Carbenoxolone inhibits junctional transfer and upregulates connexin43 expression by a protein kinase A-dependent pathway
Abstract
We have been investigating the function and gene expression of connexins by vascular wall cells, especially Connexin43 (Cx43) in bovine aortic endothelial cells (BAEC). In this study, we tested the effects of carbenoxolone (CBN), a gap junction communication (GJC) blocker on the junctional transfer of Lucifer yellow in BAEC. CBN is a water-soluble derivative of the liquorice-root extract 18-α-glycyrrhetinic acid. CBN rapidly abolished dye-transfer in the scrape-load transfer assay (a measure of GJC) in a reversible and dose-dependent fashion. We then asked whether the BAEC might somehow compensate for the loss of junctional communication by altering the expression of connexins. Thus, we treated BAEC with 100 µM CBN in serum free medium and determined the total Cx43 cellular distribution (immunostaining) and protein content (immunoblotting). Besides changes in distribution, by 6 h, Cx43 content levels increased to 166% ± 22% (P < 0.0001) of controls. RNA blot data showed two-three fold increases in Cx43 message in BAEC after 6 h of CBN treatment, suggesting transcriptional control. Since CBN has structural similarities to corticosteroids, we tested both aldosterone and prednisolone but neither drug increased Cx43 levels, suggesting that the CBN response was not due to a generalized steroid effect. Staurosporine inhibited the CBN-induced increase in Cx43 content, suggesting a role for kinases in the signaling pathway. Further studies with inhibitors indicated that PKA but not PKC was implicated. In summary, CBN blocks junctional communication and modulates Cx43 expression in BAEC. These results suggest a feedback mechanism for control of connexin expression based on junctional patency. J. Cell. Biochem. © 2006 Wiley-Liss, Inc.
Gap junctions are aggregates of inter-cellular channels, which connect the cytoplasms of adjoining cells by membrane-membrane apposition. This aqueous pathway allows the passage of second messengers, ions, metabolites [Beyer, 1993; Goodenough et al., 1996], and solutes of less than approximately 1,000 Da. Gap junctions play significant regulatory roles in embryonic development, electrical coupling, metabolic transport in non-vascularized tissue, apoptosis, differentiation, and tissue homeostasis [Willecke et al., 2002; Saez et al., 2003].
Structurally, gap junctions are composed of integral membrane proteins called connexins (Cx). There are 20 known connexin gene family members in humans, several of which have been cloned and characterized [Kumar and Gilula, 1996; Willecke et al., 2002; Saez et al., 2003].
During inter-cellular channel formation, six connexin proteins oligomerize into a hexameric hemichannel, which is also known as a connexon, traffics to the plasma membrane. Basically, one-hemichannel docks with its counterpart on the opposing cell to form an intact channel. These channels can be gated in response to several stimuli, including modifications in voltage, pH, and connexin phosphorylation [Saez et al., 2003].
The presence of gap junctions has been documented among and between vascular cells. Various reports of their exact connexin composition suggest that the distribution of connexins vary among vascular beds and vessel types, and is differentially regulated in response to injury and growth [Larson et al., 1987; Sweet et al., 1988; Little et al., 1995]. It is clear that homo and hetero-cellular gap junctions may contribute to control mechanisms in metabolism, growth, and vasoactivity. However, the role of junctional communication in the vascular wall per se remains somewhat speculative [Larson et al., 1990, 1997].
We have been studying the expression of different connexins in vascular wall cells in bovine aortic endothelial cells (BAEC), with the goal of understanding why these cells express multiple connexins and how they control the expression of different connexins [Larson et al., 1997]. As part of this work, we have determined that BAEC expresses only Cx43, and at the highest levels, when they are sub confluent, while they express both connexins 43 and 37 (Cx43 and Cx37) at confluency. One possible mechanism for controlling connexin expression might be some sort of cellular sensor, which could detect whether connexons in the membrane were in patent channels. We decided to explore this possibility using sub confluent BAECs (so that only Cx43 would be expressed, and at relatively high levels), which were treated with carbenoxolone (CBN), a compound known to block junctional channels [Goldberg et al., 1996; Winmill and Hedrick, 2003].
