Hydrogen peroxide and endothelin-1 are novel activators of betacellulin ectodomain shedding
Abstract
The betacellulin precursor (pro-BTC) is a novel substrate for ADAM10-mediated ectodomain shedding. In this report, we investigated the ability of novel physiologically relevant stimuli, including G-protein coupled receptor (GPCR) agonists and reactive oxygen species (ROS), to stimulate pro-BTC shedding. We found that in breast adenocarcinoma MCF7 cells overexpressing pro-BTC, hydrogen peroxide (H2O2) was a powerful stimulator of ectodomain shedding. The stimulation of pro-BTC shedding by H2O2 was blocked by the broad-spectrum metalloprotease inhibitor TAPI-0 but was still functional in ADAM17 (TACE)-deficient stomach epithelial cells indicating the involvement of a distinct metalloprotease. H2O2-induced pro-BTC shedding was blocked by co-culturing cells in the anti-oxidant N-acetyl-L-cysteine but was unaffected by culture in calcium-deficient media. By contrast, calcium ionophore, which is a previously characterized activator of pro-BTC shedding, was sensitive to calcium depletion but was unaffected by co-culture with the anti-oxidant, identifying a clear distinction between these stimuli. We found that in vascular smooth muscle cells overexpressing pro-BTC, the GPCR agonist endothelin-1 (ET-1) was a strong inducer of ectodomain shedding. This was blocked by a metalloprotease inhibitor and by overexpression of catalytically inactive E385A ADAM10. However, overexpression of wild-type ADAM10 or ADAM17 led to an increase in ET-1-induced pro-BTC shedding providing evidence for an involvement of both enzymes in this process. This study identifies ROS and ET-1 as two novel inducers of pro-BTC shedding and lends support to the notion of activated shedding occurring under the control of physiologically relevant stimuli. J. Cell. Biochem. 99: 609–623, 2006. © 2006 Wiley-Liss, Inc.
Abbreviations used:
ADAM, a disintegrin and metalloprotease; APMA, p-aminophenylmercuric acetate; AR, amphiregulin; BTC, betacellulin; CM, conditioned media; DMEM, Dulbecco's modified Eagle's medium; DMSO, dimethyl sulfoxide; EGF, epidermal growth factor; EIA, enzyme-linked immunoassay; ER, epiregulin; ET-1, endothelin-1; FBS, fetal bovine serum; GPCR, G-protein coupled receptor; HB-EGF, heparin-binding EGF-like growth factor; HRP, horse radish peroxidase; MMP, matrix metalloprotease; NRG, neuregulin; PBS-T, phosphate buffered saline-tween; PSF, penicillin/streptomycin/fungizone; ROS, reactive oxygen species; SD, standard deviation; TACE, tumor necrosis factor-α-converting enzyme; TBS-T, Tris-buffered saline-tween; TGFα, transforming growth factor α; VSMC, vascular smooth muscle cell.
Betacellulin (BTC) belongs to the epidermal growth factor (EGF)-like family of cytokines. Other mammalian EGF-like factors include EGF, transforming growth factor α (TGFα), heparin-binding EGF-like growth factor (HB-EGF), epiregulin (ER), amphiregulin (AR), epigen and a complex family of neuregulins (NRGs) [Dunbar and Goddard, 2000]. BTC and other EGF-like growth factors are synthesized as transmembrane proforms with an extracellular N-terminal ectodomain and a cytoplasmic C-terminus. The ectodomain is proteolytically cleaved to release a mature soluble factor in a process called ectodomain shedding [Sanderson et al., 2005]. The shed BTC ectodomain mediates diverse cellular responses such as proliferation, growth inhibition, migration, and differentiation [Dunbar and Goddard, 2000] via the binding of ErbB receptors, in particular ErbB1 (EGFR) and ErbB4 (HER4) [Olayioye et al., 2000].
Both genetic and biochemical experiments have implicated metalloprotease subtypes of the ADAM (a disintegrin and metalloprotease) [Blobel, 1997], membrane type matrix metalloprotease (MT-MMP) [Schlondorff et al., 2001], and matrix metalloprotease (MMP) [Li et al., 2002] families in the ectodomain shedding of a variety of transmembrane proteins. Previously, we and others have identified ADAM10 as the sole mediator of pro-BTC shedding in a range of different cell lines [Hinkle et al., 2004; Sahin et al., 2004; Sanderson et al., 2005]. This distinguishes BTC from other EGF-like factors including TGFα, HB-EGF, ER, AR, and several NRG isoforms which are predominantly ADAM17 substrates [Montero et al., 2000; Hinkle et al., 2004; Sahin et al., 2004; Horiuchi et al., 2005].
Shedding of pro-BTC and other EGF-like factors is up-regulated by chemical stimuli including the calcium ionophore A23187 and the metalloprotease activator APMA [Pandiella and Massague, 1991; Merlos-Suarez et al., 2001; Sanderson et al., 2005]. In addition, phorbol 12-myristate 13-acetate (PMA) exposure increases ectodomain shedding of most EGF-like factors with the exception of pro-BTC and possibly pro-EGF [Le Gall et al., 2003; Hinkle et al., 2004; Sahin et al., 2004; Sanderson et al., 2005]. PMA in particular remains one of the most widely scrutinized experimental activators of ectodomain shedding. However, for the most part, the physiological relevance of such stimuli to activated shedding events that may occur in vivo is poorly understood. On the other hand, endogenous or environmental stimuli such as G-protein coupled receptor (GPCR) agonists [Prenzel et al., 1999], cytokines [Tanida et al., 2004], cell adhesion [Gechtman et al., 1999], irradiation [Takenobu et al., 2003], reactive oxygen species (ROS) [Zhang et al., 2001], carcinogens [Richter et al., 2002], wounding [Xu et al., 2004], or infectious agents [Lemjabbar and Basbaum, 2002] can also stimulate shedding of transmembrane molecules and these enhanced shedding events are of physiological relevance.
