Codominant expression of genes coding for different sets of inducible salivary polypeptides associated with parotid hypertrophy in two inbred mouse strains
Abstract
Experimental mouse parotid hypertrophy has been associated with the expression of a number of isoproterenol-induced salivary proline-rich polypeptides (IISPs). Mouse salivary proline-rich proteins (PRPs) have been mapped both to chromosomes 6 and 8. Recently, mice of two inbred strains (A/Snell and A.Swiss) have been found to differ drastically in the IISPs. In this study, mice of both strains were used for cross-breeding experiments addressed to define the pattern of inheritance of the IISP phenotype and to establish whether the IISPs are coded on a single or on several chromosomes. The IISP phenotype of individual mice was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) of whole saliva collected after three daily stimulations by isoproterenol. Parental A/Snell and A.Swiss mice were homogeneous for distinctive strain-associated IISP-patterns. First filial generation (F1) mice obtained from the cross of A/Snell with A.Swiss mice expressed with no exception both the A/Snell and A.Swiss IISPs (coexpression). In the second filial generation (F2) both parental IISP phenotypes reappeared together with a majority of mice expressing the F1-hybrid phenotype (1:2:1 ratio). Backcrosses of F1 × A/Snell and F1 × A.Swiss produced offsprings displaying the F1 and the corresponding parental phenotypes with a 1:1 ratio. No recombinants were observed among F2 mice or among mice resulting from backcrosses. Thus, genes coding for the IISPs that are expressed differentially in both mouse strains are located on the same chromosome, probably at the same locus (alleles) or at quite closely linked loci (nonalleles). © 2005 Wiley-Liss, Inc.
Abbreviations used:
IISPs, isoproterenol-induced salivary proline-rich polypeptides; PRPs, proline-rich proteins; PSP, parotid secretory protein; kDa, kilodalton; SDS, sodium dodecyl sulfate; PAGE, polyacrylamide gel electrophoresis; TCA, trichloroacetic acid; χ2, Chi-square value; df, degrees of freedom; Mr, relative molecular mass; F1 and F2, first and second filial generations, respectively.
As in other rodents, isoproterenol provokes marked trophic effects in the parotid glands of mouse [Novi and Baserga, 1971; Maeda et al., 1991]. Concomitantly, in the parotid glands of an inbred mouse strain (A/Snell) isoproterenol induces a group of polypeptides that have been considered as markers of the trophic response on the basis of their accumulation in the glands in strict correlation with the intensity of the hypertrophic gland enlargement [López Solís and Miranda Wilson, 1986; López Solís et al., 1987, 1990]. Those polypeptides (named as C, D, E, F, and G; relative molecular mass (Mr) 65, 61, 51.5, 38, and 37 kilodalton (kDa)) are secretory in nature [López Solís et al., 1989]. Thus, polypeptides C–G can be observed as early as 24 h after a single trophic stimulation by isoproterenol both in the fluid collected directly from cannulated hypertrophic parotid glands and in whole saliva [López Solís et al., 1989, 2003a]. Besides, those isoproterenol-induced salivary polypeptides are part of the highly complex family of proline-rich proteins (PRP) and so they display both a characteristic metachromatic staining after Coomassie blue and a high solubility in trichloroacetic acid (TCA) [Muenzer et al., 1979; Mehansho et al., 1987; López Solís et al., 1993; González et al., 2000]. Recently, the induction of a different group of isoproterenol-induced salivary proline-rich polypeptides (IISPs) (named as P, Q, Q′, R, S, and T; Mr 59, 57, 54.5, 46, 36, and 34 kDa) during the trophic response in parotid glands was observed in a second inbred mouse strain (A.Swiss) [López Solís et al., 2003b]. These polypeptides have been observed both in parotid gland homogenates and in the saliva collected from chronically isoproterenol-stimulated mice. Thus, another strain-associated invariant profile of IISPs has emerged [López Solís et al., 2003b].
