Hyperthermia inhibits cell proliferation and induces apoptosis: Relative signaling status of P53, S100A4, and Notch in heat sensitive and resistant cell lines
Abstract
The effects of hyperthermia on the expression of p53, the apoptosis-associated genes Bax and Bcl-2, Notch and S100A4 have been studied in the HepG2 cell line and the HUT cell line derived from HepG2, adapted for growth in hyperthermic conditions. Hyperthermia inhibits cell proliferation and induces apoptosis. HepG2 and HUT cells differed in respect of anchorage to growth surface, degree of proliferation and apoptosis and expression of p53, Bax, Bcl-2, Notch, and S100A4 genes. The induction of apoptosis and the inhibition of cell proliferation occurred independently of p53, and independently also of involvement of the apoptosis family genes Bax and Bcl-2. We demonstrate novel and marked differences between transient heat shock and heat adaptation in respect of pathways of signaling and generation of phenotypic effects in vitro. Different signaling patterns have been identified here. Pathways of signaling by S100A4, by its interaction with and sequestration of p53, and by Notch also seem differentially operational in the induction of apoptosis, and both appear to be activated as alternative pathways in the context of hyperthermia signaling independently of p53. J. Cell. Biochem. 103: 212–220, 2008. © 2007 Wiley-Liss, Inc.
Hyperthermia has been investigated over the past several years as a possible mode of cancer treatment on account of the perceived sensitivity of cancer cells to hyperthermia and inhibition of tumor growth by hyperthermia [Yatvin et al., 1987; Wallen et al., 1989; Albertazzi et al., 1998]. These effects are conceivably attributable to the ability of hyperthermia to markedly alter the expression of several genes related to cell proliferation and differentiation. Cell proliferation, cell cycle progression and apoptosis, are intricately linked processes. The expression of the cell cycle regulator gene p53 and its down stream targets has been shown to be modulated by hyperthermia [Abe et al., 2001; Mikheeva et al., 2004]. The expression of the metastasis promoter S100A4 gene, which is involved in cell cycle regulation in conjunction with p53, is also known to be modulated by hyperthermia [Cajone et al., 1994; Albertazzi et al., 1998; Sherbet and Lakshmi, 2006]. However, there are no reports concerning the effects of hyperthermia on the proliferation and differentiation signaling by Notch. We describe here the effects of hyperthermia on the expression of p53, the apoptosis-associated genes Bax and Bcl-2, Notch and S100A4. We show further that the alterations in their expression induced by hyperthermia reflect changes in their signaling pathways.
MATERIALS AND METHODS
Cell Line and Treatments
HepG2 is a human hepatoma cell line, which is normally grown at 37°C. HUT is a variant cell lines derived from HepG2. It is capable of growing at 42°C. The HUT cell line is a variant of HepG2 cell line selected by exposure of HepG2 cells to heat treatment as described previously [Albertazzi et al., 1998]. The HepG2 cells were cultured at 37°C in a humidified atmosphere with 5% CO2 in Eagle's MEM (Sigma-Aldrich, M4655) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich) and antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin) (Sigma-Aldrich). Cells were maintained by subculturing every 4 days at an initial density of 4 × 105 cells per T-25 tissue culture flask reaching a final density of 2–2.5 × 106 cells/flask. HUT cells were cultured as a monolayer at 37°C in a humidified atmosphere with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich, D6546) supplemented with 10% FBS (Sigma-Aldrich), antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin) (Sigma-Aldrich), 0.2 M L-glutamine (Sigma-Aldrich), 0.1 M sodium pyruvate and 1 M N-2-hydroxyethyl-piperazinyl-N′-2-ethanesulfonic acid (HEPES) (Sigma-Aldrich) pH 7.2. Cells were maintained by subculturing every 2 days at an initial density of 4 × 105 cells per T-25 tissue culture flask reaching a final density of 1.5–2 × 106 cells/flask.Cells were exposed to hyperthermia [42°C] for different times as indicated in results.
Cell Growth Analysis
Cells (0.4 × 106) were seeded in T-25 tissue culture flasks. After 0–24–48 h the cells were counted in a Bürker's chamber. The experiment was performed in triplicate.
Assay of Apoptosis
Cells were washed twice with PBS and incubated for 5 min at room temperature with Binding Buffer 1× (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl and 2.5 mM CaCl2), Annexin V-FITC (200 µg/ml) (MBL) and propidium iodide (1 mg/ml) (MBL). Viable and stained cells were detectable by fluorescence microscopy. The experiment was performed in triplicate.
