Increased expression and altered subunit composition of proteasomes induced by continuous proteasome inhibition establish apoptosis resistance and hyperproliferation of Burkitt lymphoma cells
Abstract
The proteasome is the main protease for extralysosomal protein degradation in eukaryotic cells, and constitutes a sophisticated high molecular mass proteinase complex underlying a tightly coordinated expression and assembly of multiple subunits and subcomplexes. Here we show that continuous inhibition of proteasomal chymotrypsin-like peptidase activity by the proteasome inhibitor bortezomib induces in human Namalwa Burkitt lymphoma cells increased de novo biogenesis of proteasomes accompanied by increased expression of the proteasome maturation protein POMP, increased expression of 19S-20S-19S proteasomes, and abrogation of expression of β1i, β2i and β5i immunosubunits and PA28 in favor of increased expression of constitutive proteolytic β1, β2 and β5 subunits and 19S regulatory complexes. These alterations of proteasome expression and subunit composition are accompanied by an increase in proteasomal caspase-like, trypsin-like and chymotrypsin-like peptidase activities, not inhibitable by high doses of bortezomib. Cells harboring these proteasomal alterations display rapid proliferation and cell cycle progression, and acquire resistance to apoptosis induced by proteasome inhibitors, γ-irradiation and staurosporine. This acquired apoptosis resistance is accompanied by de novo expression of anti-apoptotic Hsp27 protein and the loss of ability to accumulate and stabilize pro-apoptotic p53 protein. Thus, increased expression, altered subunit composition and increased activity of proteasomes constitute a hitherto unknown adaptive and autoregulatory feedback mechanism to allow cells to survive the lethal challenge of proteasome inhibition and to establish a hyperproliferative and apoptosis-resistant phenotype. J. Cell. Biochem. 103: 270–283, 2008. © 2007 Wiley-Liss, Inc.
The highly conserved ubiquitin-proteasome pathway is the principal system for nuclear and extralysosomal cytosolic protein degradation in eukaryotic cells [Glickman and Ciechanover, 2002; Ciechanover, 2006]. The central proteolytic machinery of this system constitutes the 26S proteasome, a large multicatalytic multisubunit protease complex that degrades and processes essential cell proteins by limited and controlled proteolysis, thereby governing basic cellular processes [Baumeister et al., 1998; Voges et al., 1999; Glickman and Ciechanover, 2002; Naujokat and Hoffmann, 2002].
The proteolytic activities of the 26S proteasome occur in a barrel-shaped 20S catalytic core complex composed of an axially stacked formation of two outer seven-membered α rings and two inner seven-membered β rings, giving the 20S complex the general stoichiometry of α1–7β1–7β1–7α1–7 [Baumeister et al., 1998; Voges et al., 1999]. Only β1, β2, and β5 subunits are proteolytically active, harbor proteolytic sites formed by N-terminal threonine residues that face the central cavity of the 20S complex, and possess caspase-like, trypsin-like, and chymotrypsin-like peptidase activity, respectively [Dick et al., 1998; Kisselev et al., 1999].
During de novo biogenesis and assembly of 20S complexes, the constitutively expressed β1, β2, and β5 subunits can be replaced by IFN-γ-inducible homologous counterparts, the so-called immunosubunits β1i (LMP2), β2i (MECL1), and β5i (LMP7), leading to altered proteasomal cleavage site preference and increased proteasomal production of antigenic peptides for MHC class I presentation [Kloetzel, 2001; Krüger et al., 2003]. However, 20S complexes are incapable of degrading ubiquitin-conjugated and folded substrate proteins and require for this task 19S or 11S regulatory complexes capped at both ends of the 20S complex, leading to the assembly of 26S proteasome holoparticles [Baumeister et al., 1998; Voges et al., 1999]. 19S complexes exhibit sophisticated multisubunit assemblies required for recognition, deubiquitination, unfolding and translocation of substrate proteins destined to be proteolytically degraded in the 20S complex [Glickman et al., 1998]. The 11S complex, also termed proteasome activator PA28, is an IFN-γ-inducible ring-shaped heptameric assembly that enhances proteasomal production of antigenic peptides for MHC class I presentation, independently of the presence of immunosubunits in the 20S complex [Schwarz et al., 2000; Krüger et al., 2003].
Mammalian cells usually harbor a heterogeneous population of 20S complexes, which contain either the constitutive β1, β2, and β5 subunits or the IFN-γ-inducible β1i, β2i, and β5i immunosubunits, or a subunit composition intermediate between constitutive and immuno 20S complexes. Such 20S compexes can be further divided into subtypes that differ in their enzymatic properties and tissue distribution [Dahlmann et al., 2000]. Similar to the diversity of 20S complexes, 26S proteasomes exhibit three major species with different regulatory complex assemblies and enzymatic characteristics: 19S-20S-19S, PA28-20S-PA28, and 19S-20S-PA28 [Tanahashi et al., 2000; Shibatani et al., 2006]. However, except for the IFN-γ-induced expression of β1i, β2i, and β5i immunosubunits and PA28, mechanisms regulating subunit composition, proteolytic activity and cellular content of proteasomes under steady-state or stress conditions are largely unclear.
