Role of PTEN and Akt in the regulation of growth and apoptosis in human osteoblastic cells
Abstract
Cancer cells are characterized by either an increased ability to proliferate or a diminished capacity to undergo programmed cell death. PTEN is instrumental in regulating the balance between growth and death in several cell types and has been described as a tumor suppressor. The chromosome arm on which PTEN is located is deleted in a subset of human osteosarcoma tumors. Therefore, we predicted that the loss of PTEN expression was contributing to increased Akt activation and the subsequent growth and survival of osteosarcoma tumor cells. Immunoblot analyses of several human osteosarcoma cell lines and normal osteoblasts revealed relatively abundant levels of PTEN. Furthermore, stimulation of cell growth or induction of apoptosis in osteosarcoma cells failed to affect PTEN expression or activity. Therefore, routine regulation of osteosarcoma cell growth and survival appears to be independent of changes in PTEN. Subsequently, the activation of a downstream target of PTEN activity, the survival factor Akt, was analyzed. Inappropriate activation of Akt could bypass the negative regulation by PTEN. Analyses of Akt expression in several osteosarcoma cell lines and normal osteoblasts revealed uniformly low basal levels of phosphorylated Akt. The levels of phosphorylated Akt did not increase following growth stimulation. In addition, osteosarcoma cell growth was unaffected by inhibitors of phosphatidylinositol-3 kinase, an upstream activator of the Akt signaling pathway. These data further suggest that the Akt pathway is not the predominant signaling cascade required for osteoblastic growth. However, inhibition of PTEN activity resulted in increased levels of Akt phosphorylation and enhanced cell proliferation. These data suggest that while abundant levels of PTEN normally maintain Akt in an inactive form in osteoblastic cells, the Akt signaling pathway is intact and functional. © 2003 Wiley-Liss, Inc.
Abbreviations used:
Akt, protein kinase B; EGF, epidermal growth factor; IGF-1, insulin-like growth factor 1; LOH, loss of heterozygosity; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide; OS, osteosarcoma; PI-3 kinase, phosphatidylinositol 3′ kinase; PTEN, phosphatase and tensin homologue; Rb, retinoblastoma.
Osteosarcoma (OS) is the most common form of primary bone cancer and occurs predominantly in children and young adults. This cancer has a high rate of metastasis and patients identified as having metastatic disease at diagnosis experience a 5-year survival rate as low as 20% [Meyers et al., 1993; Saeter et al., 1995]. During the last few decades, a combination of surgical and chemotherapeutic treatments of OS primary tumors has brought the survival of patients without metastatic disease to nearly 80% [Meyers et al., 1992]. Clearly, a better understanding of the disease process in these patients is necessary to target treatment and consistently improve prognosis.
OS results from dysregulated growth control of osteoblast-like cells. Several studies of OS primary tumors and cell lines have identified the overexpression of genes such as c-fos, c-myc and mdm2 [Ladanyi et al., 1993; Rabbani et al., 1997]. In addition, loss of heterozygosity (LOH) studies have identified genetic loss within 3q, 10q, 13q, 17p, and 18q [Toguchida et al., 1989; Scheffer et al., 1991; Yamaguchi et al., 1992; Kruzelock et al., 1997]. Complimentary studies have established that the loss on 13q and 17p involve inactivation of the tumor suppressor genes RB and p53, respectively [Toguchida et al., 1988; Wadayama et al., 1994]. The loss of genetic information on chromosome 10q in OS may suggest a role for PTEN (phosphatase and tensin homologue, 10q23.3) [Li et al., 1997; Steck et al., 1997].
The PTEN protein (also referred to as MMAC1 for ‘mutated in multiple advanced cancers’ [Steck et al., 1997]) has been identified as a tumor suppressor in a variety of cancers including prostate, breast, and endometrial [Steck et al., 1997; Whang et al., 1998; Kanamori et al., 2001]. Several studies have evaluated tumor samples for mutations and deletions of the PTEN gene, yet abnormalities of protein expression have also been documented. PTEN is a pivotal protein regulating the balance between cell growth and death, therefore, the loss of PTEN is instrumental in the survivability of cancer cells in several systems [Dahia et al., 1999, 1999; Ramaswamy et al., 1999]. PTEN is a 60-kDa dual specificity phosphatase [Myers et al., 1997] that can dephosphorylate serine, threonine and tyrosine residues as well as phosphatidylinositols [Maehama and Dixon, 1998; Myers et al., 1998]. The lipid phosphatase activity of PTEN is necessary for the protein to function in the capacity of a tumor suppressor [Myers et al., 1998] and the protein phosphatase activity induces G1 cell cycle arrest by down-regulating cyclin D1 [Weng et al., 2001a].