Assay of Gene and Protein Expression
Western blotting
Total proteins were extracted by RIPA Buffer Modified (50 mM Tris-HCl pH 7,4; 150 mM NaCl, 1% Triton X-100, 0,1% SDS, 1% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4 (sodium orthovanadate), 2 µg/ml aprotinin, 2 µg/ml Leupeptin, 1 µg/ml Pepstatin A, 50 µg/ml N-Tosyl-L-lysine chloromethyl ketone (TLCK), 100 µg/ml phenylmethylsulfonyl fluoride (PMSF) and 2 mM NaF). Extracts were loaded onto a SDS–polyacrylamide gel (Sigma-Aldrich, A3574) (8% for Notch1, 10% for p53, 12% for Bax e Bcl-2). Every gel was run at 20 mA in a Bio-Rad mini-gel system. Proteins were transferred to nitrocellulose membrane (100 V, overnight, 4°C) and blocked for 1 h in 0.05% Tween 20, 5% non-fat milk at room temperature. Specific antibodies were used (anti-NOTCH-IC, 1:500, 2 h of incubation at room temperature, Santa Cruz; anti-p53, 1:500, 2 h of incubation at room temperature, Sigma-Aldrich; anti-Bax, 1:400, 2 h of incubation at room temperature, Santa Cruz; anti-Bcl-2, 1:200, over night incubation at 4°C, Santa Cruz) and proteins were visualized by enhanced chemiluminescence (ECL) according to the instructions of the manufacturer (Amersham-Pharmacia).
RT-PCR
Total RNA was extracted by Sacchi-Chomczynski method and was retrotranscribed. Specific primers were used for RT-PCR [GAPDH: left 5′-CCA TGG AGA AGG CTG GGG-3′, right 5′-CAA AGT TGT CAT GGA TGA CC-3′, 177 bp; NOTCH1: left 5′-CGC CTT TGT GCT TCT GTT CT-3′, right 5′-CCC ACT CAT TCT GGT TGT CG-3′, 215 bp; HES1: left 5′-ACG ACA CCG GAT AAA CCA AA-3′, right 5′-CGG AGG TGC TTC ACT GTC AT-3′, 200 bp; S100A4: left 5′-GGTGTCCACCTTCCACAAGT-3′, right 5′-GCTGTCCAAGTTGCTCATCA-3′, 157 bp; P53: left 5′-GCGCACAGAGGAAGATC-3′, right 5′-GAGTTCAAGGCCTCATTCA-3′, 189 bp].
Quantification of Intensity of Bands
Densitometric analysis of bands was performed by using Kodak software. Results were confirmed by repeating the experiment at least three times.
RESULTS
The effects of hyperthermia on cell adhesion, growth, and apoptosis was assessed on the two cell lines together with the expression of a number of genes related to cell cycle regulation, apoptosis and differentiation.
Effects of Hyperthermia on Cell Anchorage and Apoptosis
Marked differences were noticed in the adhesion to the growth substratum of thermosensitive HepG2 and the heat resistant HUT cells upon exposure to hyperthermia (Fig. 1). Whilst HUT cell attachment to the substratum was unaffected, the number of adherent HepG2 cells was gradually reduced by 50% over a 6-h exposure to hyperthermia.
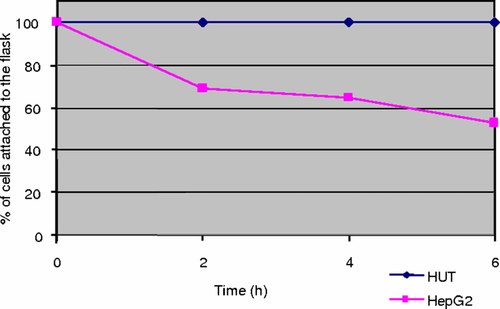
Shows the effects of hyperthermia on adhesion of HepG2 and HUT cells to substratum. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The extent of cell anchorage and the degree of apoptosis was then compared in HepG2 and HUT cells. Apoptosis of HepG2 cells attached to the growth surface increased over the 6-h exposure to hyperthermia reaching about 46% (Fig. 2). In contrast, apoptosis of adherent HUT remained at a low level (about 15%) for up to 24 h (Fig. 3). There was an increase of apoptosis to 36% at 48 h without changing of culture medium. The growth of HUT cells was reduced by 50% after 48 h of hyperthermia (Fig. 4). Thus, a clear relationship can be documented between hyperthermia and its growth inhibitory effect actuated by the induction of apoptosis.