Studies in EL-4 T cell lymphoma cells revealed that continuous inhibition of proteasomal proteolysis results in the selection of cells which lack proteolytically active proteasomes, down-regulate proteasome expression, and express a giant protease identified as tripeptidyl peptidase II (TPP II) [Glas et al., 1998; Geier et al., 1999; Wang et al., 2000]. In such cells, TPP II can substitute for certain proteasome functions as demonstrated by TPP II-mediated proteolysis of proteasome-specific substrates [Glas et al., 1998; Geier et al., 1999; Wang et al., 2000]. Moreover, proliferation of such cells is inhibited in the presence of a TPP II inhibitor, demonstrating a compensatory role for TPP II in promoting proliferation and cell cycle progression when proteasome function is continuously inhibited [Glas et al., 1998].
We now provide evidence for a different compensatory mechanism in human Namalwa Burkitt lymphoma cells adapted to the proteasome inhibitor bortezomib which has recently entered clinical trials for the treatment of lymphomas and solid tumors [Rajkumar et al., 2005; Richardson et al., 2006]. These adapted cells exhibit abundant de novo biogenesis and expression of proteasomes with altered subunit and subcomplex composition, leading to increased proteasome activity and the establishment of a hyperproliferative and apoptosis-resistant phenotype.
MATERIALS AND METHODS
Antibodies and Reagents
Recombinant human (rh) Interferon-γ (IFN-γ) was purchased from AL-Immunotools (Friesoythe, Germany). The fluorogenic oligopeptidyl proteasome substrates Z-GGL-amc, Boc-LRR-amc and Boc-LLE-amc were purchased from Biomol (Hamburg, Germany). Anti-β-actin antibody (mouse monoclonal, AC-15) was purchased from Sigma (Taufkirchen, Germany), anti-POMP (rabbit polyclonal), anti-β1 (mouse monoclonal), anti-β1i (LMP2) (rabbit polyclonal), anti-β2 (mouse monoclonal), anti-β2i (MECL-1) (rabbit polyclonal), anti-β5 (rabbit polyclonal), anti-β5i (LMP7) (rabbit polyclonal), anti-α-subunits (mouse monoclonal), anti-α3 (mouse monoclonal), anti-β4 (rabbit polyclonal), anti-β6 (rabbit polyclonal), anti-α/β subunits (rabbit polyclonal), anti-Rpt5 (mouse monoclonal), anti-Rpn2 (mouse monoclonal), anti-PA28β (rabbit polyclonal) and anti-polyubiquitinated proteins (mouse monoclonal, FK2) were purchased from Biomol, anti-Hsp27 (mouse monoclonal, F-4), anti-c-myc (rabbit polyclonal, N-262) and anti-p53 (mouse monoclonal, DO-1) were purchased from Santa Cruz (Heidelberg, Germany), and anti-TPPII (chicken polyclonal, WL-26) was purchased from Immunsystem AB (Uppsala, Sweden). The proteasome inhibitor bortezomib (PS-341, Velcade®) was purchased from Millenium Pharmaceuticals (Cambridge, USA). Lactacystin, protein C kinase inhibitor staurosporine and TPP II inhibitor AAF-cmk were purchased from Biomol. Inhibitors were dissolved in DMSO and stored in stock solutions at −20°C.
Cell Lines
Namalwa cells and multidrug resistant MES-SA/Dx5 human uterine sarcoma cells (ATCC, Rockville, USA) were maintained in culture medium (CM) consisting of RPMI1640 (Gibco-Invitrogen, Karlsruhe, Germany), 2 mM L-glutamine (Sigma), 100 IU penicillin and 100 µg/ml streptomycin (Gibco-Invitrogen) and supplemented with 10% fetal calf serum (FCS, Gibco-Invitrogen). Namalwa cells adapted to bortezomib were maintained continuously in CM supplemented with 12.5 nM bortezomib.
Establishment of Bortezomib-Adapted Cells
Bortezomib-adapted Namalwa cells were generated by continuous exposure of Namalwa cells to 12.5 nM bortezomib that corresponds to the plasma concentration measured in patients after intravenous application of therapeutically effective doses of bortezomib [Papandreou et al., 2004]. CM containing 12.5 nM bortezomib was exchanged every 24 h. Most of the cells treated in this manner underwent apoptosis, but became resistant to apoptosis induced by bortezomib and started to grow again after 3–4 weeks of continuous treatment with 12.5 nM bortezomib. These viable and growing cells adapted to bortezomib were termed Namalwaad cells.
Measurement of Proteasome Activity in Permeabilized Cells
The inhibitory profiles of inhibitors toward proteasomal peptidase activities in permeabilized Namalwa cells were measured as described previously [Princiotta et al., 2001] with minor changes. Briefly, cells (4.8 × 105/ml) were incubated for 90 min at 37°C in culture medium containing different inhibitor concentrations or DMSO as a control. Cells were washed twice in phosphate buffered saline (PBS) and then resuspended in 50 mM Tris (pH 7.4) containing 5 mM MgCl2, 0.035% SDS and 0.2 mg/ml digitonin. Cells were transferred into black 96-well flat-bottom plates at a final concentration of 2 × 104 cells in 80 µl in each well. Thereafter, 20 µl of fluorogenic substrate was added to each well. After incubation for 3 h at 37°C, fluorescence was measured at excitation of 380 nm wavelength and emission of 460 nm wavelength using a SpectrafluorPlus 96-well plate reader equipped with the Magellan software (Tecan, Crailsheim, Germany). Values determined in cells incubated with proteasome inhibitors were evaluated against those determined in cells incubated with DMSO, which were defined as 100% of proteasomal peptidase activities.