Our preliminary findings demonstrated that CBN blocked transfer of the dye, Lucifer yellow CH (LYCH) in these cells. Similar results have been shown by others for different cell types [e.g., Martin et al., 1991; Goldberg et al., 1996]. CBN is a water-soluble derivative of the liquorice root extract, 18-α-glycyrrhetinic acid and is known to bear structural similarities with corticosteroids (Armanini et al., 1983). We hypothesized that blocking gap junctional communication (GJC) with CBN would induce a change in the gene expression of gap junctional peptide/s, specifically Cx43, as a feedback control mechanism. Among the two connexins Cx37 and 43 expressed in BAEC, only Cx43-mediated channels are known to be permeable to LYCH [Steinberg et al., 1994; Veenstra et al., 1995]. Further, Cx43 is the primary GJC-connexin expressed at this stage of cellular growth. The general approach to testing our hypothesis of an effect of CBN treatment on Cx43 expression was to treat the cells for several hours and then assay for Cx43 distribution (immunostaining), content (immunoblotting) and message (Northern blotting). Since we found an upregulation of Cx43 expression in the treated BAECs, we tested for possible protein kinase mediation in the signaling pathway and found Protein Kinase A (but not Protein Kinase C) dependency.
MATERIALS AND METHODS
Materials
Unless otherwise noted all chemicals were from Sigma Chemical, Co. (St. Louis, MO) and were ACS or Cell Culture Certified or Molecular Biology grade. Organic solvents were purchased from Fisher Scientific (Springfield, NJ) and were ACS grade or better.
Cell Culture
Bovine (calf) aortic endothelial cells were isolated by intimal scraping, plated and cultured in low glucose (100 mg/L) Dulbecco's Modified Eagle Medium (DMEM, GIBCO, Grand Island, NY) plus 10% calf serum (Hyclone, Logan, UT) and penicillin/streptomycin (ICN Biomedicals, Costa Mesa, CA) as previously reported [Larson et al., 1990, 1997]. Culture medium was changed every other day. Although some cultures were tested for endothelial nature by vital staining with DiI-Ac-LDL (Biomedical Technologies, Stoughton, MA, not shown), purity was usually assessed by visual inspection of cell and culture morphology.
Scrape-Load Dye Transfer Assay
Confluent cultures of BAEC were treated with 100 µM CBN for 30 min. Control and treated cells were washed with D-PBS and covered with 0.5% Lucifer yellow/0.5% RITC-DEXTRAN (70S). Linear scrapes were made and the dyes were rinsed off, after 30 s. After an additional 5 min of incubation, the diffusion of the dye LYCH (CH corresponds to the carbohydrazide group) was assayed. Dye-containing positive rows of cells, from several separate experiments were counted and plotted.
Antibodies to Cx43
We used a commercial mouse monoclonal antibody (aCx43MAb, Chemicon, Temecula, CA). The aCx43MAb recognizes an epitope on the carboxyl-terminus of Cx43 [Larson et al., 1997].
Immunoblots
Cultures were washed in isotonic saline, and the cells were scrape-harvested, and pelleted. Pellets were resuspended in Laemmli sample buffer without bromophenol blue or β-mercaptoethanol. Protein content was determined using the DC-BioRad protein assay (BioRad) and then bromophenol blue and β-mercaptoethanol were added. Immunoblots were otherwise carried out as described [Larson et al., 1997]. Briefly, samples (usually 50 µg of protein/lane) were run on 12% SDS–PAGE gel and separated peptides were electrophoretically transferred to nitrocellulose in 25 mM Tris, 192 mM glycine, 5% methanol, and 0.05% SDS. Membranes were blocked in 5% non-fat dried milk in Tris buffered saline with 0.1% Triton X-100 (TBS-T), incubated with aCx43MAb, washed with TBS-T, incubated with HRPO-conjugated donkey anti-mouse IgG and washed again. Immune complexes were detected using enhanced chemiluminescence (Amersham or Pierce kits). Densitometric quantitation was carried out using video digitization (PCVISIONplus, Imaging Technologies, Inc.) of backlit films, with analysis using GelPro (Media Cybernetics). The densest spots/bands on films were compared with fogged film to make sure they were not saturated. The film was not preflashed.
cDNA Probes
The cDNA probes used in this study consisted of DNA fragments containing primarily coding sequences [1–1,392 of rat Cx43, Beyer et al., 1989] prepared by digestion with appropriate restriction enzymes and isolated by electrophoresis in low melting-point agarose. Probes were 32P-labeled using the Klenow fragment of DNA polymerase I and random hexanucleotide primers.