Insofar, no much genetic data are available with regard to the IISP phenotype expressed either in the A/Snell strain or in the A.Swiss strain. In mouse, genes coding for salivary PRPs, such as the nonallelic MP2 and M14 genes, were located first on chromosome 8 according to evidence derived from DNA analysis of somatic cell hybrids [Azen et al., 1984; Ann et al., 1988] and, later, on chromosome 6 on the basis of in situ hybridization and high resolution genetic mapping [Azen et al., 2000; Bachmanov et al., 2001]. In human, loci coding for various subfamilies of salivary PRPs have been mapped both to proximal chromosome 12 (PRB1–PRB4, PRH1, PRH2) and to distal chromosome 4 (PROL3 and PROL5) [Kim et al., 1993; Isemura and Saitoh, 1997]. Thus, different analytical strategies, different molecular probes used by various laboratories, different PRP genes actually located on different chromosomes, and a combination of all those possibilities underlie most of the information presently available with regard to chromosomal location of PRP-coding genes in mouse and in other mammals. In addition, the assignment of specific PRP genes to their salivary protein products or, vice versa, the assignment of specific salivary PRPs to the genes that encode them, is a complex matter that has been intensively studied in the last years both in human and mouse [Azen et al., 1984, 1989, 2000; Ann et al., 1988; Lyons et al., 1988; Azen, 1993; Kim et al., 1993; Isemura and Saitoh, 1997; Bachmanov et al., 2001]. In mouse, MP2 and M14 genes have not been associated with a particular set of inducible salivary PRPs. In this context, the availability of two pure mouse strains that are variants for the IISP character [López Solís et al., 2003b] together with the sensitive and unambiguous detection of the IISPs in whole saliva produced by mice bearing hypertrophic salivary glands [López Solís et al., 2003a] might constitute highly valuable tools to conduct studies addressed to characterize genetically the expression of the salivary PRPs in mice derived from experimental matings between parents previously phenotyped for the IISP character. In the present sialogenetic study, the identification of the IISPs in the saliva of individual mice of various hybrid generations produced by crossing the parental A/Snell and A.Swiss strains is reported. Coexpression of the A/Snell and A.Swiss IISPs in first filial generation (F1), reappearance of the parental phenotypes with predominance of the hybrid phenotype in second filial generation (F2), the expression of both the hybrid phenotype and the corresponding parental phenotype with a ratio of 1:1 among mice produced by backcrosses and, finally, the absence of recombinants, suggested that the whole set of IISPs in both inbred mouse strains are coded by a single locus or by loci that are closely linked on a single chromosome.
MATERIALS AND METHODS
Animals
Male and female mice of the A/Snell and A.Swiss strains, inbred in our laboratory since 1960 and weighing 22 ± 2 g were used when 2–3 months old. The animals were maintained on an alternating 12-h light and 12-h dark schedule and fed ad libitum.
IISP Phenotypes
Isoproterenol (0.16 μmol/g body weight) was administered intraperitoneally every 24 h for 3 days [López Solís et al., 2003a]. Control mice consisted of animals that were injected with saline. After 24 h of the last administration of isoproterenol, samples of whole saliva were collected. To do so, microvolumes (10–20 μl) of 4% pilocarpine were instilled directly into the mouse mouth. Once salivation became visible (about 4–5 min later), aspiration of the salivary fluid was initiated by means of a tuberculine syringe fitted with a bent micropipette tip [López Solís et al., 2001]. Saliva from every single mouse was accumulated in preweighed Eppendorf tubes that were maintained in ice. At the end of the collection procedure, the Eppendorf tubes were weighed again in order to estimate the amount of collected saliva by assuming a specific gravity of 1.00 g/ml. Aliquots were taken for protein quantitation and gel electrophoresis as described below. Routinely, samples of saliva from individual mice were stored at −80°C. The IISPs were identified by comparing the electrophoretograms of salivas produced by unstimulated versus stimulated single mice. Two procedures were used with no differences between them. Either groups of samples of saliva collected from individual unstimulated mice were contrasted against groups of samples of saliva collected from individual isoproterenol-stimulated mice or, alternatively, samples of saliva collected from individual unstimulated mice were contrasted against salivary samples obtained from the same groups of animals after isoproterenol stimulation. The whole group of IISPs expressed by every single mouse constituted the salivary phenotype of the animal.