Western Blot Analysis
Cells were pelleted and lysed in radio immunoprecipitation assay (RIPA) buffer in the presence of a protease inhibitor mixture (Roche, Penzberg, Germany). Protein content was quantified using the protein assay kit from Sigma–Aldrich (Taufkirchen, Germany). Samples with equivalent amounts of total protein were boiled in SDS-PAGE sample buffer, separated on 12% Tris–HCl gels from BioRad (Munich, Germany), and transferred onto nitrocellulose membranes (Amersham Life Science, Braunschweig, Germany). For analysis of mono- and polyubiquitinated proteins, samples were separated on 7.5% Tris–HCl gels. Equal protein loading was verified by staining with Ponceau S (Sigma–Aldrich). After blocking the membrane with 3% (w/v) milk-PBS for 2 h, the membrane was incubated for 1 h with the respective specific antibody followed by incubation with horseradish peroxidase-conjugated (HRP) anti-rabbit, anti-goat, anti-chicken or anti-mouse IgG (Santa Cruz). Equal protein loading was confirmed by blotting β-actin. As a loading control of 20S proteasome abundance, blots of whole 20S particles were performed. Signals of targeted proteins were detected by the Super Signal West Pico Chemiluminescence reagent (Pierce, Bonn, Germany) and recorded on ECL Hyperfilm (Amersham Life Science) in the linear detectable range.
Proliferation Assay
Proliferation of the cells was determined by [3H]thymidine incorporation for 24 h according to standard methods. Cells were washed and resuspended at a concentration of 2.5 × 105 cells/ml in CM containing different concentrations of bortezomib, lactacystin, AAF-cmk or 5 µl/ml DMSO. Cells were then cultured for 24 h in 96-well plates after the addition of 20 µl [3H]thymidine per well, yielding a specific activity of 5 Ci/mmol. Thereafter, cells were harvested without a precipitation step onto glass fiber filters (Dunn, Asbach, Germany) using an automated cell harvester (Inotech, Nabburg, Germany). Incorporation of [3H]thymidine was quantified using a scintillation β-counter (Inotech).
Irradiation of Cells
Cells were γ-irradiated with a dose of 30 Gy using an irradiator containing a 137Cs source (OB 29/4, STS Steuerungstechnik und Strahlenschutz GmbH, Braunschweig, Germany). Cells were then incubated for 24 or 48 h at 37°C in CM. Apoptotic cells were subsequently determined by flow cytometry as described below.
Detection of Apoptotic Cells
Cells were incubated for 24 or 48 h at 37°C in CM containing different concentrations of bortezomib, lactacystin, AAF-cmk, 2 µM staurosporine or 5 µl/ml DMSO as a control. Staining of apoptotic cells was performed using the annexin V-fluorescein isothiocyanate (FITC)/PI apoptosis assay kit (BD Pharmingen, Heidelberg, Germany) according to the manufacture's instructions. Determination of apoptotic cells was performed by flow cytometry using a FACScan® flow cytometer and the Cellquest® software (Becton Dickinson, Heidelberg, Germany).
Calculation of Specific Apoptosis
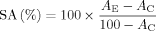
Cell Cycle Analysis
Cells cycle analysis was performed as described previously [Nicoletti et al., 1991; Naujokat et al., 2003] with minor changes. Cells were washed once with PBS, pelleted by centrifugation and resuspended in PBS containing 50 µg/ml propidium iodide, 0.1% Triton X-100, and 0.1% sodium citrate. Samples were stored at 4°C for 30 min and gently vortexed before analysis by flow cytometry using a FACScan® flow cytometer and the Cellquest® software (Becton Dickinson).
Flow Cytometry
Flow cytometric analysis of P-glycoprotein expression in non-adapted cells, Namalwaad cells and multidrug resistant MES-SA/Dx5 uterine sarcoma cells was performed with standard staining and analysis procedures using a FITC-conjugated monoclonal mouse IgG2b antibody raised against P-glycoprotein (BD Pharmingen, Heidelberg, Germany) and a FACScan® flow cytometer equipped with the Cellquest® software (Becton Dickinson).
Rhodamine 123 Efflux Assay
Cells (5 × 105/ml) were incubated with 10 µM rhodamine 123 (Sigma) for 3 h. Thereafter, cells were washed in PBS, and the fluorescence intensity of intracellular rhodamine123 was detected by flow cytometry.
RESULTS
Namalwa Cells Adapted to Bortezomib Exhibit Increased Proteasomal Proteolytic Activities
Exposure of human Namalwa Burkitt lymphoma cells to 12.5 nM bortezomib that corresponds to plasma concentrations measured in patients after intravenous application of therapeutically effective doses of bortezomib [Papandreou et al., 2004] resulted in induction of apoptosis and complete growth inhibition (data not shown). After 3–4 weeks of continuous treatment with 12.5 nM bortezomib, the cells became resistant to apoptosis induced by bortezomib and started to grow in the presence of 12.5 nM bortezomib. Compared to cells not adapted to bortezomib, Namalwaad cells exhibited increased proteasomal caspase-like, trypsin-like and chymotrypsin-like peptidase activities as determined by measuring the hydrolysis of the fluorogenic oligopeptidyl substrates Z-GGL-amc (for chymotrypsin-like peptidase activity), Boc-LRR-amc (for trypsin-like peptidase activity) and Boc-LLE-amc (for caspase-like peptidase activity) in permeabilized cells (Fig. 1A). Even high doses of bortezomib (50 nM), which markedly inhibited chymotrypsin-like peptidase activity in non-adapted cells, failed to affect the increased chymotrypsin-like peptidase activity in Namalwaad cells (Fig. 1B). Lactacystin, which markedly inhibited caspase-like, trypsin-like and chymotrypsin-like peptidase activities in non-adapted cells, inhibited chymotrypsin-like peptidase activity, but not caspase-like and trypsin-like peptidase activities in Namalwaad cells (Fig. 1C).