It has become apparent that ectodomain shedding stimuli lead to transactivation of ErbB receptors. This is a process whereby stimuli increase the activity of sheddases, such as ADAMs [Prenzel et al., 1999] or MMPs [Hao et al., 2004], leading to EGF-like factor shedding and subsequent binding of the shed ectodomain to ErbB receptors, in either an autocrine or paracrine fashion. In most cases this leads to cellular effects such as proliferation, migration, or inhibition of apoptosis [Fischer et al., 2003; Zhang et al., 2004]. Transactivation has been most widely described with HB-EGF and TGFα [Wetzker and Bohmer, 2003] but in at least one report the glucagon-like peptide 1 (GLP-1) was proposed to stimulate pro-BTC shedding and ErbB1 transactivation at the surface of insulinoma cells [Buteau et al., 2003]. In some cases, transactivation of ErbB receptors in response to stimuli has been linked to pathophysiological processes such as atherosclerosis, chronic kidney disease, and cancer [Higashiyama, 2004; Lautrette et al., 2005].
In this report, the action of physiologically relevant stimuli such as GPCR agonists and ROS on the rate of metalloprotease mediated pro-BTC ectodomain shedding was investigated. We identify endothelin-1 (ET-1) and hydrogen peroxide (H2O2) as novel pro-BTC shedding stimuli. In addition, we show that H2O2 and calcium ionophore (A23187) are unique in their mechanisms of metalloprotease activation by relying on ROS and calcium influx, respectively. We also identify ADAM10 and ADAM17 as ET-1-activated pro-BTC sheddases, providing the first cellular evidence that ADAM17 may be involved in pro-BTC processing. These findings indicate that stimuli which relate to normal and pathophysiological processes dependent on ET-1 and ROS may function in part via enhanced shedding of ErbB ligands such as BTC.
EXPERIMENTAL PROCEDURES
Materials
Anti-human BTC ectodomain Asp32–Tyr111 polyclonal (antigen purified) antibody, mouse anti-human BTC ectodomain antibody, and recombinant human BTC were purchased from R&D Systems (Minneapolis, MN). A23187, phorbol 12-myristate 13-acetate (PMA), p-aminophenylmercuric acetate (APMA), and N-acetyl-L-cysteine were purchased from Sigma-Aldrich. TAPI-0 was purchased from Peptides International (Louisville, KY). GM6001, the MMP2/MMP9 inhibitor (2R)-2-[(4-biphenylylsulfonyl) amino]-3-phenylpropionic acid, the MMP3 inhibitor N-isobutyl-N-(4-methoxyphenylsulfonyl) glycylhydroxamic acid and all other inhibitors were purchased from Calbiochem (Merck, Darmstadt, Germany). ET-1 was obtained form Bachem (Torrance, CA).
Generation of an Antibody Against the Human Pro-BTC C-Terminus
New Zealand white rabbits were injected with the peptide DITPINEDIEETNIA corresponding to the human pro-BTC C-terminal residues Asp164–Ala178. The peptide was generated by Mimotopes Pty Ltd (Victoria, Australia) and was conjugated to the Diphtheria Toxoid super antigen [Del Giudice et al., 1998]. Conjugation required the additional presence of a cysteine residue at the N-terminus of the peptide. The first immunization was performed by subcutaneous injection of 300 µg of peptide conjugate mixed with 600 µl of Freund's complete adjuvant (Sigma-Aldrich). Subsequent immunizations were performed at 2-week intervals with 300 µg of peptide conjugant in sterile water. Following four immunizations, rabbits were bled for serum collection. Antibody generation was approved by the Animal Ethics Committee of the Women's and Children's Hospital, Adelaide, SA, Australia.
Cell Culture and Harvesting of Conditioned Media and Cell Lysates
The MB52 cell line is derived from the stable transfection of MCF7 human breast epithelial adenocarcinoma cells with the human pro-BTC cDNA [Tada et al., 1999]. Generation and propagation of wild-type and ADAM17ΔZn/ΔZn conditionally immortalized stomach epithelial (STOM) cells transduced with a retroviral vector for expression of C-terminally HA tagged pro-BTC have been described previously [Sanderson et al., 2005]. Rodent vascular smooth muscle cells (VSMCs) were obtained from Dr. Satoru Eguchi (Temple University School of Medicine, PA) and were transduced with the same pro-BTC retroviral vector as described [Sanderson et al., 2005]. MB52 and VSMCs were cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and penicillin/streptomycin/fungizone (PSF).
CM was harvested and then centrifuged at 12,000g to remove particulates. Soluble/shed BTC in CM was detected using the BTC EIA or ELISA and/or Western blot (see below). Cells were lysed directly in 2 × Tris-tricine PAGE loading buffer (0.9 M Tris-HCl, 24% Glycerol, 8% SDS, 0.015% Coomassie blue G, 5% β-mercaptoethanol, pH 8.45). Lysates were then heated at 95°C for 5 min, sheared five times through a 25 × 1″ gauge needle, and then centrifuged at 12,000g for 10 min to remove insoluble material. Cell lysates were used in Western blots.