RNA Blots
Total RNA was harvested from cultures and prepared for RNA blotting using Trizol (Gibco). High stringency blots were prepared as previously described [Larson et al., 1990]. Blots were prehybridized in 0.75M Na2HPO4 (pH 7.2), 5% SDS, 100 mg/ml salmon sperm DNA (Sigma) for 1 h at 65°C and then hybridized overnight in the same buffer plus the labeled probe at 65°C. Blots were then washed in 0.03 M Na2HPO4 (pH 7.2) with 1% SDS at 65°C before auto radiographic exposure to Kodak XAR-5 film at −80°C with an intensifying screen. RNA blots, prepared under the above conditions, show complete specificity of hybridization for Cx43 mRNA at 3.3 kb. Quantitation of autoradiograms was carried out by video image analysis as described above. Equal loading was checked by ethidium bromide staining. Blots were stripped and reprobed for the housekeeping gene (human fibroblast γ-actin) according to Larson et al. [1997].
Immunofluorescence
BAEC were plated onto glass coverslips in complete medium. At various time points, cells were fixed and permeabilized as previously described [Laing and Beyer, 1995; Larson et al., 1997]. Briefly, slides or coverslips were rinsed in PBS, fixed for 2 min in methanol:acetone (1:1) at room temperature, rinsed in PBS and the cells permeabilized in PBS with 1% Triton X-100 (PBS/T) for 10 min at room temperature. Cells were stained with aCx43MAb (1:400 in PBS/T) overnight at 4°C, washed, stained with goat anti-mouse-IgG conjugated with Cy3 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA; 1:800 in PBS/T) for 1 h at room temperature, washed again and coverslipped. Slides were examined and photographed using a Nikon Diaphot or Microphot-FXA.
Data Analysis and Statistics
Where appropriate, statistical analyses were carried out by t-test or ANOVA using SigmaStat for Windows (ver 1, Jandel Scientific) with appropriate post-tests as noted.
RESULTS
CBN Inhibits GJC
We have previously reported that CBN blocks gap junction mediated dye transfer in BAEC [Larson et al., 1990; Sagar et al., 1997]. In the present study, we used the scrape-load transfer assay to test the ability of BAEC to transfer the dye LYCH. Pooled data shown in Figure 1A from several dose-response experiments of BAEC when treated with CBN for 30 min resulted in a dose-dependent decrease in the LYCH dye transfer capability. The observed block in transfer was rapid (<5 min) and reversible (data not shown). In considering these data, we speculated as to whether BAEC might have a feedback mechanism for detecting junctional patency or loss thereof. Based on this notion, we formulated a hypothesis that BAEC would compensate for changes in junctional communication by regulating the expression of connexins.
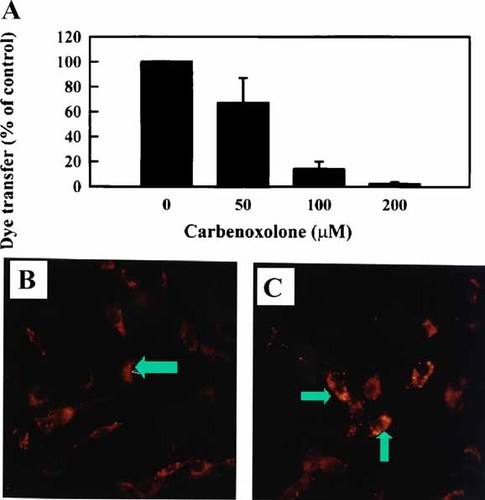
A: Dose-response effects of CBN on the scrape-load dye transfer assay in confluent BAEC. Cells were treated with increasing amounts of CBN for 30 min. The diffusion of the dye LYCH was assayed 5 min after scraping. Pooled and normalized data of several such experiments (means ± SD), as quantitated by counting rows of dye-containing cells (see Materials and Methods). B: Shows Cx43 cellular distribution in control BAEC in serum free medium (SFM), and (C), 100 µM CBN treated BAEC in SFM. Cells were immunostained with anti Cx43 after 6 h of treatment. Note the bright vesicular staining of treated BAEC (arrows). A representative of seven photographic fields per treatment from two individual experiments is shown. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
These scrape-load transfer assays were carried out on confluent BAEC and the following experiments were done on sub confluent cells. We made this shift in models for two reasons: (a) Cx43 expression is highest in sub confluent cells, while Cx37 is not expressed [Larson et al., 1997], (b) Cx43 channels are permeable to LYCH while Cx37 channels are not [Steinberg et al., 1994; Veenstra et al., 1995]. Hence, we maximized expression of the connexin involved in dye transfer.