Increased proteasomal proteolytic activities in Namalwaad cells in the presence and absence of proteasome inhibitors. Non-adapted Namalwa cells and Namalwaad cells were incubated for 90 min with proteasome inhibitors or DMSO. Subsequently, proteolytic activities of proteasomal caspase-like, trypsin-like and chymotrypsin-like peptidase activities were determined in permeabilized cells as described in Materials and Methods. Proteolytic activities in non-adapted cells incubated with DMSO were defined as 100%. Proteasomal proteolytic activities in cells without inhibitors (A). Proteasomal proteolytic activities in cells treated with bortezomib (B), and lactacystin (LC) (C). Data are given as mean values ± SD of three independent experiments in triplicate.
Namalwaad Cells Fail to Accumulate Polyubiquitinated Proteins in Response to Treatment With Proteasome Inhibitors
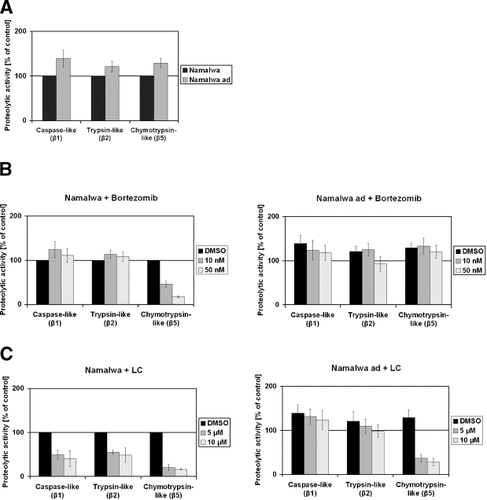
Accumulation of polyubiquitinated proteins is a functional consequence of inhibition of proteasomal protein degradation [Wang et al., 2000; Princiotta et al., 2001]. To investigate the extent of inhibition of proteasomal protein degradation in functional terms, we performed Western blot analysis of polyubiquitinated proteins from non-adapted Namalwa cells and Namalwaad cells treated for 7 h with bortezomib, lactacystin or the TPP II inhibitor AAF-cmk. Although Namalwaad cells displayed elevated levels of polyubiquitinated proteins under steady state conditions that might reflect increased turnover of proteins destined to undergo proteasomal degradation, the cells failed to accumulate polyubiquitinated proteins in response to treatment with proteasome inhibitors or AAF-cmk (Fig. 2). By contrast, non-adapted cells displayed low steady state levels of polyubiquitinated proteins that markedly accumulated in response to treatment with bortezomib or lactacystin (Fig. 2). However, from these results, we cannot completely rule out that proteasomes in Namalwaad cells cannot be inhibited anymore.
Detection of polyubiquitinated proteins in Namalwaad cells and non-adapted cells exposed to proteasome inhibitors or AAF-cmk. Cells were treated for 7 h with 20 nM bortezomib (Bor), 10 µM lactacystin (LC), 20 µM AAF-cmk (AAF) or DMSO prior to Western blot analysis using the FK2 monoclonal antibody which detects polyubiquitinated proteins. One representative experiment out of three independent experiments is shown.
Increased Expression of Proteasomal Proteolytic Subunits and Altered Proteasome Subunit Composition in Namalwaad Cells
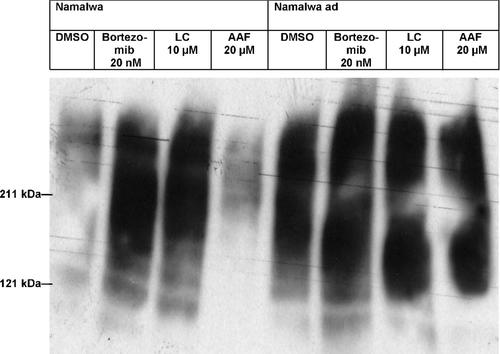
To investigate whether the increased proteasomal proteolytic activities in Namalwaad cells might be linked to altered proteasomal subunit expression and composition, we investigated the expression of a series of proteasomal subunits and subcomplexes in Namalwaad cells by Western blot analysis. Putative alterations of the capability of the cells to express proteasomal subunits and subcomplexes in response to IFN-γ, which induces expression of β1i, β2i, β5i and PA28, were investigated by Western blot analysis of proteasomal subunits and subcomplexes from cells treated for 24 h with 1,000 U/ml IFN-γ. First, we observed that expression of proteasome maturation protein POMP is increased in Namalwaad cells. Expression of POMP is further increased by treatment of Namalwaad cells with IFN-γ that was not observed in non-adapted cells (Fig. 3A). Since POMP associates with precursor intermediates during 20S proteasome biogenesis and its expression is essential for de novo biogenesis of proteasomes [Witt et al., 2000; Krüger et al., 2001], increased expression of POMP detected in Namalwaad cells demonstrates increased de novo biogenesis of proteasomes in the cells. According to the increase in proteasomal proteolytic activities in Namalwaad cells (Fig. 1A), the cells displayed increased expression of the constitutive proteolytic subunits β1, β2 and β5 and completely down-regulated expression of the corresponding immunosubunits β1i, β2i and β5i whose expression could not be induced anymore by IFN-γ (Fig. 3A). Expression of PA28β, an IFN-γ-inducible subcomplex required for proteasomal production of antigenic peptides for MHC class I presentation, was completely abrogated, but expression of Rpt5 and Rpn2, ATPase and non-ATPase subunits of the 19S regulatory complex, respectively [Lam et al., 2002; Wendler et al., 2004], was up-regulated in Namalwaad cells (Fig. 3B). These results demonstrate that Namalwaad cells completely abrogate expression of immunoproteasomes and PA28-20S-PA28 proteasomes in favor of abundant expression of 19S-20S-19S proteasomes. Expression of non-proteolytic α and β subunits (α subunits, α3, β4 and β6) was markedly up-regulated in Namalwaad cells (Fig. 3B), indicating that the cells abundantly express and assemble complete 20S complexes. However, Namalwaad cells abundantly express β1, β2 and β5 proteolytic subunits (Fig. 3A), but only slightly increase proteolytic activities (Fig. 1A), suggesting that the cells harbor proteasomes with lower specific proteolytic activities. As a loading control that matches the 20S proteasome in abundance we performed Western blot analysis of whole 20S particle expression (α/β subunits) that was markedly increased in Namalwaad cells (Fig. 3A and B). Expression of TPP II was markedly down-regulated in Namalwaad cells (Fig. 3B).
