2,3,7,8-tetrachlorodibenzo-p-dioxin increases bovine herpesvirus type-1 (BHV-1) replication in madin-darby bovine kidney (MDBK) cells in vitro
Abstract
Dioxin—2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a common environmental toxin of current interest. In the last years, higher levels of TCDD than those permitted in UE [European Commission. 2002. European Commission Recommendation 2002/201/CE. Official Gazette, L 67/69] were detected in milk samples from cow, water buffalo, goat, and sheep raised on some areas of Campania Region (South Italy). Dioxin often causes immunosuppression and might render the animal liable to viral infections. In addition, viral infections are able to alter the pattern of dioxin distribution in different organs of the exposed animals. Bovine Herpesvirus type-1 (BHV-1) is a widespread pathogen, which causes infectious rhinotracheitis and infectious pustular vulvovaginitis in cattle. Herein, we have studied the effects of TCDD and BHV-1 infection, in Madin-Darby Bovine Kidney (MDBK) cells, alone as well as in association, so as cellular proliferation, apoptosis, and virus replication. We have observed an increase in cell viability of confluent monolayers at low TCDD concentrations. TCDD treated cells demonstrated increased viability compared to controls as evaluated by MTT test. TCDD exposure increased cell proliferation but induced no changes on apoptosis. Cells exposed to TCDD along with BHV-1 showed a dose-dependent increase in cytopathy, represented by ample syncytia formation with the elimination of the cellular sheets and increased viral titer. These results suggest that TCDD increases viral replication in MDBK cells while BHV-1 further decreases viability of TCDD exposed cells. Since very low concentrations (0.01 pg/ml) are sufficient to augment BHV-1 titer, TCDD may contribute to reactivate BHV-1 from latency, leading to recurrent disease and increase virus transmission. J. Cell. Biochem. 103: 221–233, 2008. © 2007 Wiley-Liss, Inc.
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), commonly known as dioxin, is one of the congeners of polychlorodibenzodioxin, a vast family of organochlorides, which are widespread and persistent environmental contaminants. TCDD exposure, in addition to inducing tumors, teratogenesis, hepatotoxicity, epithelial dysplasia, reproductive toxicity, and thymic atrophy, also causes immunosuppression [Poland and Knutson 1982; Safe, 1986; Mukerjee, 1998]. In the last years, higher levels of TCDD than those permitted in UE [European Commission, 2002] were detected in milk samples from cow, water buffalo, goat, and sheep raised on some areas of Campania Region (South Italy) [Diletti et al., 2003; Santelli et al., 2006], where investigations in some sheeps exposed to high dioxin levels during pasturage have revealed increased chromosome abnormalities [Iannuzzi et al., 2004; Perucatti et al., 2006]. Toxicity studies have evidenced TCDD as the most dangerous compound able to modify cellular mechanisms controlling the growth and development, inducing a large variety of tissue- and species-specific effects in man and animals. The toxic effects so far recorded in human are chloracne and immunosuppression [Mocarelli et al., 1986; Bock, 1994; Kerkvliet 1995; Baccarelli et al., 2005; Bock and Kohle, 2005]. In laboratory animals, the most common non-carcinogenic effects of TCDD are immunosuppression and increased susceptibility to infectious agents [Thigpen et al., 1975; House et al., 1990; Yang et al., 1994]. In particular, several studies have indicated that TCDD exposure leads to an enhanced mortality in mice infected with influenza virus [Burleson et al., 1996], increased gene expression of human immunodeficiency virus type-1 (HIV-1) in chronically infected cells [Gollapudi et al., 1996] and an activation of cytomegalovirus replication in human fibroblasts [Murayama et al., 2002]. Furthermore, Ohata et al. [2003] have observed that TCDD activates HIV-1 replication in OM 10.1 cells, promyelocytic cell line latently infected with HIV-1.