CBN Causes a Change in the Cx43 Immunofluorescent Staining Pattern
First, we started out to study the distribution of Cx43 in CBN-treated BAEC using immunostaining. As shown in Figure 1 B versus C, 100 µM CBN treatment for 6 h (C) resulted in an increase in intracellular vesicular staining (arrows) over the controls (B). The altered pattern of cells with bright vesicular staining was assessed for several microscopic fields. The marked increase in cells with bright intracellular vesicular staining suggested an altered distribution of Cx43 as well as an increase in Cx43 content.
CBN Treatment Increases Cx43 Expression
We followed up on the immunostaining data by testing for altered Cx43 content. Figure 2A is the pooled, normalized densitometric data from several immunoblot experiments, which show a statistically significant increase in Cx43 protein content up to 166% (±22%) of the controls. Figure 2B is a representative immunoblot from these experiments in which Cx43 protein content was increased in BAEC treated with 100 and 200 µM CBN, compared to the control BAEC. The response appeared to be maximal at 100 µM CBN. Since we determined that CBN treatment altered the cellular distribution of Cx43 (Fig. 1B,C) and increased Cx43 content (Fig. 2A,B), we then tested to find out if it altered Cx43 gene expression.
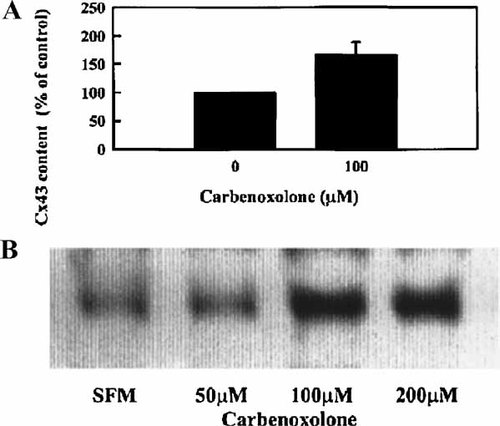
Effect of CBN-treatment on Cx43 protein content, as determined by immunoblot analyses. A: Bars represent means ± SD of normalized, pooled densitometric data (n = 13; *P < 0.0001, Student's t-test). B: A typical immunoblot showing dose-response, where BAEC were treated for 6 h with 0, 50, 100, and 200 µM CBN in SFM. Equal amounts of protein from each sample were used for immunoblotting with anti-Cx43.
CBN Treatment Regulates Cx43 Gene Expression
RNA blot analyses detected a CBN-mediated increase in Cx43 mRNA levels as demonstrated in the pooled, normalized densitometric data (Fig. 3A), which shows that 100 and 200 µM CBN increased Cx43 mRNA levels by two-three fold. Again, a maximal response was elicited with 100 µM CBN treatment. Figure 3B, is a representative Northern blot of these studies. The increases in Cx43 mRNA content suggested that CBN treatment increased Cx43 gene transcription.
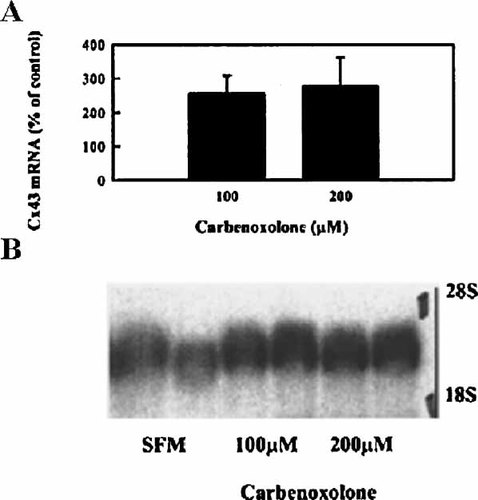
Effect of CBN-treatment on Cx43 mRNA levels. A: Bars denote means ± SD of normalized, pooled densitometric data from identical experiments (n = 4; *P < 0.02, Student's t-test). B: A typical Northern blot showing dose-response, where BAEC were treated for 6 h, with 0, 100, and 200 µM CBN in SFM, duplicate samples are shown. RNA was collected and blots were probed for Cx43 mRNA. Equal loading was checked by ethidium bromide staining. Further, the blots were stripped and reprobed for the housekeeping gene (human fibroblast γ-actin), to confirm a stable expression of its message.