Increased expression of proteasomal proteolytic subunits and altered proteasome subunit composition in Namalwaad cells. A: Expression of the proteasome maturation protein POMP, proteasomal constitutive proteolytic subunits (β1, β2 and β5), immunosubunits (β1i, β2i and β5i), and whole 20S particles (α/β subunits) in Namalwaad cells and non-adapted cells with or without prior treatment of the cells with rh IFN-γ. B: Expression of non-proteolytic α and β subunits of the 20S proteasome, expression of proteasome activator PA28β, 19S regulatory complex subunits Rpt5 and Rpn2, and TPP II in Namalwaad cells and non-adapted cells with or without prior treatment of the cells with IFN-γ. Where indicated, cells were treated for 24 h with rh IFN-γ (1,000 U/ml; 3 × 105 cells/ml) prior to Western blot analysis. Anti-β-actin antibody was used as a control for equal protein loading. All blots show results obtained from one experimental set and one cell lysate of either Namalwaad cells or non-adapted cells. Each protein was detected in a separate blot. One representative experimental set out of three independent experimental sets is shown.
Hyperproliferation of Namalwaad Cells
To investigate the functional consequences of increased proteasomal proteolytic activity and increased expression of 19S-20S-19S proteasomes in Namalwaad cells, we determined the proliferative activity of the cells in the presence and absence of bortezomib, lactacystin, and the TPP II inhibitor AAF-cmk. Compared to non-adapted cells, Namalwaad cells displayed rapid proliferation as determined by the incorporation of [3H]thymidine for 24 h (Fig. 4A). This hyperproliferation was only slightly inhibited by high doses (50 and 100 nM) of bortezomib, whereas bortezomib at 12.5, 50 and 100 nM markedly inhibited proliferation of non-adapted cells (Fig. 4A). Lactacystin at 5 and 10 µM inhibited the hyperproliferation of Namalwaad cells, but the extent of lactacystin-induced inhibition of proliferation was more prominent in non-adapted cells (Fig. 4B). AAF-cmk inhibited proliferation of non-adapted cells, but failed to inhibit hyperproliferation of Namalwaad cells at 5 and 10 µM (Fig. 4C), indicating that TPP II activity is dispensable for hyperproliferation of Namalwaad cells. Rapid cell cycle progression and accelerated cell cycle transitions are associated with increased proteasomal activity [Naujokat and Hoffmann, 2002], and inhibition of proliferation and cell cycle progression induced by proteasome inhibitors is accomplished by the induction of apoptosis and cell cycle arrest at the G2/M phase [Naujokat et al., 2000; Naujokat and Hoffmann, 2002; Yin et al., 2005]. Therefore, we determined the induction of G2/M cell cycle arrest in Namalwaad cells and non-adapted cells in the presence of bortezomib, lactacystin and AAF-cmk. Treatment with bortezomib or lactacystin resulted in the induction of G2/M arrest in non-adapted cells, whereas induction of G2/M arrest was partially suppressed in Namalwaad cells (Fig. 4D). G2/M arrest induced by AAF-cmk was much more prominent in non-adapted cells than in Namalwaad cells (Fig. 4D), suggesting that partial resistance to induction of G2/M arrest allows Namalwaad cells to hyperproliferate in the presence of proteasome inhibitors and AAF-cmk.