Protein Content
Ten-microliter aliquots of saliva from every single mouse were spotted onto cellulose disks, dried at room temperature, fixed in 5% TCA, and washed successively (changes every 5 min) in 5% cold TCA, 80% ethanol, and 3:1 (v/v) ethanol/ether. The disks were air-dried and incubated at 45°C in 0.25% Coomassie blue R-250 for 30 min, drained and washed exhaustively in several changes of 7% acetic acid until clear background. The disks were dried under a lamp, introduced into Khan tubes, and eluted in 3 ml of 66% methanol–0.25% ammonia. Eluates were read at 610 nm in a double-beam Shimadzu UV-160 spectrophotometer. A standard curve was prepared by including disks containing 10–50 μg of bovine serum albumin. Blank disks contained no protein [Bramhall et al., 1969; Durham and López Solís, 1979].
Protein Electrophoresis
Aliquots of whole mouse saliva containing 40 μg of protein were mixed with sample buffer and electrophoresed in SDS–polyacrylamide slab gels (11%) as specified elsewhere [Laemmli, 1970; López Solís and Miranda Wilson, 1986; López Solís et al., 2003a]. To calibrate the electrophoretic separations, molecular weight standards were run in parallel. Following the electrophoretic separation, gels were fixed overnight in 15% isopropanol/10% acetic acid, rinsed twice in the same solution, and stained for at least 15 h in 0.25% Coomassie blue R-250 dissolved in 45% isopropanol/10% acetic acid. The gels were rinsed in 10% isopropanol/10% acetic acid until clear background. After destaining, the gels were rinsed in three 5-min changes of distilled water and scanned in an AGFA-Snapscan 1236 device.
Materials
Pilocarpine was obtained as a 4% solution from pharmaceutical suppliers (Licarpin™, Saval Laboratories, Chile). (+/−)-Isoproterenol-HCl (molecular weight 247.7), reagent-grade chemicals, and protein molecular weight standards for gel electrophoresis were acquired from Sigma (St. Louis, MO). Protein molecular weight standards were phosphorylase b (97 kDa), phosphofructokinase (84 kDa), ovotransferrine (78 kDa), bovine serum albumin (66 kDa), α-amylase (55 kDa), ovoalbumin (45 kDa), carbonic anhydrase (30 kDa), and α-lactalbumin (14.2 kDa). Solvents used for gel processing were purchased from Merck (Santiago, Chile). Cellulose disks (Whatman grade 1; 2.5 cm diameter) were obtained from Whatman (Maidstone, England).
RESULTS
Effect of Isoproterenol on the Polypeptide Profile of Saliva From A/Snell and A.Swiss Mice
Groups of 20 inbred A/Snell mice and 20 inbred A.Swiss mice (10 male and 10 female per strain) were separated for breeding experiments. Saliva obtained from control unstimulated mice of both the A/Snell strain and the A.Swiss strain displayed as major features both the parotid gland-produced polypeptide band A (α-amylase, Mr 55.000), polypeptide band B (parotid secretory protein (PSP), Mr 21.000), and polypeptide band X (40 kDa) (Fig. 1A). By contrast, saliva from A/Snell mice bearing hypertrophic parotid glands after 3 days of stimulations with isoproterenol displayed, with no exception, marked changes in the polypeptide profile. Those changes involved basically the induction of the metachromatically stained IISP polypeptide bands C (Mr 64.500), D (Mr 60.000), E (Mr 51.500), F (Mr 37.000), and G (Mr 36.000) in addition to a significant diminution in the intensities of the normal polypeptide bands A and B (Fig. 1A). The intensities of the rest of the salivary polypeptide bands, including polypeptide band X, remained fully unaltered after the treatment with isoproterenol. Thus, taken the whole set of IISPs as a single phenotype, the group of A/Snell mice constituted a fully homogeneous representative of the parental generation