Aldosterone and Prednisolone Do Not Alter Cx43 Expression
CBN bears some structural similarities to corticosteroids (Armanini et al., 1983). In order to determine whether the CBN-mediated changes in Cx43 expression were due to a generalized steroid effect, we treated BAEC with a mineralocorticoid (aldosterone) and a glucocorticoid (prednisolone) at the same molar concentrations as we used for CBN. Pooled data from multiple experiments with these steroids (Fig. 4A), do not demonstrate any effects on Cx43 expression in BAEC. A representative immunoblot in Figure 4B shows no increases in Cx43 protein content when cells were treated for 6 h with 100 and 200 µM aldosterone. Similar results with prednisolone are shown in Figure 4C.
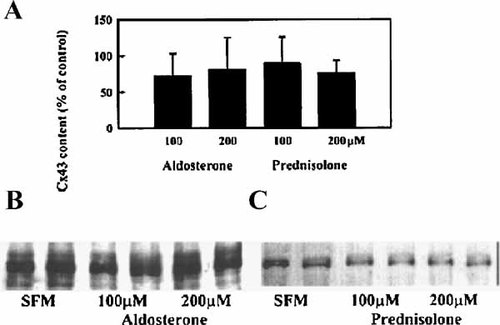
Effect of corticosteroids on Cx43 protein content. A: Bars represent means ± SD of the normalized, pooled densitometric data from identical experiments (n = 6). BAEC were treated with 0, 100, and 200 µM aldosterone (B) and prednisolone (C) in SFM, duplicate samples are shown. After 6 h, cells were harvested, and equal amounts of protein were loaded in each lane, electrophoresed and immunoblotted with anti-Cx43.
Evidence for a Kinase Involvement in the CBN-Mediated Increase in Cx43 Expression
Since kinases are involved in signaling pathways that alter the gene expression of connexins [Cruciani and Mikalsen, 2002; Solan and Lampe, 2005], we tested for a possible kinase involvement in the CBN-mediated increased expression of Cx43. Figure 5A, is an immunoblot, which shows that blocking protein kinase activity with staurosporine (10 pM to 1 nM) resulted in a dose-dependent inhibition in the CBN-mediated increase of Cx43 protein content (compare lanes 2 and 6–8). This inhibitory effect suggested a protein kinase involvement in the signaling pathway.
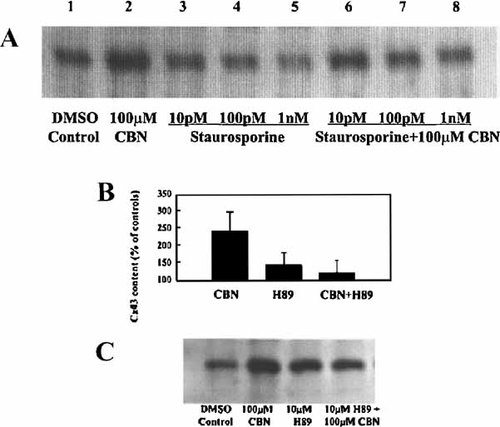
A: Effect of staurosporine on CBN-mediated regulation of Cx43 protein content: A representative immunoblot of several experiments (n = 5). BAEC in SFM were treated with DMSO (solvent control, lane 1) and with 100 µM CBN (lane 2). Lanes 3–5 correspond to 10 pM, 100 pM, and 1 nM staurosporine treatments, while lanes 6–8 correspond to staurosporine plus 100 µM CBN. After 6 h, cells were harvested, and equal amounts of protein were loaded in each lane, electrophoresed and immunoblotted with anti-Cx43. B: Effect of H89 on the CBN-mediated regulation of Cx43 protein content as determined by immunoblot analyses. Bars represent means ± SD of normalized and pooled densitometric data (n = 9; *P < 0.0001 vs. 100 µM CBN, Student's t-test). C: BAEC in SFM were treated with DMSO (solvent control, lane 1), 100 µM CBN (lane 2), 10 µM H89 (lane 3), or 10 µM H89 plus 100 µM CBN (lane 4). After 6 h, cells were harvested, and equal amounts of protein were loaded in each lane, electrophoresed and immunoblotted with anti-Cx43.