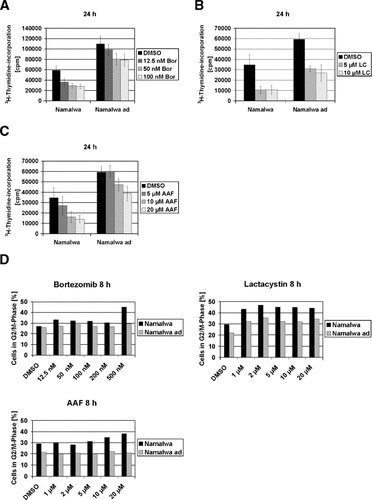
Hyperproliferation of Namalwaad cells in the presence and absence of proteasome inhibitors or AAF-cmk. Namalwaad cells and non-adapted cells were treated for 24 h with DMSO or indicated concentrations of bortezomib (Bor) (A), lactacystin (LC) (B) or AAF-cmk (C). Proliferation was determined by measuring the cellular incorporation of [3H]-thymidine for 24 h as described in Materials and Methods. Data are given as mean values ± SD of three independent experiments in triplicate. Detection of cells in the G2/M-phase of the cell cycle (D). Cells were exposed for 8 h to DMSO or to the indicated concentrations of bortezomib, lactacystin or AAF-cmk (AAF). The percentages of cells in the G2/M-phase are shown. Cell cycle analysis was performed as described in Materials and Methods. One representative experimental set out of four independent experimental sets is shown.
Apoptosis Resistance of Namalwaad Cells
We next determined the sensitivity of Namalwaad cells towards apoptosis induced by diverse stimuli. In contrast to non-adapted cells, Namalwaad cells displayed resistance to apoptosis induced by high concentrations of bortezomib (Fig. 5A). Resistance to apoptosis induced by lactacystin was apparent in Namalwaad cells, but was not as prominent as observed for bortezomib (Fig. 5B). AAF-cmk failed to induce apoptosis in non-adapted cells and Namalwaad cells (Fig. 5C). Namalwaad cells displayed resistance to apoptosis induced by γ-irradiation or staurosporine (Fig. 5D), demonstrating that increased proteasomal proteolytic activity and increased expression of 19S-20S-19S proteasomes in Namalwaad cells have allowed the establishment of an apoptosis-resistant phenotype.

Apoptosis resistance of Namalwaad cells. Namalwaad cells and non-adapted cells were exposed for 24 or 48 h to the indicated concentrations of bortezomib (A), lactacystin (LC) (B) or AAF-cmk (AAF) (C). D: Cells were incubated for 24 or 48 h with staurosporine or cells were cultured for 24 or 48 h after irradiation with a dose of 30 Gy. Apoptosis was analyzed by flow cytometry, and specific apoptosis was calculated as described in Materials and Methods. One representative experimental set out of four independent experimental sets is shown.
Namalwaad Cells and Non-Adapted Cells do not Express P-Glycoprotein and are Incapable of MDR-1/P-Glycoprotein-Mediated Drug Efflux
To exclude the possibility that the cells acquire resistance to bortezomib by expression of the mdr-1-encoded P-glycoprotein, a 170 kDa transmembrane efflux pump that eliminates various drugs and small molecules from the cytosolic compartment, we demonstrated by flow cytometry that Namalwaad cells and non-adapted cells do not express P-glycoprotein (Fig. 6, left column). Using the rhodamine 123 efflux assay, which allows the functional analysis and quantification of MDR-1/P-glycoprotein-mediated multidrug resistance [Ludescher et al., 1992], we showed that Namalwaad cells and non-adapted cells are incapable of eliminating drugs and small molecules from the cytosolic compartment by MDR/P-glycoprotein-mediated mechanisms (Fig. 6, right column). As a positive control, we used multidrug resistant MES-SA/Dx5 uterine sarcoma cells that express P-glycoprotein and eliminate rhodamine 123 by MDR-1/P-glycoprotein-mediated efflux (Fig. 6).
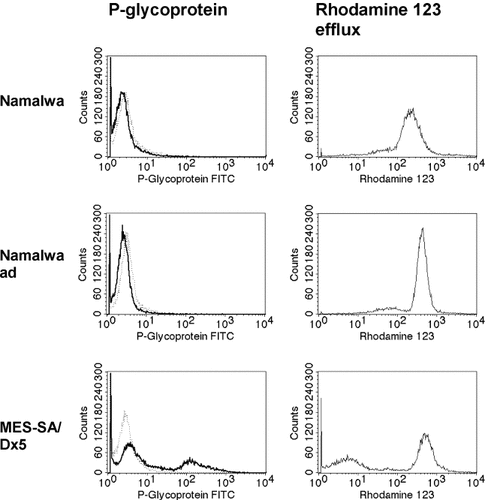
Determination of P-glycoprotein expression (left column) and rhodamine 123 efflux (right column) in Namalwaad cells and non-adapted cells. Cell surface expression of P-glycoprotein in non-adapted cells, Namalwaad cells, and multidrug resistant MES-SA/Dx5 cells were determined by flow cytometry using a FITC-labeled mouse monoclonal IgG2b antibody raised against P-glycoprotein. Rhodamine 123 efflux in non-adapted cells, Namalwaad cells, and MES-SA/Dx5 cells were determined as described in Materials and Methods. One representative experimental set out of four independent experimental sets is shown.