BTC Enzyme-Linked Immunoassay (EIA) and ELISA
In experiments in which two or more alternate cell lines were used, soluble/shed BTC in CM was detected using the BTC ELISA as described previously [Sanderson et al., 2005]. Using this assay, levels of pro-BTC expression could also be determined in cell lysates and results showing levels of soluble/shed BTC in CM could be standardized based on this determination. In experiments involving a single cell line, the BTC EIA was sufficient to measure levels of soluble/shed BTC in CM. This assay was performed as described below. Ninety microliters of CM was mixed with 10 µl of 10 × EIA coating buffer (0.15 M Na2CO3, 0.35 M NaHCO3, pH 9.3) and loaded into 96-well immunosorbent plates. Plates were coated overnight at 47°C and then blocked in 2% BSA in phosphate buffered saline-tween (PBS-T) (136 mM NaCl, 2.6 mM KCl, 1.5 mM KH2PO4, 8 mM Na2HPO4, pH 7.4, 0.1% Tween-20). The primary antibody (anti-human BTC ectodomain Asp32–Tyr111) was used at 0.2 µg/ml dilution and secondary HRP conjugated rabbit anti-goat antibody used at 1/1,000, diluted in PBS-T. Plates were washed four times with PBS-T between antibody additions. EIAs were developed with OPD substrate (1 mg/ml o-phenylamine diamine) in citrate-phosphate buffer (35 mM citric acid, 67 mM Na2HPO4, pH 5.0, plus 0.03% H2O2) and stopped with 2 N H2SO4. Absorbance was read at 490 nm. Results are expressed as the mean percentage levels of BTC in CM compared to controls +/− standard deviation (SD) of triplicate determinations.
Concentration of BTC From Conditioned Media
The levels of soluble/shed BTC in CM were not sufficient to be detected by Western blot; therefore, BTC was precipitated using heparin-agarose beads as a means of concentration. Cells were grown to confluence in DMEM/10% FBS/PSF, then transferred to serum-free DMEM and cultured for 24 and 48 h before CM collection. BTC was precipitated from CM by addition of 20 µl of heparin-agarose (50% slurry). This was incubated overnight at 4°C and then pellets were collected by centrifugation at 10,000g for 10 min at 4°C. Pellets were washed three times with 1 ml of 20 mM Tris pH 7.5, and then centrifuged as above. BTC was eluted from the pellets by heating at 95°C for 5 min in 2 × Tris-tricine PAGE loading buffer prior to Western blotting.
Western Blotting
Cell lysates and CM were run on 10–20% gradient Tris-tricine polyacrylamide gels (Invitrogen). Proteins were then transferred to Hybond-C extra nitrocellulose (Amersham Pharmacia Biotech). Membranes were then blocked in 5% non-fat milk in Tris-buffered saline-tween (TBS-T) (25 mM Tris-HCl, 500 mM NaCl, pH 7.5, 0.1% Tween-20). Anti-human BTC ectodomain Asp32–Tyr111 was used at 0.2 µg/ml dilution and anti-BTC C-terminus Asp164–Ala178 serum was used at a 1/500 dilution. Secondary-horse radish peroxidase (HRP) conjugated antibodies were used at a 1/2,500 dilution. Following primary and secondary antibody additions, membranes were washed four times for 5 min with TBS-T. Blots were developed using Supersignal West Dura Extended Duration Substrate (Pierce, Milwaukee, WI). In some cases, nitrocellulose membranes were stripped by immersing in stripping buffer (60 mM Tris-HCl, 2% SDS, 1% β-mercaptoethanol, pH 6.8) at 65°C for 30 min, then blocked and re-probed with another antibody.
Inhibition of Constitutive Pro-BTC Shedding
For determination of the effect of protease inhibitors on constitutive shedding, cells were seeded into 6-well (VSMCs) or 24-well (MB52 cells) plates and grown to confluence. Cells were then cultured for 24 or 48 h in serum-free DMEM plus either the vehicle (DMSO) or protease inhibitor. Cell lysates and CM were harvested and analyzed by Western blot and the BTC EIA or ELISA.
Activation of Pro-BTC Shedding
For investigation of the effect of activators on pro-BTC shedding, cells were grown to confluence in 24-well (MB52 cells) or 6-well (STOM cells) plates. Cells were serum-starved for 2 h then cultured for 4 h in DMEM with either vehicle (DMSO) or an activator of shedding. Pre-incubations of cells with protease inhibitors, N-acetyl-L-cysteine or calcium-free DMEM were performed for 30 min prior to addition of shedding activators. For investigation of the effect of GPCR agonists on pro-BTC shedding in VSMCs, cells were grown to confluence in 6-well plates. Cells were then incubated twice in 3 ml serum-free DMEM for 24 h (total 48 h). This was performed in order to make cells quiescent by serum deprivation. Cells were then cultured in 900 µl DMEM for 30 min and then activated by direct addition of 100 µl of a 10 × solution of shedding activators to the culture medium. Cells were then incubated for 1 h prior to collection of CM and cell lysates for analysis using the BTC ELISA.
RESULTS
Characterization of Pro-BTC Ectodomain Shedding in MB52 Cells
We have previously identified the calcium ionophore A23187 and the metalloprotease activator APMA as stimuli that activate the ADAM10-dependent shedding of pro-BTC in conditionally immortalized cell lines [Sanderson et al., 2005]. We investigated here the possibility that ectodomain shedding of pro-BTC would occur in response to physiologically relevant stimuli. We used MB52 cells which are transfected to overexpress human pro-BTC [Tada et al., 1999] and initially performed experiments to determine if the ectodomain shedding of pro-BTC was functional in these cells.