Bovine Herpesvirus type-1 (BHV-1) is an important widespread pathogen, which causes infectious rhinotracheitis (IBR) and infectious pustular vulvovaginitis (IPV). It can provoke hypofertility, abortion, and systemic infections in newborn calves and occasionally neurological diseases such as encephalitis [Gibbs et al., 1977; Jones, 2003]. BHV-1, by affecting the cattle respiratory system, could render the animals more susceptible to secondary bacterial infections, through immunosuppression, leading to pneumonia and occasionally to death [Bielefeldt Ohmann and Babiuk, 1985; Inman et al., 2001; Jones, 2003]. Increased susceptibility to secondary infections correlates with depressed cell-mediated immunity after BHV-1 infection [Griebel et al., 1987; Carter et al., 1989]. BHV-1, like other members of the family of Alphaherpesvirus, is characterized by latency, which is established in sensory ganglionic neurons of an infected host [Ackermann et al., 1982], in which the viral latent state can persist for the lifetime or can periodically be reactivated. Frequently, latent infections could be reactivated by different stress and immunosuppression conditions [Pastoret et al., 1986; Jones, 2003], such as exogenous administration of corticosteroids or elevated levels of natural corticosteroids as a consequence of stress [Sheffy and Davies, 1972; Winkler et al., 2000].
TCDD is a highly immunosuppressive compound that leads a wide range of immunologic effects in experimental animals including host resistance to infectious disease and suppressed humoral and cell-mediated immune responses (as reviewed in Mandal, 2005). Immunosuppression induced by TCDD increases the susceptibility of organisms to bacterial, and viral infections [Thigpen et al., 1975; House et al., 1990; Yang et al., 1994; Fioriti et al., 2005]. Moreover, host resistance to influenza virus has been demonstrated to be diminished in presence of TCDD [Teske et al., 2005]. In particular, experiments with Herpesvirus type II have revealed an increased mortality of TCDD-exposed mice [Clark et al., 1983]. At the same time, viral infections in animals have been shown to change uptake, tissue distribution and toxicity of several environmental pollutants and in some cases cause aggravated diseases. In fact, in a murine model of infection, human coxsackievirus B3 has been shown to cause redistribution of previously accumulated TCDD [Funseth et al., 2000] and to change uptake and tissue distribution of poly-brominated diphenyl ethers [Darnerud et al., 2005].
Actually, in Italy, BHV-1 is widespread and the eradication represent a goal still [G.U., 2004/558/CE; Ackermann and Engels 2006]. Nothing is known about the susceptibility of BHV-1 to TCDD and related compounds. Diletti et al. [2003] have reported that in Campania region, in the years 2001–2003, levels of TCDD exceeding the European Union tolerance were detected also in milk of cow. Based on the above reasons in the present research, we have studied the effects of TCDD on BHV-1 replication in Madin-Darby Bovine Kidney (MDBK), an epithelial-like cell line. In fact, studies that have examined the tumor-promoting activities of dioxin have indicated that epithelial cells are most responsive to the effect of dioxin [Poland et al., 1982]. Moreover, we have studied the effects of TCDD on cell viability, proliferation and on apoptosis in MDBK cells. This in vitro culture system avoids the eventual stress factors often encountered in animals. It is important to note that this is the first report describing the interaction between TCDD and BHV-1 in vitro.
MATERIALS AND METHODS
Materials
MDBK cells (CCL22, American Type Culture Collection) were cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM), supplemented with 2% fetal calf serum (FCS), 1% L-glutamine, 1% penicilline/streptomycine, 0.2% sodium pyruvate, and 0.1% tylosine. All cultures were maintained in an incubator at 37°C (in 5% CO2/95% air). This cell line was maintained free of mycoplasma and of bovine viral diarrhea virus.
The BHV-1 Cooper strain, kindly provided by Prof. Castrucci (University of Perugia, Italy), was used throughout the study. Virus stocks were routinely grown on MDBK cells and were also used for determination of virus titers [De Martino et al., 2003].
TCDD in toluene was purchased from Supelco (St. Louis, MO). All other chemicals were of the highest purity that is commercially available.
Effects of TCDD on Confluent Monolayers of MDBK
Cell viability
MDBK cells (2 × 104 cells/well), in 96-well plates, at confluency, were exposed to TCDD (0.01, 0.1, 1, 10, or 100 pg/ml) and incubated for 24, 48, or 72 h.
Cell viability was assayed by means of the MTT (Sigma, Milan, Italy) assay as previously described [Pagnini et al., 2004]. 3-(4,5-Dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/ml) was added to each well at different times and then incubated for 3 h. At the end of the incubation, the medium was removed and the converted dye was solubilized with acidic isopropanol (0.1N HCl in absolute isopropanol) and was measured at wavelength of 570 nm with background subtraction at 630–690 nm [Mosmann, 1983].