of one of the pure-breeding strains to be used in this study. On the other hand, isoproterenol was also a powerful inducer of new salivary polypeptides in the A.Swiss strain. Thus, after three administrations of isoproterenol given at 24-h intervals, all these mice expressed in saliva at least six induced metachromatically stained IISP polypeptide bands, namely, P (Mr 59.000), Q (Mr 57.000), Q′ (Mr 54.500), R (Mr 46.000), S (Mr 36.000), and T (Mr 34.000) (Fig. 1B). As previously reported, in response to isoproterenol, polypeptide bands A and B in this strain showed a marked diminution in their intensities although that observation was more difficult in the case of polypeptide A because it became partially masked by some of the induced polypeptides (Q and Q′). Again, the intensities of the rest of the salivary polypeptide bands, including polypeptide band X, remained unaltered after the sialotrophic treatment. Accordingly, the whole group of A.Swiss mice was also a proper representative of the parental generation of the second pure-breeding strain whose salivary electrophoretic polypeptide profile after isoproterenol became readily distinguishable from that displayed by the A/Snell strain. Thus, the isoproterenol-induced sets of salivary polypeptides or IISPs in both strains could be considered as strain-dependent discrete and constant traits. Under the present experimental conditions (electrophoretic fractionation and detection procedure), the alignment of the electrophoretograms corresponding to salivas obtained from single isoproterenol-stimulated mice of both strains allowed a proper, consistent, and unambiguous differentiation of the A/Snell and A.Swiss IISPs with the sole exception, because of size similarities, of the doublets F/G (A/Snell) and S/T (A.Swiss) (Fig. 1A,B). Accordingly, further phenotypic analyses in the breeding experiments were focused on the rest of the strain-associated IISPs, namely, the A/Snell polypeptide bands C, D, and E and the A.Swiss polypeptide bands P, Q, Q′, and R.
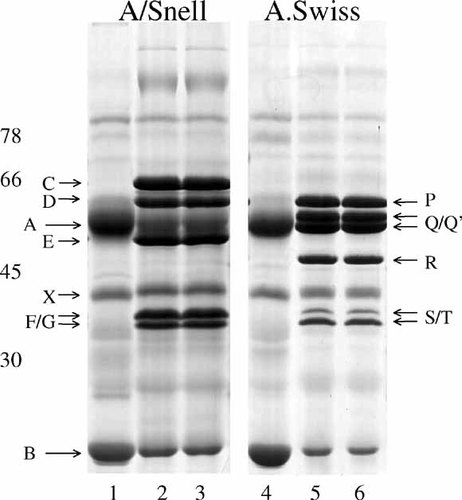
Induction of isoproterenol-induced salivary proline-rich polypeptides (IISPs) in the parental mouse strains. Male and female A/Snell and A.Swiss mice were injected intraperitoneally with isoproterenol (0.16 μmol/g body weight) at 24-h intervals. Control mice were injected for 3 days with saline. Saliva from individual mice was collected separately at 24-h after the third injection. Aliquots of saliva containing 30 μg of protein were electrophoresed. In the gels that are representative of 200 mice of the A/Snell strain (gel A) and 70 mice of the A.Swiss strain (gel B), electrophoretograms corresponding to control mice (lanes 1 and 4) and isoproterenol-stimulated mice (lanes 2, 3, 5, and 6) are shown. No differences between control salivas from both strains were observed. By contrast, the induced IISPs C (65 kDa), D (61 kDa), and E (51.5 kDa) in the A/Snell strain and the induced IISPs P (59 kDa), the duplet Q/Q′ (55 and 57 kDa), and R (46 kDa) in the A.Swiss strain are distinctive strain-associated molecular features. The IISPs F/G and S/T in A/Snell and A.Swiss mice, respectively, migrate in the range 34–38 kDa and no unequivocal resolution between them in this type of electrophoretic fractionation was achieved. In both strains, the normal secretory polypeptide bands A and B undergo downregulation during the stimulation by isoproterenol although in the case of polypeptide band A in the A.Swiss strain that specific effect was partially quenched by the induced doublet Q/Q′. Standard molecular weights: ovotransferrine (78 kDa), bovine serum albumin (66 kDa), ovoalbumin (45 kDa), and carbonic anhydrase (30 kDa).