Expression of the Apoptosis-Related Proteins p53 and Hsp27 in Namalwaad Cells
Finally, we determined the differences of expression of apoptosis-related proteins in Namalwaad cells and non-adapted cells treated for 0–24 h with 50 nM bortezomib. Regarding the expression of Bax, Bcl-2, Bcl-XL, c-IAP1, XIAP, PUMA and Noxa, proteins involved in the regulation of apoptosis induced by proteasome inhibition [Pei et al., 2003; Yang and Du, 2004; Concannon et al., 2007], we did not detect any differences in Namalwaad cells and non-adapted cells (data not shown). Namalwaad cells displayed de novo expression and bortezomib-induced accumulation of the anti-apoptotic heat shock protein Hsp27 (Fig. 7) whose constitutive or ectopic expression has been shown to confer resistance to apoptosis induced by bortezomib [Chauhan et al., 2003]. Conversely, expression of the tumor suppressor protein p53 whose accumulation and stabilization in response to proteasome inhibition contribute to the induction of apoptosis [Lopes et al., 1997; Chen et al., 2000] was markedly reduced in Namalwaad cells, and protein levels of p53 failed to accumulate in response to treatment with high doses of bortezomib (Fig. 7). Because Burkitt lymphoma cells constitutively overexpress the proto-oncogene c-myc that is linked to overexpression of TPP II and TPP II-mediated apoptosis resistance [Gavioli et al., 2001], we investigated a putative modulation of c-myc expression in Namalwaad cells. As shown in Figure 7, levels of c-myc expression were found to be equal in Namalwaad cells and non-adapted cells and remained unchanged in response to treatment with bortezomib, suggesting that c-myc does not play a role in the establishment of apoptosis resistance of Namalwaad cells.
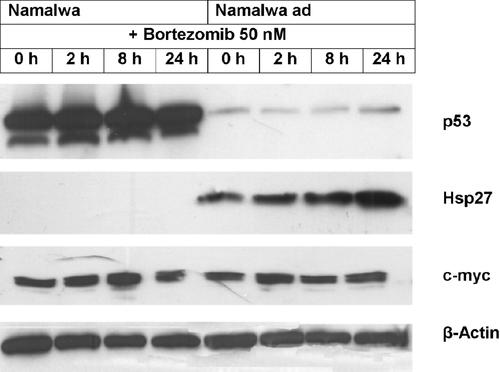
Differential expression of the apoptosis-related proteins p53 and Hsp27 in Namalwaad cells and non-adapted cells. Cells were exposed for the indicated times to 50 nM bortezomib prior to Western blot analysis. Anti-β-actin antibody was used as a control for equal protein loading. For each protein, one representative experiment out of three independent experiments is shown.
DISCUSSION
We report here a mechanism of adaptive modification of the proteasome system in response to continuous inhibition of the proteasomal chymotrypsin-like peptidase activity in human Namalwa Burkitt lymphoma cells. Cells adapted to initially lethal concentrations of the proteasome inhibitor bortezomib display abundant de novo biogenesis and expression of proteasomes with altered subunit and subcomplex composition and increased proteolytic activity, whereas expression of TPP II is down-regulated. By contrast, previous studies in mouse EL-4 T cell lymphoma cells adapted to proteasome inhibitors demonstrated down-regulation of proteasome expression and proteolytic activity as well as increased expression and proteolytic activity of TPP II as a compensatory mechanism [Glas et al., 1998; Geier et al., 1999; Wang et al., 2000; Hong et al., 2003]. However, EL-4 cells had been adapted to the proteasome inhibitors NLVS and lactacystin, which, compared to bortezomib, exhibit a different inhibitory profile toward proteasomal peptidase activities: whereas bortezomib solely inhibits chymotrypsin-like peptidase activity in Namalwa cells (Fig. 1B), NLVS and lactacystin inhibit strongly chymotrypsin-like and trypsin-like peptidase activities and weakly caspase-like activity in EL-4 cells [Wang et al., 2000].
The chymotrypsin-like peptidase activity constitutes the main proteolytic site of proteasomal protein degradation in mammalian cells [Kisselev et al., 2006], and selected and continuous inhibition of this central peptidase activity may lead to the selection of cells with modified subunit composition and increased expression and activity of proteasomes as described herein. This hypothesis is supported by results obtained in Drosophila S2 cells with impaired proteasomal chymotrypsin-like peptidase activity as a result of targeted β5 subunit expression by RNA interference for 4 days. Such cells displayed increased expression of non-targeted subunits of the 20S complex (α2 and α7) and the 19S regulatory complex (Rpt2, Rpt6 and Rpn12) [Wojcik and DeMartino, 2002]. Moreover, a study by Meiners et al. [2003] demonstrated transient and concerted up-regulation of most 26S proteasome subunit mRNAs, increased expression of proteasomal subunits β1, α6 and Rpt1, and increased de novo biogenesis of proteasomes in rat vascular smooth muscle cells exposed for 6–8 h to proteasome inhibitors targeting the chymotrypsin-like peptidase activity. These results have been complemented by a recent study demonstrating increased mRNA levels and protein expression of proteasomal α and β subunits in mouse neocortical neurons treated for 18 h with subtoxic concentrations of the proteasome inhibitor MG-132 [Lee et al., 2004].
These studies investigated changes of the proteasome system in response to short-term and subtoxic inhibition of proteasomal proteolytic activity. In our study, cells were continuously exposed to initially lethal concentrations of bortezomib. To exclude MDR/P-glycoprotein-mediated drug efflux of bortezomib, we demonstrate that the P-glycoprotein transmembrane efflux pump is not expressed in Namalwaad cells and non-adapted cells and that the cells are incapable of eliminating intracellular drugs and small molecules via P-glycoprotein-mediated efflux. However, we cannot completely rule out the low probability that bortezomib is eliminated by rare drug eliminating mechanisms mediated by other members of the ABC transporter family.