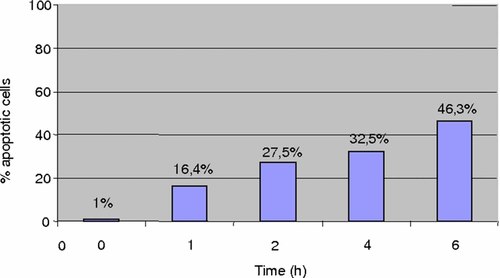
Apoptosis of HepG2 cells still attached on the flasks during hyperthermia.
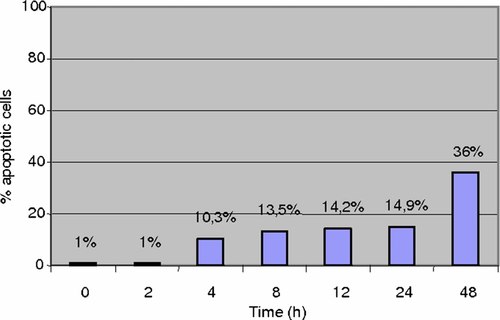
Apoptosis of HUT cells still attached on the flasks during hyperthermia.
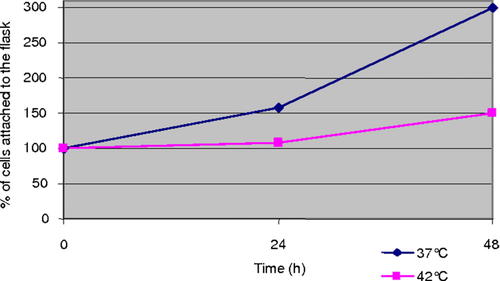
Shows the growth of HUT cells during hyperthermia as percent of seeded cells. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Effects of Hyperthermia on Gene Expression
In order to investigate if there was a genetic basis for the differential ability of HepG2 and HUT cells to survive under hyperthermia, we have compared the expression in these cell lines of some genes involved in apoptosis, cell proliferation, and cell differentiation during different times of exposure to hyperthermia; this was limited to 4 h for HepG2 cells and up to 48 h for HUT cells.
Modulation of p53 Expression
P53 is a well-studied protein involved in apoptosis and cell cycle control. We studied the changes in the expression of p53 protein and the gene by Western blot and by RT-PCR analysis in HepG2 and HUT cells. P53 protein in HepG2 cells is reduced during hyperthermia, but returned to high level when the cells were returned to 37°C for 4 h (Fig. 5). But when HUT cells are exposed to hyperthermia, the amount of p53 protein progressively increased up to 48 h and these levels remained higher than in controls when the cells were returned to 37°C. Measurement of the level of p53 mRNA by RT-PCR revealed no changes in either cell line (Fig. 6). This suggests that changes of p53 protein expression were not due to transcription.
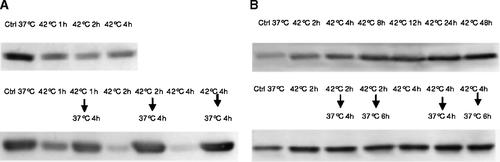
Western blot assay of expression of p53 protein in HepG2 (panel A) and Hut (panel B) cells exposed to hyperthermia.
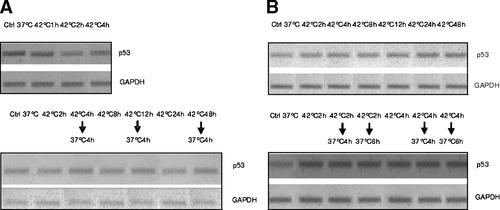
RT-PCR assay of expression fo P53 gene in HepG2 (panel A) and HUT (panel B) cells exposed to hyperthermia.
Modulation of Expression of Apoptosis Gene
The experiments described above show that under the present experimental conditions apoptosis induced by hyperthermia occurred independently of p53 (Figs. 2-5). We therefore investigated the modulation of expression of the proapoptotic Bax and antiapoptotic Bcl-2 genes. The degree of apoptosis seemed to markedly reflect the expression of Bax and Bcl-2 genes (Fig. 7). Thus, in HepG2 cells apoptosis was at 16% beginning with 1 h of heat exposure and the pro-apoptotic protein Bax increased and remained at high levels also when the cells are returned to 37°C. On the other hand, the anti-apoptotic protein, Bcl-2, is decreased by hyperthermia and the expression remained at low levels also when cells are returned at 37°C. In sharp contrast, in HUT cells neither Bax nor Bcl-2 appeared to be affected by hyperthermia. Only when the cells showed a high level of apoptosis, which was after 24 h of hyperthermia, did Bax and Bcl-2 show changes similar to those seen in HepG2 cells.
