Bmi-1 Overexpression Improves Sarcopenia Induced by 1,25(OH)2D3 Deficiency and Downregulates GATA4-Dependent Rela Transcription
Qiuyi Wang, Jingyu Zhao, Haiyun Chen these authors contributed equally to this work and should be considered co-first authors.
Abstract
Sarcopenia increases with age, and an underlying mechanism needs to be determined to help with designing more effective treatments. This study aimed to determine whether 1,25(OH)2D3 deficiency could cause cellular senescence and a senescence-associated secretory phenotype (SASP) in skeletal muscle cells to induce sarcopenia, whether GATA4 could be upregulated by 1,25(OH)2D3 deficiency to promote SASP, and whether Bmi-1 reduces the expression of GATA4 and GATA4-dependent SASP induced by 1,25(OH)2D3 deficiency in skeletal muscle cells. Bioinformatics analyses with RNA sequencing data in skeletal muscle from physiologically aged and young mice were conducted. Skeletal muscles from 2-month-old young and 2-year-old physiologically aged wild-type (WT) mice and 8-week-old WT, Bmi-1 mesenchymal transgene (Bmi-1Tg), Cyp27b1 homozygous (Cyp27b1−/−), and Bmi-1TgCyp27b1−/− mice were observed for grip strength, cell senescence, DNA damage, and NF-κB-mediated SASP signaling of skeletal muscle. We found that muscle-derived Bmi-1 and vitamin D receptor (VDR) decreased with physiological aging, and DNA damage and GATA4-dependent SASP activation led to sarcopenia. Furthermore, 1,25(OH)2D3 deficiency promoted DNA damage-induced GATA4 accumulation in muscles. GATA4 upregulated Rela at the region from −1448 to −1412 bp at the transcriptional level to cause NF-κB-dependent SASP for aggravating cell senescence and muscular dysfunction and sarcopenia. Bmi-1 overexpression promoted the ubiquitination and degradation of GATA4 by binding RING1B, which prevented cell senescence, SASP, and dysfunctional muscle, and improved sarcopenia induced by 1,25(OH)2D3 deficiency. Thus, Bmi-1 overexpression improves sarcopenia induced by 1,25(OH)2D3 deficiency, downregulates GATA4-dependent Rela transcription, and sequentially inhibits GATA4-dependent SASP in muscle cells. Therefore, Bmi-1 overexpression could be used for translational gene therapy for the ubiquitination of GATA4 and prevention of sarcopenia. © 2023 American Society for Bone and Mineral Research (ASBMR).
Introduction
Sarcopenia is characterized by decreased muscle mass, strength, and function and is a progressive and degenerative systemic skeletal muscle disease.(1, 2) Age has been reported to increase the risk of sarcopenia, and its prevalence rises to 30% in the population over 80 years of age.(3) With an ever-increasing aging population, skeletal muscle aging has become a major public health problem, and the mechanism of sarcopenia needs to be addressed, along with the design of novel treatments.
Vitamin D (VD) is converted to 25-hydroxylated VD by the action of hepatic 25-hydroxylase and then converted to active VD [1,25(OH)2D3] catalyzed by 1α-hydroxylase [encoded by Cyp27b1], which plays a biological regulatory role via binding to the VD receptor (VDR).(4) 1,25(OH)2D3 plays a critical role in downregulating the rate of aging,(5) and it has been reported that processes related to cellular aging, such as inflammation, oxidative stress, and DNA damage, can all be induced by 1,25(OH)2D3 deficiency,(6-8) which not only accelerates the rate of aging but also elevates the incidence of senescence-associated diseases.(9) 1,25(OH)2D3 functions to help control blood calcium and phosphate homeostasis, thereby supporting metabolic functions, neuromuscular transmission, and bone mineralization.(10-12) It is widely believed that 1,25(OH)2D3 supplementation could help prevent bone loss caused by aging.(13) A 1,25(OH)2D3 deficiency also contributes to a reduction in muscle mass.(14) The rate of the decline in strength and age-dependent atrophy in skeletal muscles can be predicted by serum VD levels in elderly individuals,(15) where VDR levels decrease in the muscles with aging.(16) In addition, 1,25(OH)2D3 can regulate the speed at which skeletal muscle progenitors access the area of an injury for repair and remodeling.(17) However, the exact mechanism of 1,25(OH)2D3 deficiency in the regulation of sarcopenia and the relationship to cellular senescence remain unclear. Whether 1,25(OH)2D3 deficiency could cause cellular senescence and a senescence-associated secretory phenotype (SASP) in skeletal muscle cells to induce sarcopenia needs to be further investigated.
Cellular senescence involves multiple molecular mechanisms, and DNA damage promotes many stimuli that trigger senescence.(18) DNA damage is a key inducer of oxidative stress-associated cellular senescence that results in the production of damage signals and activates ataxia telangiectasia and Rad-3-related protein (ATR) as well as ataxia telangiectasia-mutated (ATM) genes.(19) In response to ATR and ATM, the zinc finger transcription factor GATA4, a member of the GATA family, is upregulated significantly during the senescence process.(20) GATA4 has been reported to activate nuclear factor kappa-B (NF-κB) signaling, which mediates the expression of secreted inflammatory cytokines to induce SASP.(21, 22) However, how GATA4 mediates NF-κB signaling remains unclear and whether GATA4 could be upregulated by 1,25(OH)2D3 deficiency to promote SASP in skeletal muscle cells awaits further clarification.