Cross-Breeding Experiments
As indicated above, the A/Snell and A.Swiss strains of mice differ sharply in the IISP phenotype. Male and female A/Snell mice were crossed, respectively, with female and male A.Swiss mice to produce hybrid F1 mice (n = 50). When F1 mice were 2 months old, they were subjected to a chronical (3-day) administration of isoproterenol and a day later to molecular typing for the IISPs. With no exception the whole group of hybrid F1 mice coexpressed all the IISPs that were characteristic of the parental A/Snell and A.Swiss strains (Fig. 2). By contrast, when male and female of the first filial generation (F1) were crossed together and the resulting mice of the F2 generation (n = 61) were subjected individually to molecular typing for the IISPs, three different phenotypes were observed (Fig. 3). In effect, about a half of the F2 offspring (n = 30) expressed the same hybrid phenotype as the F1 mice while the other half expressed in roughly equal frequencies either the A/Snell (n = 14) or the A.Swiss (n = 17) phenotypes. No other phenotype was identified among F2 mice.
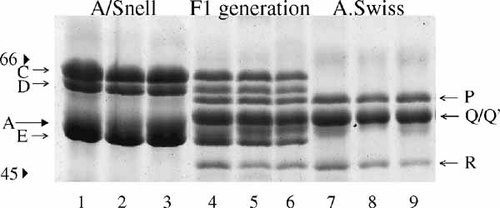
Coexpression of the parental IISPs in hybrid first filial generation (F1) mice. Mice of the F1 generation produced by crossing A/Snell and A.Swiss mice were isoproterenol-stimulated and their salivas were individually collected for IISP typing, as described in “Materials and Methods.” The gel shows electrophoretograms corresponding to salivas from isoproterenol-treated male or female mice that were taken at random out of 14 A/Snell parents (lane 1, male; lanes 2, 3, females), 14 A.Swiss parents (lane 7, male; lanes 8, 9, females), and 50 A/Snell × A.Swiss F1 mice (lane 4, male; lanes 5, 6, females). With no exception the whole F1 progeny showed an invariant IISP phenotype consisting in the simultaneous expression of the A/Snell IISPs and the A.Swiss IISPs.
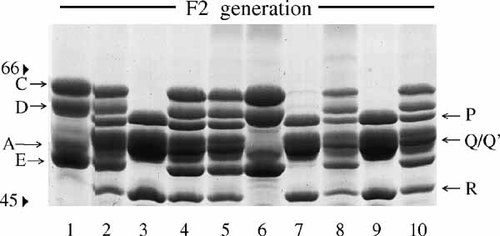
Reappearance of the parental IISP phenotypes together with the hybrid IISP phenotype in second filial generation (F2) mice. Mice of the F2 generation produced by a single cross between two F1 mice were isoproterenol-stimulated and their salivas were individually collected for IISP typing. The gel, which is representative of 12 identical offsprings consisting of 61 F2 mice, displays the IISP phenotypes of 10 different F2 mice (lanes 1–10). Mice 1 and 6 exhibit the A/Snell IISP phenotype; mice 3, 7, and 9 exhibit the A.Swiss IISP phenotype, and mice 2, 4, 5, 8, and 10 exhibit the hybrid IISP phenotype.
Finally, backcrosses F1 × A/Snell and F1 × A.Swiss were also conducted and the corresponding offsprings were tested for the identity of the IISPs. To do so, male or female F1 mice were crossed, respectively, with female or male mice of each of the parental strains. In the offspring of the F1 × A/Snell backcrosses (n = 102), 44 mice displayed the F1 phenotype and 58 mice expressed the A/Snell phenotype, that is, roughly a 1:1 ratio (Fig. 4A). No other phenotype was observed. Likewise, in the offspring of the F1 × A.Swiss backcrosses (n = 70), 42 mice displayed the F1 phenotype and 28 mice displayed the A.Swiss phenotype, that is, again roughly a 1:1 ratio between both resulting phenotypes (Fig. 4B). Once more, no other phenotype was observed. A summary of the phenotypes observed in the various generations is presented in Table I.