Cell proliferation
MDBK cells (31 × 104 cells/flask) were plated in 25 cm2 flask, after 24 h, at confluency, were exposed to TCDD (0.01, 0.1, 1, 10, and 100 pg/ml) and incubated for 24, 48, or 72 h. At different times of incubation, adherent cells, removed from the culture substrate by treatment with trypsin-EDTA solution, were mixed with cells previously collected by centrifugation in supernatant from the same flask and resuspended at an adequate concentration in PBS. Thus, the entire cell population of the culture was reconstituted for determination of the cell number by counting them in a Burker chamber.
Evaluation of apoptosis
Morphological analysis
MDBK cells in 24-well plates, at confluency, were exposed to TCDD (0.01, 0.1, 1, 10, and 100 pg/ml) and incubated for 24 h and up to 7 days (TCDD medium was changed every 48 h). At different times of incubation, adherent cells cultured on coverslips underwent to morphological analysis following fluorescence staining with acridine orange (Sigma, St. Louis, MO). Cells were collected from the above groups and washed once, resuspended in PBS, then 25 µl of the cell suspension was mixed with 1 µl of a dye mixture containing acridine orange (100 µg/ml) in PBS. One drop of the stained cell suspension was placed on a microscope slide and observed under fluorescence microscope. Briefly, a minimum of 600 cells, including those showing apoptotic characteristics, were counted using a fluorescence microscope. The identification of apoptotic cells was based on the presence of uniformly stained nuclei showing chromatin condensation and nuclear fragmentation. Positive controls were obtained using camptothecin (10 µg/ml) [Darzynkiewicz et al., 1992].
DNA fragmentation assay
Monolayers of MDBK cells were grown in 25 cm2 flask, after 24 h, at confluency, were exposed at all concentrations of TCDD (0.01–100 pg/ml) and incubated for 24 h and up to 7 days (TCDD medium was changed every 48 h). Positive controls were obtained using camptothecin (10 µg/ml), as described above. DNA from treated or not-treated cells was extracted using a commercial Qiagen DNeasy tissue kit (Qiagen S.p.A., Italy), according to the manufacturer's instructions for cultured cells. Five micrograms of each DNA sample was electrophoresed on a 1.5% agarose gel containing 0.1 mg of ethidium bromide per ml. The DNA was visualized under UV light, and the sizes of the respective amplified products were estimated by comparing the mobilities with a 100-bp ladder (Invitrogen, Milan, Italy).
Effects of TCDD in Presence of BHV-1 on Confluent Monolayers of MDBK
Cell viability
MDBK cells in 96-well plates, at confluency, were washed with DMEM and then infected with BHV-1, at multiplicity of infection (MOI) of 0.1, in presence of different concentrations of TCDD (0.01, 0.1, 1, 10, or 100 pg/ml). After 1 h of absorption at 37°C, the cells were incubated for 24, 48, or 72 h and then analyzed by MTT test as described above.
Cytopathic effects (CPE)
MDBK cells in 24-well plates, at confluency, were infected with BHV-1 at MOI 0.1, in presence of different concentrations of TCDD (0.01, 0.1, 1, 10, or 100 pg/ml). At 24, 48, or 72 h post-infection (p.i.) MDBK cells were observed under light microscope to evaluate the cytopathic effects, represented by ample syncytia formation along with elimination of the cellular sheets.
Virus titration
MDBK cells in 24-well plates, at confluency, were infected with BHV-1 at MOI 0.1, in presence of different concentrations of TCDD (0.01, 0.1, 1, 10, or 100 pg/ml). After 24, 48, and 72 h post-infection, cell extracts, obtained by three cycles of freezing and thawing, were collected, and stored in aliquots at −80°C. Virus titers were assayed by TCID50 method according to Reed and Muench [1938].
Statistical analysis
Data are presented as mean ± SEM. The paired Student's t-test was used for comparison between control and experimental groups. P-value <0.05 was considered statistically significant.