We used a commercial anti-BTC ectodomain Asp32–Tyr111 antibody and an anti-BTC C-terminus Asp164–Ala178 antibody, which we generated, in order to characterize the BTC protein bands in MB52 cells lysates. The specificity of the anti-BTC C-terminus Asp164–Ala178 antibody was confirmed by its ability to detect, by Western blot, pro-BTC in MB52 cells (transfected with pro-BTC) but not in the parental MCF7 cells (data not shown). Consistent with previous reports, five characteristic BTC bands of 40, 30, 25, 19, and 12 kDa were identified by Western blot analysis of MB52 cell lysates using the anti-BTC C-terminus Asp164–Ala178 antibody (Fig. 1A) [Shing et al., 1993; Watanabe et al., 1994; Sanderson et al., 2005]. However, only the four largest bands were detected using the anti-BTC ectodomain Asp32–Tyr111 antibody, indicating that the smallest 12 kDa band was the BTC cytoplasmic remnant which is generated following ectodomain shedding.
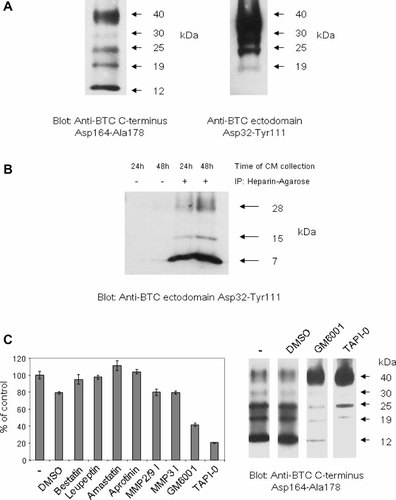
Characterization of pro-BTC ectodomain shedding in MB52 cells. A: MB52 cell lysates were analyzed by Western blot using the anti-BTC C-terminus Asp164–Ala178 antibody (left) and anti-BTC ectodomain Asp32–Tyr111 antibody (right). The anti-BTC ectodomain Asp32–Tyr111 antibody blot was overexposed in order to detect the 19 kDa cellular pro-BTC form. This band was weakly detected by the anti-BTC ectodomain Asp32–Tyr111 antibody indicating that it most likely contains less of the ectodomain sequence than the larger 40 kDa pro-BTC species, owing to N-terminal proteolysis. B: MB52 cells were cultured in DMEM for 24 and 48 h and CM collected. Soluble/shed BTC ectodomains were concentrated using heparin-agarose beads. The molecular sizes of neat or concentrated BTC ectodomains in CM was analyzed by Western blot with the anti-BTC ectodomain Asp32–Tyr111 antibody. C: MB52 cells were cultured for 48 h in the presence or absence of DMSO (vehicle) or inhibitors of aminopeptidases (bestatin or amastatin at 10 µM) [Ino et al., 2000; Scornik and Botbol, 2001], serine and cysteine proteases (leupeptin at 100 µM) [McConnell et al., 1993], trypsin, chymotrypsin, kallikrein, and plasmin (aprotinin at 2 µg/ml) [Trautschold et al., 1966], MMP2 and MMP9 ((2R)-2-[(4-biphenylylsulfonyl) amino]-3-phenylpropionic acid at 10 µM) [Tamura et al., 1998], MMP3 (N-isobutyl-N-(4-methoxyphenylsulfonyl) glycylhydroxamic acid at 10 µM) [MacPherson et al., 1997] or the broad-spectrum metalloprotease inhibitors GM6001 (10 µM) [Hao et al., 1999] or TAPI-0 (50 µM) [Mohler et al., 1994]. Levels of soluble/shed BTC ectodomains in CM were detected by EIA using the anti-BTC ectodomain Asp32–Tyr111 antibody (left). Results are expressed as mean ± SD of triplicate determinations. Lysates were collected from MB52 cells cultivated for 48 h in serum-free DMEM with or without DMSO (vehicle), GM6001 (50 µM), or TAPI-0 (50 µM) and the degree of pro-BTC ectodomain shedding analyzed by Western blot using the anti-BTC C-terminus Asp164–Ala178 antibody.
BTC shedding was confirmed by analysis of shed/soluble BTC isoforms in CM. Three BTC ectodomains (28, 15, 7 kDa) were identified using the anti-BTC ectodomain Asp32–Tyr111 antibody (Fig. 1B). The inability to detect these soluble forms with an anti-BTC C-terminus Asp164–Ala178 antibody indicated that these BTC species are most likely generated by ectodomain shedding (data not shown). Similar soluble BTC forms have been identified previously in the CM of mouse A9 fibroblast cells transfected with pro-BTC [Watanabe et al., 1994]. The 7 kDa shed BTC isoform was by far the most abundantly represented species. This is in stark contrast to results in conditionally immortalized cells in which the 28 kDa shed BTC species is virtually represented alone in CM [Sanderson et al., 2005].