Functionally, PTEN affects cell phenotypes by indirectly inactivating members of signaling programs such as Akt, a kinase identified based on oncogenic effects of the AKT8 virus [Staal, 1987; Stambolic et al., 1998; Wu et al., 1998; Cantley and Neel, 1999; Dahia et al., 1999; Weng et al., 2001b]. Phosphatidylinositol(3,4,5)P3 mediates cell growth and survival by transducing extracellular signals to intracellular cascades involving molecules such as the serine–threonine kinase Akt [Burgering and Coffer, 1995; Klippel et al., 1996; Stiles et al., 2002]. Upon the appropriate signaling, Akt translocates to the plasma membrane where it interacts with phosphatidylinositol via its pleckstrin homology domain [Klippel et al., 1997]. This membrane localization enables Akt to be phosphorylated by a phosphoinositide-dependent kinase (PDK1) [Cantley, 2002] with a subsequent stimulation of catalytic activity. Akt has several downstream targets that promote cell growth and survival, including inactivation of the apoptosis-inducing molecule BAD [del Peso et al., 1997; Kulik et al., 1997] and down regulating p27, an inhibitor of cyclin-dependent kinases [Sun et al., 1999]. Akt activation is counter balanced by the lipid phosphatase activity of PTEN which provides a negative regulation of PI-3 kinase signaling [Wu et al., 1998] and reduces the level of phosphatidylinositol(3,4,5)P3 available for Akt recruitment.
The importance of the signaling cascades involving Akt and PTEN in regulating normal osteoblastic growth, apoptosis and differentiation has not been fully elucidated. Akt can be activated by growth factors that stimulate bone growth or differentiation such as PDGF or BMP-2, respectively, but not by factors such as FGF-2 [Chaudhary and Hruska, 2001; Ghosh-Choudhury et al., 2002]. Akt also appears to be differentially activated by bone morphogenetic protein-7 and IGF-1 during osteoblastic differentiation [Shoba and Lee, 2003] and may be minimally affected by calcium flux in osteoblasts under mechanical stress [Danciu et al., 2003]. In addition, the contribution of PTEN mutations in bone malignancy is inconclusive. PTEN mutations are rare in human chondrosarcomas [Lin et al., 2002] but have not been evaluated in human OS. Mutations and loss of expression have been documented in canine OS, yet many molecular changes associated with this disease differ from the human condition [Levine et al., 2002]. The studies described herein were designed to evaluate whether the loss of PTEN expression in human osteoblastic cells contributes to a proliferative advantage. We examined the basal levels of PTEN protein as well as expression levels following growth and apoptotic stimuli in human osteoblast-like cells. We also evaluated the expression and activity of a downstream target of PTEN, the survival factor Akt. In combination with inhibitor studies, these data suggest that regulation of neither Akt nor PTEN is required for cell growth or apoptosis and is not providing a growth advantage in the OS cell lines that we analyzed.
MATERIALS AND METHODS
General Materials and Reagents
All chemicals were obtained from Sigma (St. Louis, MO) and cell culture plastics were obtained from Midwest Scientific (Valley Park, MO) unless otherwise indicated.
Human Cell Culture
The cell lines SaOS2, MG63, MNNG/HOS, and U2OS were obtained from the American Type Culture Collection (Rockville, MD). SaOSLM2 [Radinsky et al., 1994] and SaOSLM7 [Jia et al., 1999] are variants of SaOS2 and were kindly provided by Dr. R. Radinsky and Dr. E. Kleinerman, respectively, of the M.D. Anderson Cancer Center, Houston, Texas. 99-1 was initiated from a highly aggressive primary human OS (Institutional Review Board approval—protocol no. 99-662). All cells were maintained in RPMI (Mediatech CellGro, Herndon, VA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), nonessential amino acids, MEM vitamins, sodium pyruvate and sodium bicarbonate (Mediatech). Normal human osteoblasts (hOST) were purchased from Clonetics (BioWhittaker, Walkersville, MD) and maintained in OBM media provided by the manufacturer supplemented with 10% fetal bovine serum and ascorbic acid. All cell lines were subcultured at 80% confluence and routinely maintained in a humidified incubator at 37°C with 5% CO2.
Cell Proliferation Assays
Cell growth was analyzed using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) assay kit (Roche Molecular Biochemicals, Indianapolis, IN). Cells were plated in quadruplicate in 96-well plates (1,250 cells/well). After 18 h, the monolayers were washed with PBS and subjected to growth conditions as described. At the indicated time-points, MTT reagent was added to the media at a final concentration of 1.2 mM. The cells were allowed to incubate for an additional 4 h at 37°C with 5% CO2 at which time the formazan crystals were solubilized by the addition of an equal volume of solubilization buffer (10% SDS, 0.01 M HCl). Optical density was recorded at 570 nm in a Microplate reader (BioRad, Hercules, CA) and values were normalized to untreated cells 24 h after plating.