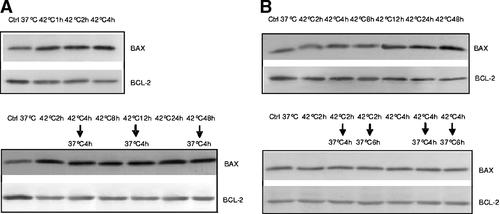
Western blot assay of expression of Bax and Bcl-2 protein in HepG2 (panel A) and HUT (panel B) cells exposed to hyperthermia.
Hyperthermia and S100A4 Signaling in Proliferation and Apoptosis
We demonstrated previously by using Northern blot analysis that hyperthermia downregulated S100A4 in HUT cells [Cajone et al., 1994]. We confirm this finding here by RT-PCR and also report for the first time that the expression of S100A4 mRNA returns to normal level when HUT cells are returned to 37°C (Fig. 8). This constitutes further evidence that changes of expression of S100A4 gene could be related to the control of the cell cycle. Also evident here is that the down regulation of S100A4 is accompanied by increased apoptosis. In HepG2 cells the S100A4 level had not changed in response to hyperthermia, although p53 level had changed.
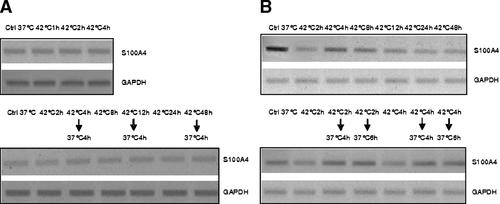
RT-PCR assay of expression of S100A4 gene in HepG2 (panel A) and HUT (panel B) cells exposed to hyperthermia.
Modulation of Notch Expression by Hyperthermia
The expression of Notch was also differentially influenced by transient and sustained hyperthermia. Figure 9 shows the quantification by Western blot analysis of the portion of Notch1 protein that is cleaved by proteases. In HepG2 cells the quantity of this protein increased after 1 h of hyperthermia and remained at this level even after 4 h. When the cells were returned to 37°C for 4 h the Notch1 protein levels returned to control levels. In HUT cells, hyperthermia markedly reduced the Notch1 protein; the levels further decreased when cells were maintained under hyperthermia for long periods. Also in HUT cells the Notch1 protein returned to control levels when cells were incubated at 37°C for 4 h.
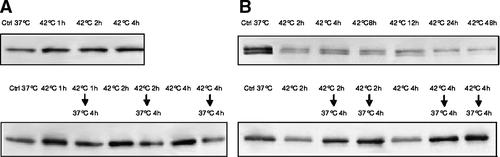
Western blot assay of expression of Notch1 protein in HepG2 (panel A) and HUT (panel B) cells exposed to hyperthermia.
We have also been able to relate these changes of levels of Notch1 protein in HepG2 and HUT cells under hyperthermia to changes of Notch gene transcription (Fig. 10). When HepG2 and HUT cells were treated in the same conditions, the changes in Notch1 mRNA levels detected by RT-PCR corresponded with Notch1 protein levels.
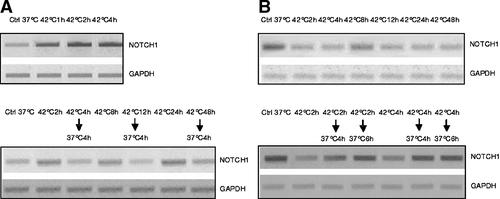
RT-PCR assay of expression of Notch1 gene in HepG2 (panel A) and HUT (panel B) cells exposed to hyperthermia.
The functional relevance of these alterations in Notch1 protein levels was checked by assaying the activation of Hes1 gene which is a down stream effector of Notch. Hes1 mRNA in HepG2 and HUT was quantified by RT-PCR in order to see if changes of Notch1 protein were reflected by the activation of Hes1. This was found to be the case as shown in Figure 11. The changes of HES1 mRNA corresponded with those of Notch1 protein and mRNA.
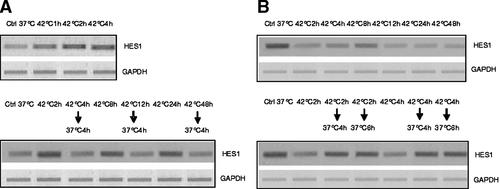
RT-PCR assay of expression of HES1 gene in HepG2 (panel A) and HUT (panel B) cells exposed to hyperthermia.