To determine the involvement of different protease subtypes in pro-BTC shedding in MB52 cells, cells were cultured for 48 h in serum free DMEM with or without protease inhibitors and the level of shed BTC in CM evaluated using the BTC EIA. Only the broad-spectrum metalloprotease inhibitors GM6001 and TAPI-0 reduced the level of BTC ectodomains detected in CM (Fig. 1C, left panel). This was found to occur in a dose-dependent fashion with a peak inhibition between 50–100 µM for each compound (data not shown). Accordingly, Western blot analysis indicated that incubation with GM6001 or TAPI-0 resulted in a decrease in the level of the 12 kDa BTC cytoplasmic remnant as well as the 25 and 19 kDa pro-BTC forms, which are generated by N-terminal processing of the 40 kDa pro-BTC form (Fig. 1C, right panel) [Sanderson et al., 2005]. Concurrently, there was an accumulation of the major cell surface 40 kDa pro-BTC form. Together these results indicate that in MB52 cells, pro-BTC undergoes efficient metalloprotease-dependent ectodomain shedding and that these cells are a suitable experimental system for investigation of this process.
Metalloprotease-Dependent Pro-BTC Shedding is Activated by Hydrogen Peroxide
Whilst the ability of pharmacological agents including A23187 and APMA to induce pro-BTC ectodomain shedding in vitro has been clearly established [Sanderson et al., 2005], the role of physiological stimuli is unclear. Hydrogen peroxide (H2O2) is a generator of oxidative stress and is involved in a variety of physiological processes such as signal transduction through GPCRs and growth factor receptors as well as pathophysiological processes such as cancer, angiogenesis, and hypertension [Slupphaug et al., 2003; Touyz, 2003; Touyz et al., 2004; Ushio-Fukai and Alexander, 2004]. Short-term (4 h) treatment of MB52 cells with 1 mM H2O2 led to an increase in levels of soluble/shed BTC ectodomains in CM (Fig. 2A), as measured using the BTC EIA. This effect was approximately twofold higher than that observed for the previously established pro-BTC shedding stimuli APMA and A23187. Treatment with all stimuli led to a decrease in levels of the 40 kDa pro-BTC isoform and an increase in the level of the 12 kDa cytoplasmic remnant, indicating that pro-BTC shedding was activated (Fig. 2B). Levels of the 25 kDa pro-BTC band were also increased by pro-BTC shedding activators. This indicates that unlike in conditionally immortalized cells, these stimuli activate N-terminal proteolysis of the 40 kDa major pro-BTC form to a similar extent to the activation of ectodomain shedding in MB52 cells.
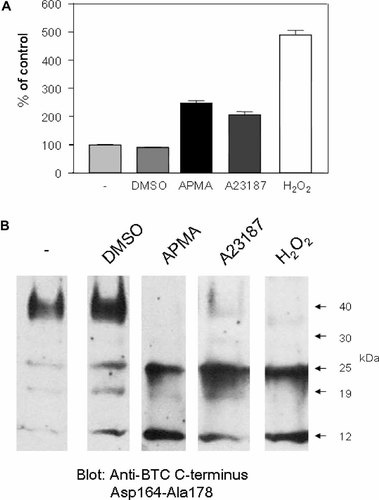
The novel stimuli hydrogen peroxide activates pro-BTC shedding. A: MB52 cells were cultured in serum-free DMEM in the presence or absence of DMSO (vehicle), APMA (0.5 mM), A23187 (1 µM), or H2O2 (1 mM) for 4 h and then level of soluble/shed BTC in CM was detected by EIA using the anti-BTC ectodomain Asp32–Tyr111 antibody. Results are expressed as the mean ± SD of triplicate determinations. B: Cell lysates from the treatment described in A were harvested and analyzed by Western blot using the anti-BTC C-terminus Asp164–Ala178 antibody.
Pre-incubation of MB52 cells with the broad-spectrum metalloprotease inhibitor TAPI-0, but not specific inhibitors of MMP2 and MMP9 or MMP3, blocked the shedding of BTC ectodomains into CM as measured using the BTC EIA (Fig. 3A). Consistent with an inhibition of ectodomain shedding, Western analysis of lysates using the anti-BTC C-terminus Asp164–Ala178 antibody showed that the decrease in levels of the 40 kDa pro-BTC fragment, characteristic of activated pro-BTC shedding, in response to H2O2 was impaired in the presence of TAPI-0 (Fig. 3B).
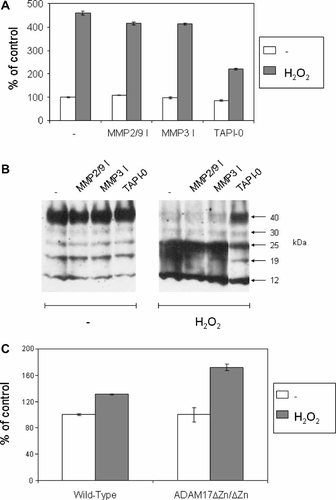
H2O2-activation of pro-BTC shedding involves a metalloprotease other than ADAM17. A: MB52 cells were pre-incubated in serum-free DMEM with or without DMSO, MMP2/9 inhibitor (10 µM), MMP3 inhibitor (10 µM), or TAPI-0 (50 µM). The specificity of each of these inhibitors is described in the legend to Figure 1. The BTC-shedding activator H2O2 was then added directly to the culture media to a final concentration of 1 mM and cells cultured for a further 4 h. Levels of soluble/shed BTC in CM was determined using the BTC EIA with the anti-BTC ectodomain Asp32–Tyr111 antibody. Results are expressed as the mean ± SD of triplicate determinations. B: Cell lysates from the treatment described in A were harvested and analyzed by Western blot using the anti-BTC C-terminus Asp164–Ala178 antibody. C: STOM cells expressing pro-BTC from wild-type and ADAM17ΔZn/ΔZn mutant mice were cultured in serum-free DMEM with or without 100 µM H2O2 for 4 h. CM was collected and levels of shed/soluble BTC ectodomains measured using the BTC ELISA. Results are expressed as the mean ± SD of quadruplicate determinations.