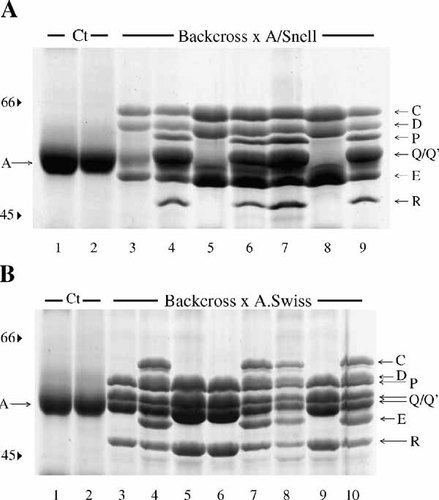
Two IISP phenotypes only in mice derived from backcrosses. Mice produced by backcrossing F1 mice with either A/Snell or A.Swiss mice were isoproterenol-stimulated and their salivas were individually collected for IISP typing. Gel A, which is representative of 19 offsprings consisting of 102 mice, shows the IISP phenotypes of seven isoproterenol-stimulated mice of a single offspring derived from a F1 × A/Snell backcross (lanes 3–9). Note among these mice the occurrence of the hybrid IISP phenotype (lanes 4, 6, 7, and 9) and the A/Snell IISP phenotype (lanes 3, 5, and 8) only. For comparison, the electrophoretograms of two unstimulated mice produced in this backcross are included (lanes 1 and 2). In gel B, the IISP phenotypes of eight isoproterenol-stimulated mice of an offspring derived from a F1 × A.Swiss backcross are shown (lanes 3–10). In these crosses, only hybrid IISP phenotypes (lanes 4, 7, 8, and 10) and A.Swiss IISP phenotypes (lanes 3, 5, 6, and 9) are observed. Again, saliva from two unstimulated mice produced in this backcross are included for comparison (lanes 1 and 2). This gel is representative of 14 offsprings consisting of 70 mice.
| IISPs | A/Snell, Phe A | A.Swiss, Phe B | First filial generation (F1), Phe AB | Second filial generation (F2) | F1 × A/Snell | F1 × A.Swiss | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phe A | Phe AB | Phe B | Phe AB | Phe A | Phe AB | Phe B | ||||
| C | + | − | + | + | + | − | + | + | + | − |
| D | + | − | + | + | + | − | + | + | + | − |
| E | + | − | + | + | + | − | + | + | + | − |
| P | − | + | + | − | + | + | + | − | + | + |
| Q | − | + | + | − | + | + | + | − | + | + |
| Q′ | − | + | + | − | + | + | + | − | + | + |
| R | − | + | + | − | + | + | + | − | + | + |
| No. | 200 | 70 | 50 | 14 | 30 | 17 | 44 | 58 | 42 | 58 |
| Ratio | 1 | 1 | 1 | 1:2:1 | 1:1 | 1:1 | ||||
- An IISP phenotype (Phe) consists of a group of IISP electrophoretic bands. The parental A/Snell strain expressed IISPs C, D, E (Phe A) and the parental A.Swiss strain expressed IISPs P, Q, Q′, R (Phe B). With no exception, each F1 mouse (n = 50, obtained from 14 independent crosses) expressed all the IISPs of both parents (coexpression) (Phe AB). In the F2 generation (n = 61, from 12 independent crosses), the parental IISP phenotypes reappeared together with a majority of mice expressing the hybrid phenotype (1:2:1 ratio). Finally, offsprings of both the F1 × A/Snell backcross (n = 102, from 19 independent crosses) and the F1 × A.Swiss backcross (n = 70, from 14 independent crosses) expressed the corresponding parental IISP phenotype and the hybrid phenotype with a 1:1 ratio. No recombinant phenotypes, expressing only a subset of the parental IISPs, were observed. Presence and absence of individual IISPs in mouse saliva are indicated by symbols “+” and “–,” respectively. Ratios indicated at the bottom line of the table were validated with the χ2 test (P > 0.05).