Summary of Changes in Cell Proliferation, Apoptosis and Gene Expression
The effects of hyperthermia on gene expression were quantified by densitometry and they are summarized in Table I, together with data on cell proliferation and apoptosis.
| Proliferation | Apoptosis (%) | p53 | Bax | Bcl-2 | S100A4 | Notch1 | |
|---|---|---|---|---|---|---|---|
| HepG2 | |||||||
| Ctrl 37°C | = | 1.0 | = | = | = | = | = |
| 1 h 42°C | ↓[−11.6%] | 16.4 | ↓[−53%] | ↑[+64%] | ↓[−27%] | = | ↑[+72%] |
| 2 h 42°C | ↓[−31.2%] | 27.5 | ↓[−66%] | ↑[+70%] | ↓[−27%] | = | ↑[+91%] |
| 4 h 42°C | ↓[−37.6%] | 32.5 | ↓[−69%] | ↑[+98%] | ↓[−50%] | = | ↑[+100%] |
| HUT | |||||||
| Ctrl 37°C | = | 1.0 | = | = | = | = | = |
| 2 h 42°C | ND | 1.0 | ↑[+180%] | = | = | ↓[−80%] | ↓[−64%] |
| 4 h 42°C | ND | 10.3 | ↑[+210%] | = | = | ↓[−50%] | ↓[−47%] |
| 8 h 42°C | ND | 13.5 | ↑[+220%] | = | = | ↓[−64%] | ↓[−68%] |
| 12 h 42°C | ↑[+3.6%] | 14.2 | ↑[+280%] | = | = | ↓[−70%] | ↓[−75%] |
| 24 h 42°C | ↑[+6.7%] | 14.9 | ↑[+300%] | ↑[+30%] | ↓[−20%] | ↓[−74%] | ↓[−90%] |
| 48 h 42°C | ↑[+50.0%] | 36.0 | ↑[+370%] | ↑[+40%] | ↓[−36%] | ↓[−75%] | ↓[−90%] |
DISCUSSION
Hyperthermia was shown some years ago to markedly inhibit cell proliferation and tumor growth. The effects appear to be produced by the activation of apoptosis pathways. We explored the operation of conventional signaling via the p53 and Bcl pathway and have formulated, on the basis of our experimental findings, the thesis that exposure to heat of cells adapted to hyperthermia and transient exposure of cells not adapted for hyperthermia activate different signaling pathways leading to apoptosis. Furthermore, we demonstrate the activation of novel signaling pathways in background of p53-independent apoptosis.
Hyperthermia Induces Apoptosis and Inhibits Growth Independently of p53
Both p53-dependent and -independent mechanisms have been evoked in the induction of apoptosis by hyperthermia [Abe et al., 2001; Dugyala et al., 2002; Guan et al., 2002; Mikheeva et al., 2004; Yasumoto et al., 2003, 2004; Nashimoto et al., 2005]. The mediation of apoptosis by p53 frequently involves activation down stream of Bax [Tamamoto et al., 2003].
Here we describe for the first time a marked difference in response with respect to p53 expression between transient heat shock and heat adaptation. We found that transferring HepG2 cells to hyperthermic conditions downregulated the expression of p53 and upregulated Bax. When these cells were transferred back to normal growth temperature the expression of p53 was reversed but Bax remained over expressed. In contrast, p53 expression was upregulated when HUT cells growing at 37°C were transferred to and maintained at 42°C. We found no correlation between expression of p53 protein and p53 transcription. The reduction of p53 protein in cells during exposure to hyperthermia has been attributed by some to its instability under high temperature. However, here heat did not seem to induce denaturation of p53 protein because its levels showed a progressive increase in HUT cells under hyperthermia. This suggests that there are marked differences in hyperthermia signaling in respect of phenotypic consequence, between cells transiently exposed to hyperthermia and those adapted to hyperthermia.
We have presented here data which shows that exposure to hyperthermia markedly influences the expression of p53 as well as apoptosis, but clearly hyperthermia-induced upregulation of p53 expression and apoptosis seem to be inversely related. These data unequivocally support the conclusion that under the conditions employed here apoptosis occurs independently of p53. Furthermore, we show for the first time that the growth inhibitory effect of hyperthermia is due to the induction of apoptosis by a p53-independent pathway.