RESULTS
Effects of TCDD on Confluent Monolayers of MDBK
Cell viability
Figure 1 shows the results of three independent experiments in which cell viability was evaluated by MTT test. The spectrophotometric analysis of solubilized formazan indicates increased MDBK viability, in confluent monolayer, in presence of different concentrations (0.01, 0.1, 1.0, 10, and 100 pg/ml) of TCDD as compared to untreated controls or those treated with toluene. As shown, cell viability significantly increases in presence of different concentrations of TCDD in a dose-dependent manner respect to untreated controls or toluene treated groups, at all time intervals (24, 48, and 72 h) studied.
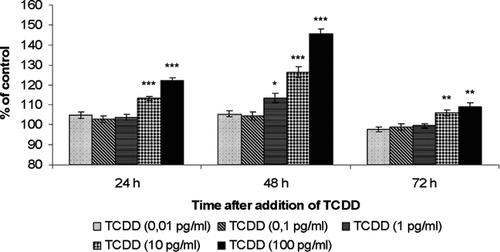
Dose–response curve of MDBK cells treated with different concentrations of TCDD and observed at different time on cell viability. Viable, adherent cells were stained with MTT at different hours of incubation and the absorbance assayed as described in the Materials and Methods Section. Data are presented as a percentage of the control, and results are expressed as the mean ± SE of three independent experiments performed in duplicate. Significant differences between control and TCDD-exposed groups are indicated by probability P. *P < 0.05, **P < 0.01, and ***P < 0.001.
Cell proliferation
Control cells seeded 31 × 104/flask almost doubled in number in 24 h (58 × 104) and reached a plateau giving rise to 83 × 04 and 125 × 104 by 48 and 72 h, respectively. Under treatment with TCDD the number of cells significantly increased at all concentrations of TCDD (0.01, 0.1, 1, 10, and 100 pg/ml) and at all time intervals (24, 48, and 72 h) studied (Fig. 2). TCDD significantly increases cell number at all concentrations used and at all time intervals studied.
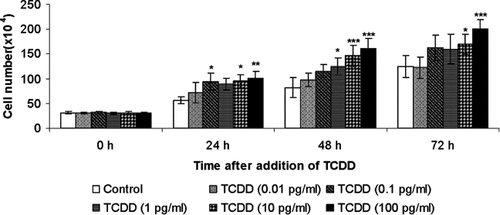
Effect of different concentrations of TCDD on MDBK cell proliferation. MDBK cells were exposed to TCDD (0.01, 0.1, 1, 10, and 100 pg/ml) and incubated for 24, 48, or 72 h. At different times of incubation, the number of cells was evaluated by counting in a Burker chamber. Cells treated with TCDD demonstrated higher proliferation rate than those of controls. Data are presented as mean ± SE of three independent experiments performed in duplicate. Significant differences between control and TCDD-exposed groups are indicated by probability P. *P < 0.05, **P < 0.01, and ***P < 0.001.
Apoptosis
The effect of different concentrations of TCDD (0.01–100 pg/ml) on apoptosis was then evaluated, at 24 h and up to 7 days in MDBK cells, respectively by acridine orange staining (Fig. 3), and DNA laddering (Fig. 4), as described in Materials and Methods Section. In these experiments, cells treated with the apoptosis inducing agent camptothecin (10 µg/ml) was used as positive control. MDBK untreated cells (control) stained with acridine orange after 24 h appear with nuclei in green and cytoplasms in orange red stain (data not shown). Camptothecin treated cells (positive control) show a different monolayer organization. In fact cells are mostly found as clumped groups of cells surrounded by abundant spaces. Positive control shows apoptotic features in which the chromosomes appear condensed in the form of semicircle at the extremity of the nucleus or with bright spheres of nuclei altered in size and morphology, the cytoplasm loses its normal structure and becomes more elongated (Fig. 3B).