Cell Cycle Analysis
Cell cycle analysis was performed following fluorescence labeling of cellular DNA by propidium iodide. Cells were seeded into 6-well plates and incubated overnight (50% confluence) in complete medium. Cell monolayers were rinsed with PBS and incubated for 18 h in serum-free RPMI. The media was then replaced with fresh serum-free media for an additional 1 h. When indicated, cells were incubated with aphidicolin (14.8 mM) in complete media for an additional 18 h. The cells were then released from the cell cycle block in media containing serum, insulin like growth factor (IGF-1: 400 U/ml), epidermal growth factor (EGF: 200 U/ml) or 17β-estradiol (10−8 M). At the indicated time-points, the monolayers were rinsed with PBS and trypsinized. The trypsin was inhibited by the addition of complete media and the cells were pelleted by low speed centrifugation (400g for 5 min). The supernatant was aspirated and the cells were resuspended in 1 ml of propidium iodide stain (3.8 mM sodium citrate, 69 mM propidium iodide, 0.01% NP40, 0.01 mg/ml RNase) and incubated overnight at 4°C. Cell cycle analysis was performed by the University of Colorado Health Sciences Cancer Center Flow Cytometry Core facility.
Induction and Detection of Apoptosis
Apoptosis was measured using differential cellular incorporation of fluorescent dyes [Mishell et al., 1980]. Cells were seeded in 6-well plates and incubated overnight in complete media. Cell cultures were exposed to ultraviolet light with a wavelength of 312 nm (Transilluminator, Fisher Scientific, Pittsburgh, PA) for 10 min. The cells were then incubated at 37°C. At the indicated time-points, the cultures were analyzed for floating cells, washed with PBS and stained with acridine orange and ethidium bromide (10 mg/ml each in PBS). Following a 10 min incubation, the cells were rinsed with PBS and analyzed by fluorescence microscopy using a Nikon TE200 inverted microscope (Nikon, Melville, NY) at magnification 200×.
Protein Isolation and Immunoblot Analysis
Cells were grown to 80% confluence, unless otherwise indicated. Cell monolayers were rinsed twice with cold PBS and lysed in RIPA buffer (150 mM NaCl, 50 mM Tris, pH 8.3, 0.1% SDS, 0.5% deoxycholic acid, and 1.0% Igepal) containing 1 mM sodium vanadate on ice. Cell residue was physically disrupted with a cell scraper and collected in a microcentrifuge tube. The DNA was removed from the cell lysate by centrifugation at 12,000g for 25 min. Soluble protein was quantitated using the BCA protein quantitation kit (Pierce, Rockford, IL) using bovine serum albumin as the protein standard.
Equal amounts of protein (25 μg/lane) were combined with five times sample buffer (50% glycerol, 0.5% bromophenol blue, 10% SDS, 250 mM Tris, pH 6.8, 72 mM β-mercaptoethanol, and 6.5 mM dithiothreitol) and heated to 94°C for 10 min. Denatured and reduced protein samples were electrophoresed through a 10% SDS–polyacrylamide gel and electrotransferred to a PVDF membrane (Schleicher & Schuell, Inc., Keene, NH) using the mini-transblotter system (BioRad, Hercules, CA). Following immobilization on nylon membranes, protein loading was evaluated by staining with Ponceau S. Nonspecific protein binding was blocked by incubating the membranes in blocking buffer (5% nonfat dry milk, 0.2% Tween-20 in PBS) for 1 h at ambient temperature. Membranes were then incubated for 18 h at 4°C with one of the following antibodies at a 1:1,000 dilution: PTEN polyclonal (Cell Signaling/Cascade Bioscience, Winchester, MA), phospho-Akt1/2 monoclonal (Cell Signaling), total Akt1/2 polyclonal (Upstate Biotechnology, Lake Placid, NY), actin monoclonal (Sigma, St. Louis, MO), phospho-Erk 1/2 polyclonal and total Erk 1/2 polyclonal (Cell Signaling, Beverly, MA). Membranes were washed in PBS containing 0.2% Tween-20 (3× 20 min) and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA). Following a 2-h incubation at ambient temperature, the membranes were washed as described above. Protein-antibody interactions were visualized using chemiluminescence (KPL, Gaithersburg, MD).
PTEN Activity Assays
Phospholipid vesicles were generated by vortexing phosphatidylserine (625 μg/ml) and phosphatidylinositol (3,4,5) P3 (300 μg/ml) (Biomol, Plymouth Meeting, PA) in 10 mM HEPES, pH 7.4, 1 mM EGTA, and 1 mg/ml BSA. PTEN was immunoprecipitated from RIPA-solubilized cell lysates and combined with phospholipid vesicles (10 μl) in a final volume of 50 μl Enzyme Assay Reaction Buffer (500 mM Tris, pH 8, 10 mM dithiothreitol) in a microtiter plate. Malachite Green reagent (100 μl) (Biomol, Plymouth Meeting, PA) was added to each well and incubated for 15 min at ambient temperature. Absorbance was measured at a wavelength of 570 nm. Activity was determined by comparing the optical densities to those obtained from a phosphate standard curve in the same assay.