The metalloprotease ADAM17 is responsible for ectodomain shedding of most EGF-like factors under constitutive and activated conditions [Sunnarborg et al., 2002; Sahin et al., 2004]. In addition, several studies have shown that ADAM17 is directly activated by ROS and subsequently mediates shedding of transmembrane molecules including TNFα and the TNF receptor [Hino et al., 1999; Zhang et al., 2001; Pietri et al., 2005]. We investigated the role of ADAM17 in H2O2-induced shedding of pro-BTC using conditionally immortalized stomach epithelial cells (STOM) from wild-type and ADAM17ΔZn/ΔZn mice transduced with a retroviral construct for expression of pro-BTC [Sanderson et al., 2005]. These STOM ADAM17ΔZn/ΔZn cells carry a targeted deletion in the catalytic zinc-binding domain of ADAM17 and have previously been used to directly link ADAM17 with the ectodomain shedding of transmembrane molecules such as VCAM-1 [Garton et al., 2003] and fractalkine (CX3CL1) [Garton et al., 2001]. Cells were treated with or without 100 µM H2O2 and then CM was collected. Due to the toxicity of 1 mM H2O2 towards STOM cells, a lower dose of 100 µM was used. As a result of this sub-optimal dose, a lower induction of pro-BTC shedding of between 0.5- to 2-fold was generally observed (data not shown). H2O2-induced shedding of pro-BTC into CM was comparable between wild-type and ADAM17ΔZn/ΔZn cells (Fig. 3C). These results indicate that unlike a variety of other transmembrane molecules, pro-BTC is not a substrate for ADAM17 in response to H2O2 induction and that a separate metalloprotease such as ADAM10 is likely to be involved.
A23187 and H2O2 Activate Pro-BTC Shedding by Distinct Mechanisms
Despite an enormous amount of research into ectodomain shedding, a clear picture of the molecular mechanisms by which various chemical and physiological stimuli lead to activation of metalloproteases remains mostly uncharacterized. In the case of PMA-activated shedding, a link to PKC has been described [Pandiella and Massague, 1991], whereas for calcium-ionophore, calcium influx into the cell presumably activates calcium-dependent signaling pathways to induce shedding [Eguchi et al., 1998].
The requirement of extracellular calcium ions for H2O2-induced pro-BTC ectodomain shedding was investigated. MB52 cells were pre-incubated in DMEM or calcium-free DMEM, and then H2O2, A23187, or APMA added for 4 h. Western analysis of lysates, using the anti-BTC C-terminus Asp164–Ala178 antibody, indicated that only the A23187-mediated decrease in levels of the 40 kDa pro-BTC isoform was impaired by culture in calcium-free media (Fig. 4A). This was consistent with our previous findings on the blockage of A23187-activated pro-BTC ectodomain shedding in calcium-free media [Sanderson et al., 2005].
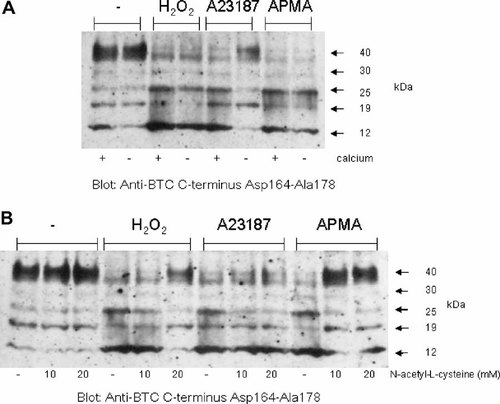
A23187 and H2O2 activate pro-BTC shedding by distinct pathways. A: MB52 cells were pre-incubated in DMEM or calcium-free DMEM for 30 min, then the pro-BTC shedding activators H2O2 (1 mM), A23187 (1 µM), or APMA (0.5 mM) added and cells cultured for a further 4 h. The level of pro-BTC shedding was assessed by analysis of cell lysates by Western blot with the anti-BTC C-terminus Asp164–Ala178 antibody. B: MB52 cells were pre-incubated in DMEM or N-acetyl-L-cysteine (10 or 20 mM) for 30 min, then H2O2 (1 mM), A23187 (1 µM), or APMA (0.5 mM) added and cells cultured for a further 4 h. Cell lysates were collected, and then analyzed by Western blot with the anti-BTC C-terminus Asp164–Ala178 antibody.
In some cases, metalloprotease activation has been proposed to occur by oxidative removal of the enzymes pro-inhibitory region by a ‘cysteine-switch mechanism’ [Nagase and Woessner, 1999; Roghani et al., 1999]. Therefore, the ability of an anti-oxidant N-acetyl-L-cysteine to block H2O2-induced pro-BTC shedding was investigated. MB52 cells were pre-incubated in N-acetyl-L-cysteine for 30 min, and then H2O2, A23187, or APMA were added for 4 h. Only activated pro-BTC shedding in response to H2O2 and APMA was impaired in the presence of N-acetyl-L-cysteine (Fig. 4B). A23187-activated shedding was unaffected by anti-oxidant treatment. These findings suggest H2O2 and APMA activate pro-BTC shedding via a calcium-independent/ROS-dependent mechanism whereas conversely A23187-activated pro-BTC shedding operates through a calcium-dependent/ROS-independent mechanism.