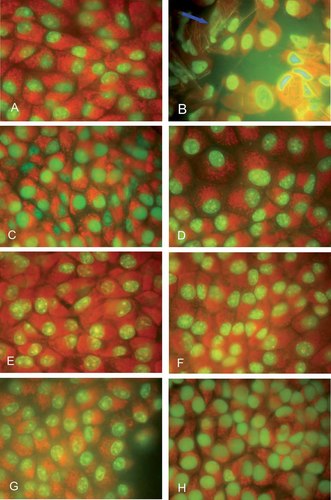
Effects of TCDD on apoptosis—morphological analysis. TCDD does not induce chromatin condensation in MDBK cells. MDBK cells were exposed to TCDD (0.01, 0.1, 1, 10, and 100 pg/ml) and incubated for 24 h and up to 7 days, as described in Materials and Methods Section (magnification, 1000×). At different times of incubation, adherent cells underwent to morphological analysis following staining with acridine orange and then observed under fluorescent microscope. A: Control cells after 7 days of culture and stained with acridine orange showing green nuclei and orange red cytoplasm. B: As a positive control, camptothecin (10 µg/ml) was used to treat cultures of MDBK cells. Arrows point to a nucleus that contains condensed chromatin. C: Cells treated with TCDD (100 pg/ml) do not reveal apoptosis after 24 h treatment. D: Cells treated with TCDD (0.01 pg/ml), (E) 0.1 pg/ml, (F) 1 pg/ml, (G) 10 pg/ml, or with (H) 100 pg/ml do not reveal apoptosis after 7 days of treatment. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
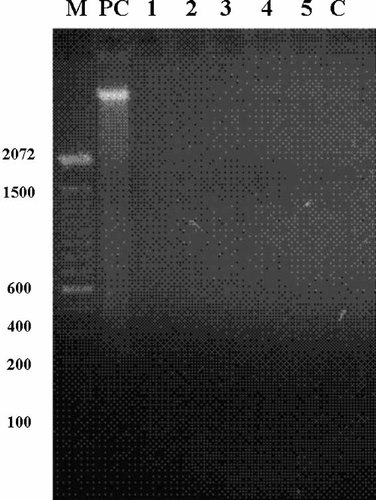
TCDD exposure do not induces DNA degradation. Agarose gel electrophoresis of DNA extracted from MDBK cells at 7 days post-treatment with TCDD (0.01, 0.1, 1, 10, and 100 pg/ml, Lanes 1,2,3,4,5, respectively). Lane C, DNA prepared from control cells; lane PC, DNA extracted from positive control cells (camptothecin—10 µg/ml); lane M, 100-bp ladder. Numbers on the left indicate base pairs. Data from one of three experiments is shown.
In all TCDD treated groups, a higher number of cells are seen, as reported above, even though their nuclei stained green similar to those of the controls and no signs of apoptosis are observed (Fig. 3C).
After 7 days of incubation, no apoptotic cells are seen in control group (Fig. 3A). Cells exposed to TCDD display evident more bright green nuclei than control, especially in groups exposed to 10 and 100 pg/ml of TCDD (Fig. 3G,H). Normal nuclei, after 7 days, are observed in 0.01, 0.1, and 1 pg/ml of TCDD treated cells. In these cells the characteristic nuclear structure is maintained while the cytoplasm appears more abundant in cells treated with 0.1 and 1 pg/ml of TCDD (Fig. 3E,F). No apoptotic cells are noticed in either cases (Fig. 3).
TCDD exposure do not induces DNA degradation, as seen in agarose gel electrophoresis of DNA extracted from MDBK cells exposed to TCDD (0.01, 0.1, 1, 10, and 100 pg/ml) at 24 h (data not shown) and 7 days (Fig. 4).
Effects of TCDD on BHV-1 Infection
Cell viability
In order to evaluate the role of TCDD on BHV-1 infection, MDBK cells were infected with BHV-1 alone or in association with different concentrations of TCDD (0.01, 0.1, 1, 10, or 100 pg/ml) and cell viability was evaluated at different hours p.i. by MTT test, as described in Materials and Methods Section. MDBK cells in monolayer, infected with BHV-1 (at MOI 0.1) after 24 h of plating and subjected to MTT test at 24 h post-infection, slightly (about 1.5%) decreased in number, while further reductions are noticed at 48 (12.52%) and at 72 h (42.32%) post-infection. Under similar experimental conditions, the viability of the cells exposed to different concentrations of TCDD at 24 h post-infection invariably is reduced, in a concentration dependent manner (Fig. 5). While at 48 h post-infection the cell number, without TCDD, decreased, but with TCDD the cell number gradually increased to reach more or less to the level of control culture treated with neither TCDD nor BHV-1. At 72 h post-infection BHV-1 drastically (49.14%) and significantly (P < 0.001) reduced the cell number and addition of TCDD (0.01 pg/ml) further reduced the cell number (P < 0.001), only to increase gradually at higher concentrations (Fig. 5).