RESULTS
OS and Osteoblast Cells Express Comparable Basal Levels of PTEN
The chromosomal loss of 10q has been documented in nearly 30% of human OS tumor samples analyzed [Yamaguchi et al., 1992]; therefore, we proposed that the loss of PTEN expression may be instrumental in OS cell growth. We evaluated the basal level of PTEN protein expression and compared it to actin levels in normal human osteoblasts and several human OS cell lines (Fig. 1A,B). We included the U2OS cell line which has shown to express PTEN by other investigators [Ramaswamy et al., 1999]. The PTEN protein was readily detected in the normal osteoblasts as well as in each of the OS cell lines analyzed. In addition, the PTEN protein is of the apparent molecular mass expected for PTEN (60 kDa), suggesting that there have not been major genetic deletions. The only other band visible on the blot was a protein of ∼35 kDa which may represent a breakdown product of PTEN. PTEN activity was assayed by malachite green detection of inorganic phosphate (Fig. 1C) [Han et al., 2000; Maehama et al., 2000]. PTEN activity varied among cell types, although each maintained active PTEN.
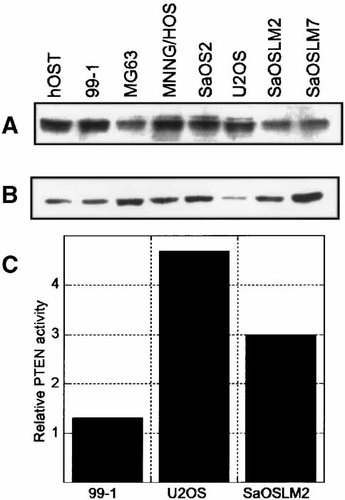
PTEN protein expression levels in several human osteoblastic cell types. A: Immunoblot analysis was used to detect the level of PTEN protein (60 kDa) in total cell lysates (25 μg) isolated from several OS and normal osteoblast cell cultures. B: The levels of actin (42 kDa) were detected by re-probing the same membrane with an actin-specific antibody. C: The level of PTEN activity was determined by evaluating phosphatase activity with Malachite green detection. Activity is expressed relative to an immunoprecipitation control.
PTEN Expression Is not Affected by Stimulation of OS Cells With Growth Factors
High basal levels of PTEN protein do not preclude it from regulating OS cell growth under stimulatory conditions. Stimulation by growth factors can diminish PTEN, lessen the antagonistic effects on Akt signaling, and thereby promote cell survival. To determine whether PTEN expression levels decrease upon stimulation of proliferation, U2OS cells were cultured in the presence of epidermal- and insulin-like growth factors (EGF, IGF-1, respectively) as well as the synthetic estrogen, 17β-estradiol. The level of PTEN expression was evaluated by immunoblot analysis 5 and 60 min following stimulation and compared to a non-stimulated control (Fig. 2A). Exposure of OS cells to EGF and 17β-estradiol did not affect PTEN expression, although the levels of PTEN were consistently diminished when cells were stimulated with IGF-1 for 60 min. We further evaluated the regulation of PTEN expression following a time course of IGF-1 stimulation (Fig. 2B). Although PTEN expression was reduced between 30–60 min following IGF-1 stimulation, long-term proliferation appeared to be unaffected (Fig. 2C). These data suggest that substantial changes in PTEN expression are not associated with long-term events such as cell growth, but do not rule out the possibility that PTEN is tightly regulated during progression of the cell cycle.
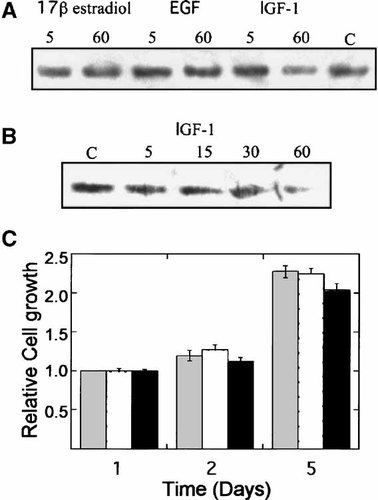
PTEN expression during growth factor stimulation of U2OS cells. A: U2OS cells were serum-starved for 18 h and immediately lysed (control ‘C’) or stimulated to grow by the addition of 17β-estradiol, EGF, or IGF-1. Cell lysates were harvested at 5 and 60 min following stimulation and 25 μg of protein was loaded per lane for immunoblot analysis of PTEN expression. B: U2OS cells were stimulated with IGF-1 following serum starvation. PTEN expression was evaluated at 0 (‘C’), 5, 15, 30, and 60 min. C: U2OS cells were cultured in the presence of serum (stippled bars), EGF (white bars), or IGF-1 (black bars) for up to 5 days. Cell growth and viability were monitored using the MTT assay and optical density values were compared to those obtained 24 h after seeding the cells.