Cyclic AMP-Dependent Protein Kinase Is Involved in the CBN-Mediated Cx43 Expression
Since staurosporine is not very kinase specific [Ruegg and Burgess, 1989], we used other inhibitors to try to determine which kinase(s) were involved. We tested specific and potent inhibitors of protein kinase C: like chelerythrine (a competitive inhibitor with respect to the phosphate acceptor) [Zheng et al., 2001], calphostin C, which is Ca2+/phospholipid-independent and competes with 1,2-diacylglycerol binding sites and Gö6979 (Ca2+-dependent selective inhibitor of protein kinase C-α and protein kinase C-β1) [Ogiwara et al., 1998], but neither of these inhibitors mirrored the effects of staurosporine (not shown). Next, we tested for a possible involvement of cAMP-dependent protein kinase (PKA) by using its specific inhibitor, H89 [Engh et al., 1996]. The pooled data from multiple immunoblots as seen in Figure 5B demonstrate that 10 µM H89 blocks 86% of the CBN-mediated increase in Cx43 protein content. A representative immunoblot shown in Figure 5C demonstrates that the increase in Cx43 protein content after CBN treatment (lane 2) is significantly reduced in the presence of H89 (lane 4). Thus, these data suggest PKA-dependency in the CBN-induced increase of Cx43 expression signaling pathway
DISCUSSION
In this investigation we have uncovered some of the cellular responses of BAEC by inducing GJC blockade with CBN (Fig. 1A). A feed back mechanism, which is likely mediated by a cellular sensor, triggers a physiological response by modifying the cellular phenotype. As a consequence, we see an increase in the distribution of Cx43 protein (Fig. 1B vs. C) its content (Fig. 2A,B) and message (Fig. 3A,B). Our data based on the use of mineralocorticoid and glucocorticoid analogues, like aldosterone and prednisolone suggest that these responses appear to be non-steroidogenic (Fig. 4A–C), and thus could be specific effects of CBN. However, caution is suggested in interpreting the data, as Lye et al. [1993] have shown an increased expression of Cx43 in the rat myometrium during labor, which has been associated with an increase in the plasma estrogen: progesterone ratio. A molecular analysis of the promoter region preceding the first exon of the Cx43 gene contains a TATA box, AP-1 and AP-2 sites. A series of half-palindromic estrogen response elements are reported in this region [Yu et al., 1994]. Further analyses with luciferase assays found a cell-specific response. Thus, tissue-specificity explains the inductive effect seen here with CBN.
Sucrose Also Regulates Cx43 Expression
While it was clear that the effect of CBN on Cx43 expression was not a generalized steroid effect, the mechanism of gene regulation remained unresolved. An important question remained unanswered, could this be a direct effect of CBN or indirect via GJC blockade? In order to address part of this question, we tested the effect of another water-soluble channel-blocking agent, sucrose [Segal and Duling, 1989; Ungvari and Koller, 2001], on Cx43 expression. We studied the protein distribution pattern and content, using immunostaining and immunoblot analyses, respectively. Our studies indicated an increase in bright intracellular vesicular staining corresponding to Cx43 distribution and Cx43 protein content (data not shown) in sucrose-treated BAEC. Our observations were comparable to the studies from CBN-treated cells as seen in Figures 1B,C and 2A,B. Thus, sucrose-induced Cx43 expression in at least two parameters: It increases Cx43 vesicular staining and Cx43 protein content. These observations suggest that if GJC is blocked by either of these two channel blockers, Cx43 expression is increased in BAEC.