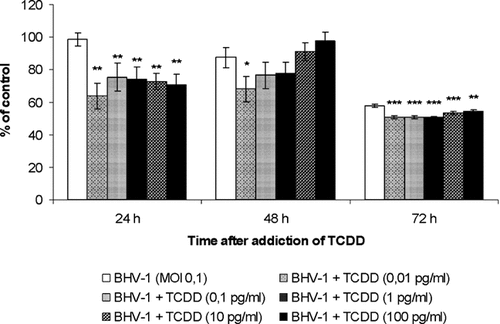
Cell viability of BHV-1-infected cells, treated or not with TCDD at different times p.i. MDBK cells were infected with BHV-1, at MOI 0.1, in presence or not of different concentrations of TCDD (0.01, 0.1, 1, 10, or 100 pg/ml) and then analyzed by MTT test. Data are presented as a percentage of the control, and results are expressed as the mean ± SE of three independent experiments performed in duplicate. Significant differences between BHV-1 infected group not exposed to TCDD and BHV-1 infected groups exposed to TCDD are indicated by probability P. *P < 0.05, **P < 0.01, and ***P < 0.001.
Evaluation of Cytopathic Effects (CPE)
MDBK cells infected with BHV-1 at MOI 0.1, in presence of different concentrations of TCDD (0.01, 0.1, 1, 10, or 100 pg/ml) are observed under a light microscope to evaluate the cytopathic effects at 24, 48, or 72 h p.i. In control untreated cells and those treated with different concentrations of TCDD we failed to see any cytopathic effects up to 72 h of culture. But in those infected with BHV-1 cytopathic effects increased with time. As shown, TCDD significantly increases BHV-1 induced cytopathic effect in MDBK cells in a time-dependent and dose-dependent manner (Fig. 6). In fact, CPE produced by BHV-1 in MDBK cells was characterized by the presence of ample syncytia formation that increased in presence of TCDD, in dose-dependent manner.
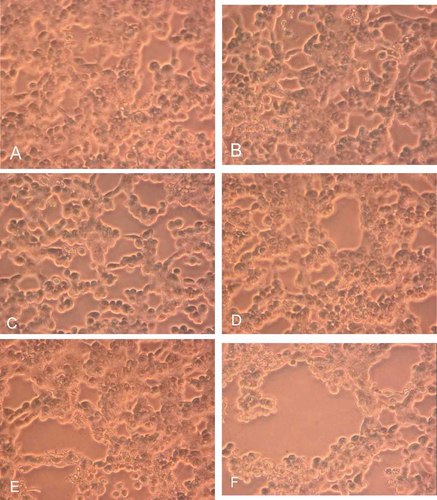
BHV-1 induced cytopathic effects in presence or not of TCDD after 48 h p.i. MDBK cells were infected with BHV-1 at MOI 0.1, in presence of different concentrations of TCDD (0.01, 0.1, 1, 10, or 100 pg/ml), and then observed under a light microscope to evaluate the cytopathic effects (100×). A: Cells infected with BHV-1. B: Cells infected with BHV-1 in presence of TCDD (0.01 pg/ml), (C) 0.1 pg/ml, (D) 1 pg/ml, (E) 10 pg/ml, and (F) 100 pg /ml. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Viral Titers
To understand role of TCDD in the life-cycle of the virus the effect of different concentrations of TCDD (0.01–100 pg/ml) on the multiplication of BHV-1 was examined. Figure 7 shows one-step growth curve of the virus in TCDD-treated and untreated cells. In presence of TCDD, after 24, 48, and 72 h post-infection, we have observed a significant increase of viral titer in treated cells at 0.01 and 100 pg/ml of TCDD (P < 0.001). These results indicate that multiplication of BHV-1 is very sensitive to TCDD treatment in vitro. In particular, we have observed an U-shaped dose responsiveness. In fact, we have observed a significant increase of viral titer in treated cells at 0.01 and 100 pg/ml of TCDD compared to 1 pg/ml of TCDD.
One step growth curve of BHV-1 in TCDD (0.01, 1, and 100 pg/ml) treated and not treated cells. MDBK were infected at MOI 0.1, at the indicated intervals, the amounts of the progeny virus were determined by TCID50 method. Data are presented as mean ± SE of three independent experiments performed in duplicate. Significant differences between BHV-1 infected group not exposed to TCDD and BHV-1 infected groups exposed to TCDD are indicated by probability P. *P < 0.05, **P < 0.01, and ***P < 0.001.
