesis is that cell–cell adhesion strength and the amount of cell–cell adhesion vary with the state of differentiation. Differentiated cells possess stable cell–cell adhesion complexes, such as tight junctions, and these would influence the scattering behavior. The use of E-cad-Fc-coated surfaces should continue to provide a good model for investigating the regulatory mechanisms underlying E-cadherin-mediated cell–cell adhesion and cell motile behavior.
Acknowledgements
We are grateful to Prof. Kozo Kaibuchi and Dr. Masato Nakagawa of Nagoya University for their generous gift of expression vectors for wild-type and mutated Rho family small G proteins. We thank Dr. Maria Carmelita Kasuya of University of Tokyo for critical reading of the manuscript. We also thank Mr. Tomofumi Tanaka of Daiichi Asubio Pharma Co., Ltd. for excellent technical assistance. This work was supported by a Grant of the 21st Century COE Program and a Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.