Endothelin-1 Activates the Metalloprotease-Dependent Shedding of BTC in Vascular Smooth Muscle Cells
A number of studies have indicated that GPCR agonists such as angiotensin II (AngII) [Eguchi et al., 2003], ET-1 [Iwasaki et al., 1998], l-α-lysophosphatidic acid (LPA) [Gschwind et al., 2003], and the glucagon-like peptide 1 (GLP-1) [Buteau et al., 2003] activate metalloprotease-dependent shedding of EGF-like factors. Therefore, the ability of GPCR agonists to stimulate pro-BTC shedding in primary VSMCs overexpressing pro-BTC was investigated. VSMCs have previously been demonstrated to shed other EGF-like factors, such as HB-EGF, in response to GPCR agonists [Mifune et al., 2004, 2005]. A number of GPCR agonists were tested but only ET-1 consistently stimulated pro-BTC shedding. This was blocked by pre-incubation with the broad-spectrum metalloprotease inhibitor GM6001 (Fig. 5A). Activation of pro-BTC shedding by ET-1 was consistently weaker than that by A23187.
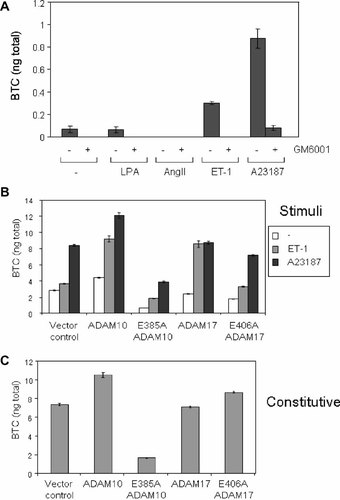
Endothelin-1 activates ADAM10- and ADAM17-dependent pro-BTC shedding in vascular smooth muscle cells. A: VSMCs expressing pro-BTC were pre-incubated for 30 min in GM6001 (50 µM) and then treated for 1 h with LPA (10 µM), AngII (1 µM), or ET-1 (10 µM). CM was collected and analyzed for soluble/shed BTC levels using the BTC ELISA. Results are expressed as mean ± SD of quadruplicate determinations. B: VSMCs expressing pro-BTC and overexpressing wild-type and E > A forms of ADAM10 and ADAM17 were cultured and treated with ET-1 (10 µM) or A23187 (5 µM). CM was collected and analyzed for levels of soluble/shed BTC using the BTC ELISA. Results are expressed as mean ± SD of quadruplicate determinations. C: The same VSMC lines expressing the vector control or wild-type or E > A forms of ADAM10 and ADAM17 in B were cultured for 24 h in serum-free media to assess the level of constitutive pro-BTC shedding. Levels of BTC in CM were determined using the BTC ELISA as above.
ADAM10 and ADAM17 are Both Involved in ET-1-activated Pro-BTC Shedding in VSMCs
To investigate the role of ADAM10 and ADAM17 in ET-1-activated pro-BTC shedding, these enzymes were overexpressed in VSMCs expressing pro-BTC. Expression of ADAMs was confirmed by Western blot (data not shown). Wild-type ADAM10 overexpression enhanced ET-1-activated pro-BTC shedding (Fig. 5B), whereas overexpression of E385A catalytically inactive ADAM10 reduced the ET-1 effect. Interestingly, activation of shedding by ET-1 was also enhanced in cells overexpressing wild-type ADAM17; however, E406A ADAM17 was without effect. This may indicate that ET-1 can activate ADAM17 to shed pro-BTC, but that in a system of dysfunctional ADAM17 activity (i.e., E406A ADAM17 overexpression), ADAM10 may be sufficient for ET-1-activated pro-BTC shedding. Similar to our previously published data in conditionally immortalized dermal fibroblasts and STOM cells [Sanderson et al., 2005], A23187-activated and constitutive shedding of pro-BTC in VSMCs was reduced by overexpression of E385A ADAM10 and increased by wild-type ADAM10 expression, but was unaffected by ADAM17 or E406A ADAM17 overexpression (Fig. 5C).
DISCUSSION
Ectodomain shedding of EGF-like growth factors is an essential process that regulates the availability of soluble ErbB ligands. Like all other EGF-like factors, pro-BTC is expressed as a transmembrane precursor that undergoes ectodomain shedding to release a soluble mature factor from the cell surface [Sanderson et al., 2005]. In this study, MB52 cells shed detectable levels of BTC ectodomains into conditioned media (CM). Three BTC ectodomain isoforms were identified in MB52 CM using an anti-BTC ectodomain Asp32–Tyr111 antibody. These forms have molecular masses similar to soluble BTC forms identified in conditionally immortalized cells and A9 mouse fibroblasts that vary in their N-terminal sequence and level of glycosylation [Watanabe et al., 1994; Sanderson et al., 2005]. Unlike in conditionally immortalized cells, in which pro-BTC shedding is highly preferred over N-terminal processing [Sanderson et al., 2005], MB52 cells appeared to mediate both cleavage events to a similar extent. This was highlighted by the equivalent accumulation of the 25 kDa pro-BTC form and 12 kDa cytoplasmic remnant in response to stimuli, and the predominant appearance of the 7 kDa shed BTC isoform in MB52 cell CM. Whilst the possibility that shed BTC forms are processed further once released into CM cannot be excluded, this alternate processing pattern may reflect an important distinction between the mechanisms of pro-BTC shedding between different cell lines, and possibly alternate regulation of sheddases.