DISCUSSION
Two congenic mouse strains, A/Snell and A.Swiss, that express during the sialotrophic response different sets of IISPs, have been used for conducting cross-breeding studies [Altman and Dittmer Katz, 1979; López Solís et al., 2003b]. This study on “experimental sialogenetics” indicates that the IISP-coding genes in both mouse strains are located on the same chromosome and suggests that in each strain either a single gene or a group of closely linked genes might code for the whole set of IISPs under consideration (C, D, and E in A/Snell and P, Q, Q′, and R in A.Swiss). These observations derive altogether from (a) the homozygous genotype of the IISP-coding genes in each one of both parental strains, (b) a homogeneous coexpression of the A/Snell and A.Swiss IISPs in the F1 offspring, (c) in the F2 offspring, the occurrence of three different IISP phenotypes, namely, A/Snell, hybrid, and A.Swiss, with a 1:2:1 ratio (χ2 = 0.31, degrees of freedom (df) = 2, P > 0.90), (d) in the offspring produced by the backcross F1 × A/Snell, the occurrence of two phenotypes, one of them corresponding to the hybrid F1 phenotype and the other to the A/Snell phenotype, with a 1:1 ratio (χ2 = 1.92, df = 1, P > 0.10), (e) in the offspring produced by the backcross F1 × A.Swiss, the occurrence of two phenotypes, one of them corresponding to the hybrid F1 phenotype and the other to the A.Swiss phenotype, with a 1:1 ratio (χ2 = 2.8, df = 1, P > 0.10), and (f) the absence of recombinants (mice expressing only a subset of the strain-associated IISPs under consideration) either in the F2 offspring or in the offsprings derived from backcrosses.
As in classical Mendelian studies, pure parental mouse strains were obtained by inbreeding, that is, by repetitious matings of siblings [Altman and Dittmer Katz, 1979]. Inbreeding of both strains has been carried out for over 40 years and so they exhibit a high index of homocigocity. Previous phenotypic analyses of the IISPs have shown no single variation among mice in either strain after screening about 300 A/Snell and A.Swiss mice altogether. Randomly organized groups of 20 A/Snell and 20 A.Swiss mice, constituting the parental generation in this study, fully reproduced our observations [López Solís et al., 2003b]. As expected, we observed no variation in the phenotypic expression of the hybrid F1 generation. Strikingly, however, all the F1 mice expressed simultaneously the whole set of A/Snell IISPs and the whole set of A.Swiss IISPs. That absence of dominance, then, allowed direct identification of the hybrids. Thus, in the F2 offspring three different phenotypes could be identified, namely, the A/Snell phenotype, the A.Swiss phenotype, and the hybrid phenotype. Accordingly, in the F2 generation, both “pure” parental phenotypes, which had fully disappeared in the F1 generation, reappeared. Furthermore, in the F2 generation, the hybrid phenotype almost doubled each of the parental phenotypes. These observations, plus the full absence of recombinants in the F2 generation, that is, mice expressing a subset of the strain-associated sets of IISPs under consideration, are consistent with the occurrence of either a single gene or several nonallele closely linked genes coding for the IISPs in each one of both homozygous strains.
In order to more fully substantiate these views, backcrosses were carried out. To do so, we first crossed F1 males with A/Snell females and, vice versa, F1 females with A/Snell males. Both types of crosses produced offsprings displaying undistinguishable results. Altogether, these backcrosses produced mice displaying F1 and A/Snell phenotypes with a 1:1 ratio. Apart from suggesting a sex-independent pattern of IISP inheritance, these results would be also consistent with the involvement of either a single gene or multiple (two or more) closely linked nonallele genes in coding the A/Snell IISPs under consideration. Backcrosses F1 × A.Swiss gave the same type of results, that is, sex-independent inheritance of the IISPs and mice displaying F1 and A.Swiss phenotypes with a 1:1 ratio. As a whole, 172 mice resulting from the backcrosses F1 × A/Snell and F1 × A.Swiss were screened. Once more, none of the backcrosses produced recombinant mice, that is, mice expressing a subset of the strain-associated sets of IISPs under consideration, thus suggesting again the involvement of a single IISP-coding locus or multiple closely linked IISP-coding loci in both strains.