Prior Cognate Studies in Our Cellular Model
Interestingly, our earlier studies have shown [Larson et al., 1997] an increase in Cx43 expression when the BAEC were sub confluent, where the cells were actively differentiating and in a log-phase growth, to form vital cell-cell contacts. On the contrary, when the BAEC cultures become confluent, the cell-cell contacts establish with respect to cell density and growth status, which would then induce a feedback control mechanism, whereby Cx43 expression is dramatically inhibited. Further, in the same investigation we have also demonstrated that exposure of BAEC to a growth inhibiting cytokine like the transforming growth factor beta1 (TGFβ1) induced a quiescent status by stalling the establishment of potential cell-cell contacts and thus affecting the formation of mature inter-cellular gap junctions. As expected, this condition triggered an upregulation of Cx43 expression. It is also noteworthy that cell-cell contact induces increased expression of Connexin37mRNA (Cx37). Cx37 is another important connexin expressed in BAEC [Larson et al., 1997, 2000]. Cellular contact inhibition controls the expression of these two connexins. The expression of both these connexins is reciprocally regulated during growth progression. Thus, TGFβ1 treated BAEC cultures which inhibited growth, also had a negative impact on the expression of Cx37 [Larson et al., 1997].
So far the accrued summary, previous [Larson et al., 1997] and current, demonstrate an increase in Cx43 expression whenever GJC is affected, as the: (A) BAEC cultures were sub confluent during growth progression, (B) BAEC cultures were subjected to quiescent stage by treating them with TGFβ1 and (C) As seen here, when GJC is blocked. Mechanistically, CBN induces a spatial disruption in the BAEC cultures, perturbing GJC, thus challenging cellular junctional patency and integrity. Hence, the BAEC would acclimatize by acquiring a strong tendency to re-organize or compensate for the loss thereof. During this physiological adaptation, at least one apparent outcome is increased Cx43 gene expression.
Other Related Studies, Future Directions, and Significance
While characterizing other regulatory attributes is beyond the scope of this article, however, there are some unanswered questions, which are of interest. Here, we demonstrate an increase in Cx43 protein distribution (increased intracellular vesicular staining) and Cx43 content (Figs. 1B,C and 2A,B), but it is not known whether the CBN-mediated increased expression of Cx43 is due to its accumulation associated with increased synthesis or decreased degradation. Exploring these would provide insights to the changes in the half-life of Cx43. We do not know as to which vesicles are involved in the CBN-induced Cx43 distribution. Answering these questions would shed some light on the regulation of Cx43 trafficking. In our previous investigation for example, we demonstrated that the increased Cx43 expression induced by TGFβ1 did regulate Cx43 trafficking, which was also associated with lysosomal compartments [Larson et al., 2001]. Notably, ubiquitin-proteasome mediated proteolytic pathway has also been implicated in Cx43 turnover in intact cells [Laing and Beyer, 1995; Musil et al., 2000].
Further studies are needed to understand the phosphorylation mechanism at the post translational level, as to which amino acid residue/s is/are involved in the CBN-induced increase of Cx43 expression. Interestingly, Richards et al. [2004] have shown that Protein kinase C spatially and temporally controls GJC during human wound repair via phosphorylation of Cx43 on serine368. They infer that this phosphorylation event is associated with a decrease in GJC. Other studies have demonstrated that cAMP enhanced junctional conductance in intact heart and isolated heart cells [De Mello, 1983; Saez et al., 1986; Burt and Spray, 1988; Musil and Goodenough, 1990]. Further, Lau et al. [1991] provide evidence that the heart gap junction protein, connexin43, from unstimulated heart tissues and cultured myocytes is stoichiometrically phosphorylated in vivo. They report that the phosphorylation of Cx43-related proteins occurs predominantly on serine residues. Regulation of Cx43 gene expression in endothelial cells is known to be mediated by cAMP response element binding activity (CREB), a transcription factor that is activated by phosphorylation and is known to be the target of a variety of signaling pathways that mediate cellular responses to extra cellular stimuli [Zhang et al., 2005].
Finally, from a functional standpoint, the goals of analyzing these molecular events is to enable this investigation to help in better understanding the role of GJC and Cx43 in vascular wound healing. In intact tissue, the cellular architecture and the gap junction integrity is normal. Here, in a BAEC sub confluent culture, GJC is perturbed, a consequential feedback control response is gap junctional reorganization. During such acclimatization the primarily affected target is Cx43 gene expression.
Acknowledgements
This work is supported by NIH grant # HL52697 to D.M.L. The authors thank Michael J. Wrobleski and Irena Zemtseva for their technical assistance during this project. We also thank Norah P. Sagar for proofreading the revised manuscript.