Ectodomain shedding of EGF-like factors has been linked to metalloproteases of the ADAM and MMP families [Blobel, 1997]. In the case of BTC, constitutive and activated shedding is primarily dependent on ADAM10, and independent of ADAM17 [Hinkle et al., 2004; Sahin et al., 2004; Sanderson et al., 2005]. This distinguishes BTC from other EGF-like factors including TGFα [Peschon et al., 1998], NRG-1 [Montero et al., 2000], HB-EGF and AR [Sunnarborg et al., 2002], and ER [Hinkle et al., 2004; Sahin et al., 2004] which are predominantly ADAM17 substrates. Consistent with this, pro-BTC shedding is not up-regulated by PMA, which is a common activator of ADAM17-dependent shedding events [Nagano et al., 2004]. In this study, metalloprotease-dependent pro-BTC shedding in MB52 cells was activated by treatment with A23187, APMA and the novel stimuli H2O2. H2O2 was consistently the most powerful activator of pro-BTC shedding.
The activation of pro-BTC shedding by APMA and H2O2 was distinct from that involving A23187. Pre-incubation with anti-oxidant blocked APMA- and H2O2-induced, but not A23187-induced, pro-BTC shedding. Whereas A23187-induced, but not APMA- or H2O2-induced, shedding was attenuated when cells were deprived of extracellular calcium. Previous work has found that shedding of EGF-like ligands can be activated by multiple stimuli that operate by seemingly independent mechanisms [Pandiella and Massague, 1991]. The mechanism by which APMA/H2O2 and A23187 lead to activation of pro-BTC shedding presumably involves the activation of metalloproteases, yet how this eventuates is likely to be divergent. APMA- and H2O2-activated pro-BTC shedding most likely occurs by direct oxidative removal of the metalloprotease pro-peptide inhibitory region. MMPs and ADAMs contain an N-terminal inhibitory pro-peptide region that blocks the catalytic domain by a ‘cysteine-switch mechanism’ [Nagase and Woessner, 1999]. ROS have previously been shown to stimulate removal of the inhibitory pro-domain of ADAMs [Zhang et al., 2001], and in some cases lead to activation of ectodomain shedding [Hino et al., 1999]. The ability of N-acetyl-L-cysteine to block APMA- and H2O2-activated pro-BTC shedding most likely implicates this mechanism of metalloprotease activation.
Interestingly, the short vasoactive peptide ET-1 was the only GPCR agonist that activated pro-BTC shedding in VSMCs. ET-1 is expressed by epithelial, mesangial, neuronal, and liver cells [Levin, 1996] and mediates cellular effects such as proliferation and migration via binding of two separate GPCRs; ETA and ETB [Sokolovsky, 1995]. Binding of ET-1 to either ETA or ETB results in receptor coupling to multiple G-proteins (Gq, Gs, and Gi) [Eguchi et al., 1993] and subsequent signal transduction through numerous effectors including phospholipases C, D, and A2, as well as adenylate and guanylate cyclases and multiple kinases [Douglas and Ohlstein, 1997]. ETA and ETB receptors have also been linked with influx of extracellular calcium [Pollock et al., 1995] and generation of ROS [Daou and Srivastava, 2004], raising the possibility that activated pro-BTC shedding in response to A23187, APMA, or H2O2 may reflect a similar signaling mechanism to the activation of shedding by ET-1.
In contrast to constitutive and A23187-activated pro-BTC shedding in VSMCs, which mapped solely to ADAM10, activation of pro-BTC shedding by ET-1 was enhanced by overexpression of both ADAM10 and ADAM17. Only E385A ADAM10 expression reduced ET-1-activated pro-BTC shedding, suggesting the ADAM10 is the primary ET-1-activated pro-BTC sheddase. ET-1 has previously been shown to transactivate the ErbB1 receptor via metalloprotease-dependent shedding of EGF-like factors [Kawanabe et al., 2004]. However, the findings here provide the first evidence of ET-1-activation of ADAM10 and ADAM17 and also the first indication of an involvement of ET-1 in pro-BTC shedding. In addition this is the first cellular evidence that under certain conditions, ADAM17 may be involved in processing of pro-BTC.
Ectodomain shedding is potentially fundamental in the development of EGF-like factor-dependent tumors [Borrell-Pages et al., 2003] and other diseases [Sasada and Igarashi, 1993; Mifune et al., 2004] involving deregulated cell growth. Our results identify ROS and ET-1 as novel stimuli towards metalloprotease-dependent ectodomain shedding of pro-BTC. This provides direction for further analysis of the role of pro-BTC and physiological stimuli in the progression of such abnormalities in vivo.
Acknowledgements
This work was supported in part by the Australian Government Cooperative Research Centers Programme to MP Sanderson CA Abbott and AJ Dunbar, and to PJ Dempsey by National Institute of Health (NIH) grants DK59778 and DK63363 and a grant from the Crohn's and Colitis Foundation of America. We thank GroPep Ltd for the use of research facilities, Roy Black and Jacques Peschon (Amgen) for providing ADAM17 reagents, Elaine Raines (University of Washington, Seattle, WA) for providing ADAM10 reagents, Garry Nolan (Stanford University) for providing the pBM retroviral vector, and Sarah Erickson and Sarah Fitzgerald (PNRI, Seattle, WA) for their excellent technical support.