PTEN Expression Does not Vary During Cell Cycle Progression of U2OS Cells
PTEN has been shown to convey a G1 block in the cell cycle [Ramaswamy et al., 1999; Weng et al., 1999; Gottschalk et al., 2001; Weng et al., 2001a]. In order to evaluate the contribution of PTEN to progression through the cell cycle, U2OS cells were synchronized using serum-starvation followed by an aphidicolin block. Cells were then released to enter S-phase by removal of aphidicolin in the presence of serum (Fig. 3A). We found that serum-starvation alone was not effective at halting the OS cell cycle progression (Fig. 3A, maximum of 60% of cells in G1), therefore, we used a double-block system to synchronize 90% of the cells in S phase. U2OS cells were harvested at various time-points following serum stimulation. At each time point, cells were subjected to cell cycle (Fig. 3A) analysis and a companion culture was lysed for immunoblot analysis (Fig. 3B,C). Within 2 h of release from aphidicolin, cells were primarily in G1 (75%), by 4–6 h the cells had almost exclusively entered S-phase (90%), and by 10 h a majority of cells had progressed to the G2/M-phases (70%). The expression of PTEN remained relatively uniform throughout progression of the cell cycle (Fig. 3B). In addition, the activity of PTEN remained relatively constant during cell cycle progression (Fig. 3D). When the cells entered the G1-phase of the cell cycle, PTEN activity increased slightly, but was not statistically significant.
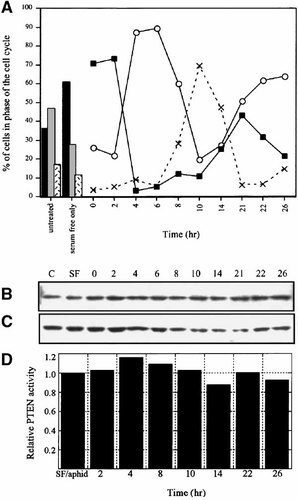
PTEN expression and activity during cell cycle progression. U2OS cells were serum-starved for 18 h, rinsed and starved for an additional 1 h (‘serum-free only,’ SF). Aphidicolin was added to the cells in the presence of complete media to provide and additional block of the cells in late G1. At time 0, aphidicolin was removed and the cells were stimulated in complete media containing 10% serum. At the indicated times, parallel cultures of cells were subjected to cell cycle, immunoblot and PTEN activity analyses. A: Cells were stained with propidium iodide and subjected to flow cytometry. Solid bars and boxes -percentage of cells in G1, gray bars and open circles—percentage of cells in S-phase, stippled bars and dotted lines—percentage of cells in G2/M-phases of the cell cycle. B: Cells were lysed in RIPA buffer and analyzed by immunoblot for PTEN expression. Control (untreated ‘C’) cells were those that were maintained in complete media throughout the experiment. C: Immunoblots from panel B were reprobed with an actin-specific antibody as a loading control. D: PTEN was immunoprecipitated from cell lysates and the lipid phosphatase activity was analyzed using the Malachite green detection system. Activity is expressed relative to that obtained for cells synchronized in late G1 (time 0).
PTEN Expression Does not Increase Coincident With the Onset of UV Light-Induced Apoptosis
PTEN is reported to have a positive effect on the ability of cells to undergo apoptosis. PTEN activity prevents the recruitment and activation of Akt, thereby stabilizing the activity of the apoptosis-inducing molecule, BAD [Datta et al., 1997]. To evaluate whether PTEN expression changes during programmed cell death, we subjected U2OS and 99-1 OS cells to ultraviolet light-induced apoptosis. Cell lysates were analyzed for PTEN expression at several time points following exposure to ultraviolet light (Fig. 4A,B). The fate of OS cells was monitored by the differential uptake of fluorescent dyes over a period of 20 h (Fig. 4C). Apoptosis appeared to progress at different rates in the two cell lines. At 6 h post UV exposure, 20–30% of the 99-1 cells were dead (ethidium bromide incorporation–red/orange), whereas 75–90% of the U2OS cells were dead. We were unable to detect an increase in PTEN expression at the time-points during which the OS cells were undergoing apoptosis. The diminishment of PTEN expression levels at later time points (6–20 h) likely represents normal protein turnover in the absence of new synthesis (half-life of PTEN is 4 h) [Tolkacheva et al., 2001].