Given the congenic character of the A/Snell and A.Swiss strains with regard to the H-2 histocompatibility gene complex, mapped to chromosome 17, it was reasonable to expect that the gene or genes coding for the IISPs in both strains were located at a single genetic region of that chromosome [Klein, 1975; Abbas et al., 1991]. However, genes coding for a diversity of proline-rich proteins have been related to several chromosomes other than chromosome 17. In effect, in mouse, PRP-coding genes have been identified on chromosomes 3 [Delhomme and Djian, 2000], 4 [Kim et al., 2002], 6 [Azen et al., 1989], 8 [Azen et al., 1984], and 15 [Yang and Mansour, 1999]. As described before, in the particular case of the salivary PRP-coding murine genes, earlier evidence derived from DNA analysis of somatic cell hybrids have shown that some of them, such as the nonallelic MP2 and M14 genes, would be located on chromosome 8 [Azen et al., 1984; Ann et al., 1988]. More recent studies based on in situ hybridization and high resolution genetic mapping, however, have assigned mouse salivary PRP genes to chromosome 6 in tight association with the sucrose octaacetate taste aversion (Soa) locus [Azen et al., 2000; Bachmanov et al., 2001]. In human, loci coding for various subfamilies of PRP-salivary proteins have been also mapped to more than a single chromosome, as it is the case of proximal chromosome 12 (PRB1–PRB4, PRH1, PRH2) and distal chromosome 4 (PROL3 and PROL5) [Kim et al., 1993; Isemura and Saitoh, 1997]. The present study has strongly suggested that at least two IISP-coding genes, which have become segregated into the inbred mouse strains A/Snell and A.Swiss, are located on the same chromosome pair and would be part, correspondingly, of a single locus or closely linked loci. It remains to be established whether the segregated IISP-coding genes might correspond to either MP2, M14, or any other insofar undescribed murine salivary PRP gene and whether they are part of either chromosomes 6 or 8.
Segregation of the IISP-coding genes into both strains might have occurred quite likely sometimes during the production of both the A/Snell strain and the allogeneic mouse strain used later to construct the congenic A.Swiss strain [Klein, 1975; Altman and Dittmer Katz, 1979]. Certainly, an alternative possibility is that initially the same IISP-coding gene or genes was or were shared by both congenic strains but later one or some of them became mutated. Further analysis of the IISPs in other mouse strains related phylogenetically to A/Snell or A.Swiss might be of relevance for getting insight into the origin of the salivary genetic differences that we have observed between the A/Snell and A.Swiss strains.
Though allelic or nonallelic relationships between the IISP-coding genes in the A/Snell and A.Swiss strains is a matter that still remains to be solved, the possibility that a single gene may code for at least three or four different polypeptides in each of both mouse strains (C–E in A/Snell and P–R in A.Swiss), as suggested by the present study, may well involve a combination of various molecular mechanisms, such as alternative mRNA splicing and post-translational processing (cleavage) of the protein product or products, as it has been described in the case of human and rodent PRPs [Maeda et al., 1985; Ann et al., 1987, 1997; Lyons et al., 1988; Azen et al., 1993, 1996; Kim et al., 1993; Miao et al., 1995; Zhou et al., 1997; Ann and Lin, 1998]. By contrast, in the eventuality that two or more closely linked genes were necessary to code for the whole set of IISPs in each parental strain, recombinants expressing just a subset of the strain-associated IISPs under consideration might occur in some of the generations analyzed in the present study (F2 and backcrosses). A screening wider than the present one would be necessary to confirm or dismiss such a possibility. As a whole, the availability of two pure mouse strains that are monomorphic for the IISP character, such as A/Snell and A.Swiss, might constitute a highly valuable tool to carry out further studies on these and other genetic relationships involved in the expression, diversity, and role of the IISPs, at least within a single species.