DISCUSSION
TCDD, one of the most potent environmental toxins, has been observed to produce a variety of cellular and molecular effects chiefly through its binding with arylhydrocarbon receptors present on cell surface. These effects range from molecular to morphological changes of the cells. The results presented herein show that TCDD alters cell viability of the MDBK cells. In fact, in MDBK cells exposed to increasing concentrations of TCDD (0.01–100 pg/ml), the viability of MDBK cells increases with increasing TCDD concentrations (Fig. 1). Several hypothesis could be proposed on the action of TCDD on the viability of MDBK. Certainly the high affinity binding of TCDD with the aryl receptor also activates the protein transcription involved in growth and differentiation of cells similar to that of activator/inhibitor plasminogen 2 (PAI-2) and interleukin-1 beta (IL-1 beta) [Bock, 1994]. For instance, through a mechanism of activation similar to that of PAI -2 or IL-1 beta induced by TCDD or an action on the genes that regulate cell cycle or on the expression of specific genes related to DNA replication. MTT test, used in the present study, which measures mitochondrial function, is a very sensitive indicator of cell function. Furthermore, we observed an increase of cell proliferation of MDBK, in presence of TCDD (Fig. 2). The basic reason for increased cell proliferation induced by TCDD could be due to the up-regulation of certain key genes related to this process as demonstrated by Ahn et al. [2005] in breast epithelial cells in vitro. Thus, increased cell viability of MDBK in presence of TCDD could be due to an increased capacity of replication of MDBK cells after 24, 48, and 72 h of incubation. It is well known that TCDD increases cell proliferation in hepatocytes and keratinocytes [as reviewed in Whysner and Williams, 1996], while other studies present contradictory results. In rat hepatocytes, TCDD increases proliferation [Moolgavkar et al., 1996], but decreased proliferation has been encountered in same type of cells by Wolfle et al. [1993]. Similarly TCDD produces an increased proliferation of human keratinocytes [Milstone and LaVigne, 1984] and of neonatal epidermal keratinocytes [Ray and Swanson, 2003]. Furthermore, TCDD increased proliferation in rat liver epithelial WB-F344 cells [Chramostova et al., 2004]. Whereas, in human keratinocytes [Loertscher et al., 2001] and in SK-N-SH human neuronal cells [Jin et al., 2004] TCDD provokes reduction of cell numbers. Murayama et al. [2002] have failed to find any effects of TCDD on the proliferation of human fibroblasts. Studies that have examined the tumor-promoting activities of dioxin have indicated that epithelial cells are most responsive to the effect of dioxin [Poland et al., 1982]. It is also known that late gestational ureteric cells respond to TCDD in vitro with the stimulation of epithelial cell growth and differentiation [Bryant et al., 2001]. In the present study, we have observed that TCDD influences replication cycle of MDBK cells, an epithelial-like cell line, increasing cell proliferation, in a dose-dependent manner.
We have demonstrated that no signs of apoptosis are observed in MDBK cells exposed to different concentration of TCDD (0.01–100 pg/ml), for 24 h and for 7 days, by acridine orange staining and DNA laddering (Figs. 3 and 4). Apoptosis, a highly regulated cell death process is characterized by biochemical and morphological events that lead to the elimination of excess cells, those abnormally developed or genetically modified [Duvall et al., 1985]. It has been demonstrated that apoptosis is not only involved in defending cells from viral infections but also it occurs as a response to other forms of stress, like thermic shock, oxidation or presence of “misfolded protein” [Vaux, 2002]. Exposition to environmental contaminants, like TCDD, in vitro, leads to contrasting effects on apoptosis. In fact, while in AtT-20 pituitary cells TCDD induces cell death with morphological and biochemical changes due both to apoptotic and necrotic cell death [Huang et al., 2005], TCDD has effects on apoptosis neither in mouse primary hepatocytes nor in human primary keratinocytes, however TCDD suppressed the apoptotic process in rat primary hepatocytes [as reviewed in Schwarz et al., 2000] and it induced apoptosis in lymphoblasts T, in the process of human leukemia [Hossain et al., 1998]. The observation that TCDD has no effects on apoptosis in our present experiments is in accord with several other authors, as reported above. Loertscher et al. [2001] have also reported that TCDD causes a reduction in normal human keratinocytes, without inducing apoptosis. It is also known that tumor-promoting agents such as dioxin are thought to counteract tumor-suppressive mechanisms by altering events that favor proliferation while inhibiting differentiation and/or apoptosis and senescence [Ray and Swanson, 2003].