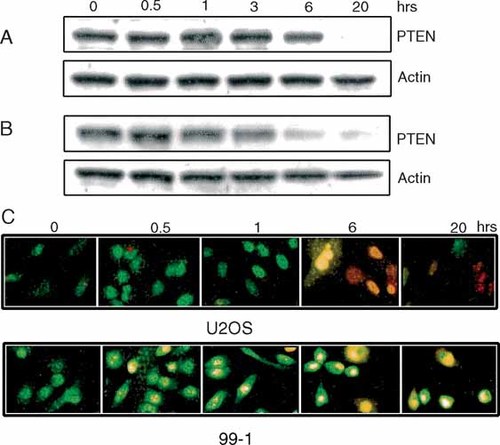
PTEN expression levels following treatment of cells with ultraviolet light. U2OS (A) and 99-1 (B) cell monolayers were exposed to ultraviolet light for 10 min to induce an apoptotic response. At the indicated times, parallel cultures of cells were lysed and subjected to immunoblot analysis for the expression of PTEN followed by actin. C: Parallel cultures were stained with ethidium bromide (enters only dead cells, yellow/red) and acridine orange (enters all cells, green) to differentiate between live cells, those undergoing apoptosis and dead cells—magnification 200×.
The goal of these studies was to identify whether there was an increase in PTEN expression concomitant with the induction of apoptosis. Therefore, we further evaluated PTEN expression in osteoblastic cells at shorter time increments during the onset of apoptosis (Fig. 5). PTEN expression levels remained relatively stable for up to 5 h (Fig. 5A,B) following UV irradiation. Apoptosis was observed within 3 h and a majority of cells were dying by 5 h post-exposure (Fig. 5C); therefore, changes in protein expression or activity (data not shown) are not required for progression of OS cells through programmed cell death.
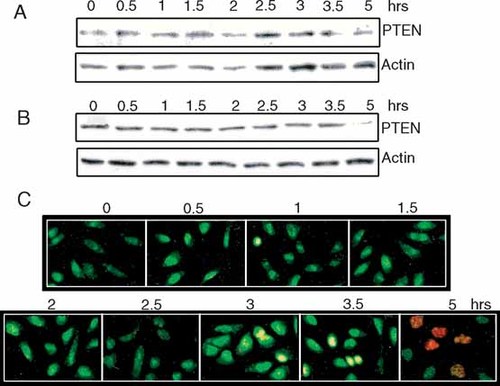
PTEN expression levels during the onset of apoptosis. U2OS (A) and 99-1 (B) cells were induced to undergo apoptosis by exposure to UV light. Parallel cultures were lysed for the detection of PTEN followed by actin. C: Companion cultures of U2OS cells were stained to detect cells undergoing apoptosis. Progression of apoptosis was monitored every 30 min over the course of 5 h. Cells were viewed at 200 × magnification.
Akt Is not Activated Following Serum Stimulation
The presence of constitutively activated Akt would have the potential to override PTEN regulation and still implicate the PTEN/Akt pathway in OS growth. We analyzed the expression of the phosphorylated form of Akt relative to total Akt1/2 during progression of the cell cycle (Fig. 6A,B). The levels of phosphorylated Akt were nearly undetectable throughout the cell cycle (detectable signal at 8, 10, and 21 h), although the inactive form of the protein was abundant. These data suggest that there is not a sustained activation of Akt during the cell cycle.
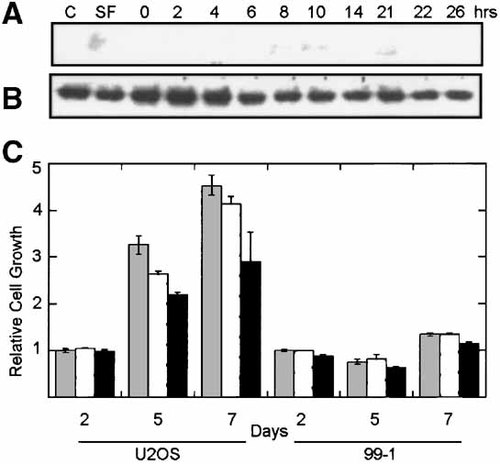
Activation of Akt during osteoblastic cell proliferation. U2OS cells were synchronized and lysed at various times during cell cycle progression. At each time point, cell lysates were generated and phosphorylated Akt1/2 (A) or total Akt1/2 (B) was analyzed by immunoblot. C: Proliferation of U2OS and 99-1 cells was evaluated over the course of 7 days in the presence of the PI-3 kinase inhibitor, wortmannin at 1 μM (white bars), 10 μM (black bars), and compared to untreated cells (stippled bars).
The studies described thus far would suggest that downregulation of PTEN or activation of Akt is not required for cell cycle progression or growth of OS cells under the conditions analyzed. To further evaluate whether regulation of Akt is necessary for proliferation we evaluated cell growth in the presence of inhibitors of PI-3 kinase. PI-3 kinase is responsible for transducing a signal from growth factor receptors to downstream signaling molecules such as Akt. The PI-3 kinase inhibitor wortmannin can be used to block Akt activation and cell survival and proliferative signals. As illustrated in Figure 6C, wortmannin (and LY294002, data not shown) had only a minimal effect on the growth of OS cells, and only then at relatively high concentrations. These data further suggest that activation of Akt, or inhibition of PTEN, is not required for normal survival and proliferation of osteoblastic cells. We were also unable to detect phosphorylated Akt when PTEN levels were diminished following an apoptotic stimulus (data not shown).