Our results indicate that, under the pressure of continuous and long-term proteasome inhibition by concentrations of bortezomib used in cancer therapy, a population of cells is selected that abundantly expresses 19S-20S-19S proteasomes to subvert the lethal effects of bortezomib. Importantly, expression of immunoproteasomes containing β1i, β2i, β5i and PA28 is completely down-regulated in Namalwaad cells in favor of increased expression of proteasomes containing β1, β2, β5 and 19S, suggesting that constitutive proteolytic β1, β2 and β5 subunits and 19S regulatory complexes are essential, whereas immunosubunits and PA28 are dispensable for metabolism and survival of cells continuously exposed to bortezomib. Increased expression of β1, β2 and β5 subunits is accompanied by increased proteasomal caspase-like, trypsin-like and chymotrypsin-like peptidase activities, and concentrations of bortezomib that markedly inhibit chymotrypsin-like peptidase activity in non-adapted cells fail to affect the increased chymotrypsin-like peptidase activity in Namalwaad cells. Similar results have been recently reported by Kraus et al. [2007] who demonstrated in bortezomib-adapted HL60 promyelocytic leukemia cells an increased activity of β1 and β5 that was only slightly inhibitable by bortezomib. However, lactacystin retains its ability to inhibit chymotrypsin-like peptidase activity, but not caspase-like and trypsin-like peptidase activity in Namalwaad cells. This might be due to the covalent binding of lactacystin to the β5 N-terminal threonine Oγ residue by the formation of an ester bond that causes irreversible β5 inhibition and prolonged residence time of lactacystin within the S1 pocket of β5 subunit [Groll and Huber, 2004]. By contrast, the boronic acid moiety of bortezomib reversibly binds to the active site of the β5 subunit by the formation of a tetrahedral boron adduct with the N-terminal threonine Oγ residue [Mc Cormack et al., 1997]. These chemically different mechanisms of β5 subunit active site inactivation exerted by bortezomib and lactacystin may explain the remaining ability of lactacystin to inhibit chymotrypsin-like peptidase activity in Namalwaad cells.
High levels of proteasome expression have been shown to correlate with rapid proliferation, accelerated cell cycle progression, apoptosis resistance, and neoplastic transformation and malignancy [Shimbara et al., 1992; Ichihara and Tanaka, 1995; Naujokat and Hoffmann, 2002]. Accordingly, we demonstrate that Namalwaad cells expressing high levels of 19S-20S-19S proteasomes display rapid proliferation and cell cycle progression as well as apoptosis resistance. Rapid proliferation and cell cycle progression is not markedly inhibited by the TPP II inhibitor AAF-cmk which also failed to induce apoptosis in Namalwaad cells, indicating that TPP II activity is not required for proliferation and viability of Namalwaad cells. By contrast, AAF-cmk has been shown to completely inhibit proliferation of NLVS-adapted EL-4 cells displaying low proteasome activity and high compensatory TPP II activity, suggesting an essential role of TPP II for proliferation and viability only when proteasome activity is down-regulated [Glas et al., 1998]. Viability, proliferation and cell cycle progression of Namalwaad cells is only partially affected by bortezomib, suggesting that increased proteolytic activity and expression of 19S-20S-19S proteasomes confer resistance to the cytotoxic effects of this proteasome inhibitor.
Namalwaad cells acquire resistance to apoptosis induced by different stimuli, including high doses of bortezomib and lactacystin, γ-irradiation and the protein C kinase inhibitor staurosporine. This general apoptosis resistance is likely due to de novo expression of the small heat shock protein Hsp27 in Namalwaad cells. Hsp27 has been shown to confer apoptosis resistance by interfering with pro-apoptotic proteins and key effectors of the mitochondrial pathway of caspase-dependent apoptosis [Garrido et al., 1999; Bruey et al., 2000]. Constitutive or ectopic expression of Hsp27 confers resistance to apoptosis induced by bortezomib in lymphoma cells [Chauhan et al., 2003], and overexpression of Hsp27 has been demonstrated to enhance proteasomal degradation of ubiquitinated proteins, such as the NF-κB-inhibitor I-κBα, leading to increased NF-κB activation that suppresses apoptosis [Parcellier et al., 2003]. Conversely, proteasome inhibition has been shown to induce rapid expression of Hsp27 [Ito et al., 2002]. Thus, it is likely that high levels of de novo expression of Hsp27 in Namalwaad cells have been evolved as a result of continuous proteasome inhibition and, in turn, perpetuate increased proteasome activity and apoptosis resistance of the cells.
Levels of tumor suppressor protein p53 are tightly regulated by proteasomal degradation of p53, and proteasome inhibition causes accumulation and stabilization of p53 that contributes to the induction of apoptosis [Maki et al., 1995; Lopes et al., 1997; Chen et al., 2000]. In contrast to non-adapted cells, which show high levels of p53, Namalwaad cells display extremely low levels of p53 and failed to accumulate p53 in response to treatment with bortezemib, suggesting that increased proteasomal activity in Namalwaad cells leads to the degradation of most p53 proteins. This failure to accumulate and stabilize p53 may also contribute to the apoptosis resistance of Namalwaad cells.
In conclusion, we report here a compensatory and autoregulatory feedback mechanism of the proteasome system in human Namalwa Burkitt lymphoma cells adapted to the proteasome inhibitor bortezomib. Our results provide some insights into the flexibility of the proteasome system in response to continuous proteasome inhibition and imply that therapeutic use of proteasome inhibitors, i.e. in cancer therapy, should be viewed more critically.
Acknowledgements
We thank Marion Miltz for excellent technical assistance.