In this study, we have observed the effects of TCDD on replication of BHV-1 in MDBK cells. The results presented herein show that TCDD alters cell viability of the BHV-1 infected cells (Fig. 5). In BHV-1 infected cells, as expected, the percentage of viable cells decreased as a function of time as determined by using MTT test to monitor viable cells. In MDBK cells, infected with BHV-1, TCDD (0.01–100 pg/ml) reduces the cell viability as compared to MDBK cells infected with BHV-1, but not treated with TCDD. Furthermore, the monolayers of BHV-1 infected MDBK, at 48 h post-infection, reveal increased cytopathic effects after incubation with TCDD as compared to controls in a time and dose-dependent manner (Fig. 6). Similarly, studies on human fibroblasts have indicated increased CPE by cytomegalovirus in presence of low doses of TCDD [Murayama et al., 2002]. One-step growth curve of BHV-1 in TCDD treated MDBK cells showed a significant increase of viral titer, in presence of TCDD. Virus titers increased at all the concentrations used (0.01–100 pg/ml), even though only results obtained with 0.01, 1, and 100 pg/ml of TCDD are presented herein (Fig. 7) indicating a modulation of BHV-1 replication in presence of TCDD with an U-shaped dose responsiveness in virus titer. These results are consistent with a recent study that reported an increase of cytomegalovirus replication in infected human fibroblasts with a concomitant increase in viral DNA replication with an U-shaped dose responsiveness in virus titer [Murayama et al., 2002]. An increase in viral gene expression in HIV-1 in cells chronically exposed to TCDD has been also reported by Gollapudi et al. [1996]. Particularly, Ohata et al. [2003] have observed that TCDD activates HIV-1 replication in OM 10.1 cells, promyelocytic cell line latently infected with HIV-1, indicating that exposure of HIV-1 infected patients to environmental pollutants such as TCDD may act as a risk factor for disease progression. It has been observed that TCDD exposure leads to an enhanced mortality in mice infected by influenza virus, but increased mortality was not correlated with increased virus titers in animal lungs [Burleson et al., 1996]. It is well known that dioxin provokes an increased susceptibility to bacteria and viruses [Thigpen et al., 1975; Clark et al., 1983; Burleson et al., 1996] leading to diminished resistance to infective agents and general immunosuppression [House et al., 1990; Yang et al., 1994]. Several hypotheses can be made on the increased BHV-1 titer observed. For example, it could be related to the increased MDBK proliferation induced by TCDD or could be due to the increased replication of the viral DNA. It is also probable that the increase of BHV-1 titer, in presence of TCDD, could be due to the activation of the extracellular signal-regulated kinase (ERK) signaling pathways, known to be involved in the increase of pseudorabies virus production, mediated by trypsin [Riteau et al., 2006]. In fact, it is also known that TCDD activates a variety of cellular signaling pathways, including ERK [Tan et al., 2004; Ahn et al., 2005]. In particular, Ahn et al. [2005] have demonstrated that TCDD activates ERK2 and increases ERK2 protein level in inverted U-shaped dose–response manner.
Recently, have been reported higher levels of TCDD than the maximum law limits in cow milk [Diletti et al., 2003] in Campania region, where BHV-1 represent a widespread pathogen. Since very low (0.01 pg/ml) concentrations are sufficient to augment BHV-1 titer, TCDD may contribute to reactivate BHV-1 from latency, leading to recurrent disease and virus transmission and these effects must be taken into account in the care of farm animal health.
Acknowledgements
This work was supported by grants by Regione Campania for the project entitled: “Contaminanti organici persistenti nell'ambiente: Studio di coorte sullo stato sanitario e sui livelli di accumulo nel latte materno in gruppi di popolazione a differente rischio di esposizione nella Regione Campania.” A.F., A.R., and A.G. were supported by Sbarro Health Research Organization.