Akt Signaling Pathway Is Inducible in Osteoblastic Cells
We evaluated whether the Akt signaling pathway was functional in osteoblastic cells, yet normally maintained in an inactive state by PTEN. For these studies, cells were treated with hydrogen peroxide to cause the reversible oxidation-induced inhibition of PTEN lipid phosphatase activity [Lee et al., 2002]. As illustrated in Figure 7A and B, Akt phosphorylation was increased in hydrogen peroxide-treated cells in a dose dependent manner relative to total Akt1/2 expression, suggesting that Akt can be activated in an environment of reduced PTEN activity. In similar experiments, we demonstrate that hydrogen peroxide treatment resulted in a proportional increase in a higher mobility form, proposed to be an oxidized form of PTEN (Fig. 7C). Treatment of osteoblastic cells with hydrogen peroxide resulted in a 40% decrease in PTEN activity and did not cause a concomitant increase in Erk1/2 phosphorylation, a MAP kinase potentially regulated by PTEN [Gu et al., 1998] (data not shown). Under these conditions, diminished PTEN activity appeared to coincide with an increase in Akt phosphorylation; therefore, we evaluated whether cells exposed to hydrogen peroxide also experienced a growth advantage. Figure 7D illustrates that upon inhibition of PTEN activity, cells have increased capacity for growth or survival. These data demonstrate that the Akt signaling pathway is functional in osteoblastic cells and can be induced, but appears not to be a predominant mechanism by which cell growth and apoptosis are normally controlled.
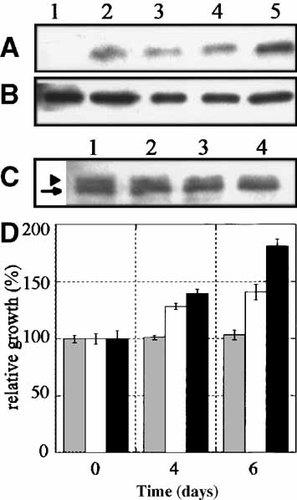
Akt activation upon inhibition of PTEN activity. Normal osteoblasts were serum-starved for 18 h, treated with hydrogen peroxide in serum-free conditions for 20 min and then stimulated by the addition of 10% FBS in the presence of hydrogen peroxide. A: Cell lysates were generated and subjected to immunoblot analysis for phosphorylated Akt. Cells were serum starved (lane 1), stimulated in the absence of hydrogen peroxide (lane 2) or stimulated in the presence of increasing concentrations of hydrogen peroxide; 50 μM (lane 3), 200 μM (lane 4), or 1 mM (lane 5). B: Total Akt1/2 was detected in the same blot from panel A. C: Cells were lysed following a 20 min treatment with varying concentrations of hydrogen peroxide; untreated (lane 1), 50 μM (lane 2), 100 μM (lane 3), and 500 μM (lane 4). PTEN was detected by immunoblot analysis. D: Proliferation of normal human osteoblasts was evaluated in the presence of hydrogen peroxide. Cells were seeded (1,000 cells per well) in 96-well plates. Hydrogen peroxide (untreated, stippled bars; 50 μM, white bars; 200 μM, black bars) was added to the wells on day 0 and replaced every third day. At the indicated times, cell proliferation was evaluated using an MTT assay. Data is expressed as percentage growth relative to untreated cells set at 100%.
DISCUSSION
Chromosomal loss of 10q has been documented in a subset of human OS tumors, but specific tumor suppressor genes residing in this region have not been implicated in the disease process [Yamaguchi et al., 1992]. PTEN is a dual specificity phosphatase that has been described as a tumor suppressor in several forms of cancer and has the genetic address of chromosome 10q23.3 [Steck et al., 1997]. The studies described herein were designed to evaluate whether PTEN may be functioning in a manner compatible with tumor suppression in human OS. Gross reductions in PTEN expression level or activity were undetectable among the cell lines examined. Recent karyotypic analyses of cells from each of these lines also indicates that chromosome 10q is retained (data not shown). Although PTEN was not lost at the genetic level in the subset of OS cells we evaluated, subtle changes in activity may still affect cell phenotype. We predicted that minor or transient changes in PTEN activity may be most easily detected by analyzing a downstream target the survival factor Akt. Therefore, the phosphorylation of Akt was evaluated in a panel of human OS cell lines. Total Akt1/2 was readily detected in each of the cell lines we analyzed, yet only minimal levels of the phosphorylated form of the serine threonine kinase could be detected. In combination, these data suggest that the PTEN/Akt pathway is not instrumental for controlling programs for either growth or death in human OS cells. This conclusion was further supported by our data in which the PI-3 kinase inhibitors had only minimal affects on the growth and survival of osteoblastic cells. We and others have shown that osteoblastic cells are relatively resistant to the effects of PI-3 kinase inhibitors. Whereas murine and human neuroblastoma cells have been shown to be sensitive to 50–100 nM wortmannin, respectively [Stambolic et al., 1998; van Golen et al., 2001], osteoblastic cells appear to require at least fivefold higher concentrations of the inhibitor to elicit a response [Kubota et al., 1998; Chaudhary and Hruska, 2001]. Indeed, OS cells continued to proliferate in the presence of 10 μM wortmannin in our studies. Therefore, osteoblastic cells appear to depend predominantly on signaling cascades distinct from those requiring PI-3 kinase activity. The relatively common loss of chromosome 10q in both human OS and chondrosarcoma suggests the presence of tumor suppressor gene(s) other than PTEN. Further studies are needed to identify and characterize these candidate tumor suppressors and better define important mechanisms involved in mesenchymal tumor formation.