There is an apparent uncoupling of the relationship between apoptosis-related genes and the actual incidence of apoptosis in HepG2 and HUT cells. This lends further support to our view that marked differences exist between effects of transient heat exposure of thermosensitive cells and effects seen in heat adapted cells. In human glioblastoma cells transient exposure results in p53/Bax mediated apoptosis [Fuse et al., 1998]. But the novelty of our findings is that in HepG2 cells hyperthermia downregulated p53 expression, but apoptosis increased together with an upregulation of Bax. The pattern of p53 expression, apoptosis and the expression of apoptosis-related genes is markedly different in HUT, where expression of p53 increased but apoptosis showed a decline without any attendant changes in the expression of apoptosis-related gene.
We recognize that the loss of p53 in HepG2 could lead to apoptosis in the absence of the genome guardian role of p53, whilst in contrast HUT cells might preserve DNA repair function and in this way contribute to heat resistance. Equally, these differing patterns of signaling could have resulted from the activation of different p53-independent apoptotic pathways in the transient exposure of heat sensitive cells and those adapted to hyperthermia. Such a switch in apoptotic pathway might be a consequence of the possible inactivation of the p53-mediated pathway, which is indeed sensitive to and might be compromised by hyperthermia [Guan et al., 2002].
S100A4 Mediated Regulation of Apoptosis
With the objective of identifying potential alternative pathways of hyperthermia-induced apoptosis we investigated the operation of the S100A4 in the regulation of apoptosis independently of p53. S100A4 is a metastasis promoter, which significantly influences cell proliferation, cell cycle control and apoptosis. S100A4 interacts with and abrogates the apoptosis inducing function of wild type p53 [Parker et al., 1994a,b; Grigorian et al., 2001; Sherbet, 2006; Sherbet and Lakshmi, 2006]. Here we have found that hyperthermia markedly downregulated S100A4 expression in HUT with parallel increases of p53 expression status but this did not happen in HepG2. This suggests that the S100A4 mediated pathway is not functional in HepG2 cells but it is switched on by chronic exposure and selection of HUT cells for thermoresistance.
Notch Signaling in Hyperthermia Mediated Regulation of Apoptosis
Notch signaling has been studied extensively in recent years and has been implicated in a wide range of normal cell function and pathogenesis. Notch has been shown to operate at different levels in development and morphogenesis, cell proliferation, differentiation, and apoptosis [Chiaramonte et al., 2006]. Notch inhibits apoptosis and induces proliferation. These effects seem to be due to altered expression of cyclins and cdk-2 together with p21 and p27 [Wang et al., 2006], implicating p53 function.
In our experiments the pattern of change of expression of Notch and p53 were diametrically opposite and hence we postulate that Notch signaling might be occurring in our experimental conditions independently of p53 and by an alternative signaling pathway. We found that exposure of HepG2 cells to hyperthermia increased Notch expression but also increased apoptosis. In HUT cells hyperthermia reduced Notch expression and reduced apoptosis. In other words, Notch signaling pathway is not operative in HepG2 cell but it is activated in the heat resistant HUT cells. So we postulate that Notch signaling might be activated in our experimental conditions in the absence of p53 and that it functions as an alternative signaling pathway, and we propose that notch signaling is differentially activated in transient exposure of thermosensitive cells and in heat adapted cells in terms of apoptosis induction.
Concluding Remarks
The present series of experiments reveal some new facets of the effects of hyperthermia in vitro. Hyperthermia influences cell proliferation and apoptosis. In thermosensitive cells the induction of apoptosis and the involvement of apoptosis family genes Bax and Bcl-2 occur independently of p53. In thermoresistent cells the level of apoptosis is low and the main effect of hyperthermia is the reduction of cell proliferation associated with a fast and durable over expression of p53. We show novel and marked differences between transient heat shock and heat adaptation in respect of pathways of signaling and generation of phenotypic effects. We have identified different signaling patterns that suggest switching of apoptotic pathway possibly consequent upon the inactivation of the p53-mediated pathway. Signaling by S100A4 given its ability to interact with and sequester p53 and the Notch pathways also seem differentially operational in the induction of apoptosis, and they appear to be activated as alternative pathways in the context of hyperthermia signaling independently of p53.
Acknowledgements
FC and PC thank Professor PP Di Fiore and Professor S. Pece for advice and providing reagents. GVS thanks Professor Bayan Sharif for providing laboratory facilities and an excellent research environment. We thank Dr. M.S. Lakshmi for reading the manuscript and making helpful suggestions.