The relevance of the PTEN/Akt signaling pathway in other forms of cancer make our results surprising, yet not without precedent. The Rb-null SaOS2 cell line has been used as a control for PTEN overexpression studies [Ramaswamy et al., 1999]. These investigators found that overexpression of PTEN did not induce a G1 block in SaOS2 cells as it did in renal carcinoma cells. In accompanying studies, another OS cell line, U2OS, was shown to have high levels of PTEN and nearly undetectable levels of phosphorylated Akt, neither of which was affected by serum stimulation. Although OS cells were used only as controls in these studies due to their Rb and p53 status, the results obtained mirror the PTEN and Akt activity we detected in our experiments. Additional reports have demonstrated that in MG-63 cells, radiation-induced apoptosis or PTHrP-induced differentiation are not affected by wortmannin, further supporting the involvement of signaling pathways other than PTEN/Akt in the expression of human OS phenotypes [Kubota et al., 1998; Carpio et al., 2001]. The list of alternative pathways utilized by osteoblastic cells and corrupted in tumor cells will undoubtedly include members of the mitogen-activated protein kinases. For example the Erk1/2 pathway has been shown to be instrumental in several aspects of osteoblastic growth and differentiation [Zhang et al., 1999; Lou et al., 2000; Lai et al., 2001; Sowa et al., 2002]. The contribution of these signaling pathways to OS cell growth will require further investigation.
The data presented here clearly illustrate that chromosomal loss of PTEN is not required for survival of human OS tumor cells, but raises interesting questions concerning the function of PTEN in these cells. The well-defined role of PTEN as a PI-3 kinase antagonist necessitates localization at the plasma membrane. By immunofluorescence, we found PTEN to be predominantly localized to the nucleus in OS cells (data not shown). Studies in thyroid and pancreatic cancer cells have also demonstrated that PTEN can be localized to the nucleus and the authors suggest that PTEN activity is at least partially controlled by subcellular localization [Gimm et al., 2000; Perren et al., 2000]. Other studies have indicated that PTEN functions at multiple sites within the cell during neuroblast differentiation [Lachyankar et al., 2000]. Although a specific nuclear function was not described in these studies, Weng et al. [2001a] have characterized a role for the protein phosphatase activity of PTEN in the downregulation of cyclin D1 and subsequent G1 arrest in breast cancer cells. Further studies are needed to investigate the physiological relevance of nuclear PTEN in human OS cells.
The improper balance between death and growth in tumor cells can involve several signaling molecules, but the PTEN/Akt pathway is commonly implicated. The experiments presented here illustrate the insufficiency of the PTEN/Akt pathway in regulating several human OS cell phenotypes and raise interesting questions: (1) What signaling pathways are critical for OS growth and death? We have found that Erk1 is activated upon serum stimulation in several human OS cell lines (data not shown). Erk1/2 can be activated by either a PI-3 kinase/protein kinase C or a ras/raf pathway [Siow et al., 1997; Park et al., 2003]; therefore, its role in regulating cell survival and proliferation will be explored relative to OS. In addition, apoptosis in SaOS2 cells has been shown to be controlled by Fas-dependent and -independent mechanisms [Fellenberg et al., 2000; LaFleur et al., 2001]. The contribution of Fas, as well as other apoptosis-inducing signaling programs to human OS, will require further investigation. (2) What is the role of the PTEN/Akt pathway in osteoblastic cells? Our data suggests that loss of PTEN function, through oxidative inhibition, allows phosphorylation of Akt and a subsequent growth advantage. These results suggest that the Akt pathway is intact and inducible yet normally maintained in an inactive state by PTEN. Although we evaluated the relationship of PTEN and Akt activity in OS cell growth and death, we are currently exploring the possibility that this signaling program is important during osteoblastic differentiation and mineralization.
Acknowledgements
We thank Sarah Stroh for her technical support and Drs. Cohen and Gardal for assistance with the apoptosis experiments.