B cell-specific Maloney murine leukemia virus insertion region 1 (Bmi-1), a polycomb gene family member, plays an important role in regulating the cell cycle and preventing cellular senescence.(23, 24) Studies have shown that, because Bmi-1 significantly activates E3 ubiquitin ligase activity of RING1B, the PRC1 complex formed by Bmi-1 and RING1B plays a crucial role in histone and protein ubiquitination.(25-27) Our recent study showes that Bmi-1-RING1B promotes GATA4 ubiquitination and its selective autophagic degradation to prevent SASP in cardiomyocyte.(28) However, it remains unclear whether Bmi-1 reduces the expression of GATA4 induced by 1,25(OH)2D3 deficiency in skeletal muscle cells.
This study demonstrates that muscular Bmi-1 and VDR decrease with physiological aging, along with DNA damage and GATA4-dependent SASP activation, leading to sarcopenia. 1,25(OH)2D3 deficiency promotes DNA damage in muscles, which increases the expression of GATA4. The upregulated GATA4 binds to its promoter and transcribes Rela, which encodes for NF-κB-p65 and induces SASP to aggravate cellular senescence in skeletal muscle cells. Bmi-1 can promote the ubiquitination and degradation of GATA4 by binding RING1B, which prevents cell senescence in skeletal muscle cells and ameliorates sarcopenia induced by 1,25(OH)2D3 deficiency.
Materials and Methods
High-throughput sequencing analysis
The gene expression profile data were downloaded from the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) database. GSE184348 contains the mRNA expression data of quadricep muscle from physiologically aged (24 months) and young (6 months) vehicle mice. Differentially expressed genes (DEGs) were analyzed using DESeq2, and pathway enrichment was analyzed using clusterProfiler in R (version 4.0.3) (R Development Core Team, Auckland, New Zealand). The correlation between genes was calculated by Pearson correlation analysis using the corrplot package.
Mice and genotyping
Cyp27b1 heterozygous (Cyp27b1+/−) and VDR heterozygous (VDR+/−) mice were generated at McGill University, as previously described.(29, 30) Originally on a BALB/c background, the Cyp27b1+/− mice or VDR+/− mice were repeatedly backcrossed with WT mice on a C57BL/6J background for over 12 generations to obtain Cyp27b1+/− mice or VDR+/− mice on a C57BL/6J background. Bmi-1Tg mice that highly expressed Bmi-1 under the control of a 2.4-kilobase (kb) Prx1 promoter were generated at Nanjing Medical University (Nanjing, China) and genotyped by PCR as described.(4) Adult Cyp27b1+/− or VDR+/− female mice were crossed with Cyp27b1+/− or VDR+/− male mice (both also maintained on a C57BL/6J genetic background) to obtain Cyp27b1−/− or VDR−/− male mice. Bmi-1Tg female mice and Cyp27b1+/− male mice were crossed to obtain male mice with the genotypes of WT, Bmi-1Tg, Cyp27b1−/−, and Bmi-1TgCyp27b1−/−. All the animals used in this study were male and were weaned at 21 days. Five male mice per cage were housed separately, and all the animals in this study were fed a normal diet, which contained 1.0% calcium, 0.67% phosphorus, and 2.2 IU vitamin D g−1 (No. 1010013; Jiangsu Province Collaborative Medicine Bioengineering Co., Nanjing, China). This study was performed in strict accordance with the guidelines of the Institute for Laboratory Animal Research of Nanjing Medical University in Nanjing of China. The protocol was approved by the Committee on the Ethics of Animal Experiments at Nanjing Medical University (Permit No. IACUC-1802007).
Cell culture
C2C12 cells
The mouse skeletal muscle cell line C2C12 was cultured in 90% DMEM (ZQ100; Zhong Qiao Xin Zhou Biotechnology, Shanghai, China) with 10% fetal bovine serum (FBS) (ZQ500-S; Zhong Qiao Xin Zhou Biotechnology), 100 U mL−1 penicillin, and 0.1 mg mL−1 streptomycin (CSP006; Zhong Qiao Xin Zhou Biotechnology).
Primary muscular fibroblasts
The extensor digitorum longus (EDL) muscles were separated from 8-week-old WT and VDR−/− mice, which were anesthetized and perfused. Then the tissue was minced and digested to generate primary muscular fibroblasts, as previously described,(31) which were cultured in 90% DMEM (ZQ100; Zhong Qiao Xin Zhou Biotechnology) with 10% FBS (ZQ500-S; Zhong Qiao Xin Zhou Biotechnology) and 100 U mL−1 penicillin and 0.1 mg mL−1 streptomycin (CSP006; Zhong Qiao Xin Zhou Biotechnology).
Administration of drugs and reagents
Muscular fibroblasts of different genotypes were divided into an untreated group, a H2O2 (100 nmol L−1) treated group, and a 1,25(OH)2D3 (1 × 10−8 M) plus H2O2 treated group. Proteins were extracted from these cells after 48 hours. The WT group was the control for the VDR−/− group under the same treatment conditions. The untreated group was the control for the H2O2 treated group and the 1,25(OH)2D3 plus H2O2 treated group of the same genotype. The H2O2 treated group was the control for the 1,25(OH)2D3 plus H2O2 treated group of the same genotype.
siRNA transfection
For GATA4 knockdown, muscular fibroblasts were transfected with siRNA targeting mouse Gata4 (5′-UCCAUCCAGUGCUGUCUGCUCUGAA-3′) or with nontargeting negative control siRNA (5′-GGCUCUAGAAAAGCCUAUGC-3′). All sequences were synthesized by Ribobio Co., in Guangzhou of China, using PRFect transfection Reagent (Proteinbio, Nanjing, China) at a final concentration of 50 nM as previously described.(24) The cells were harvested at 48 hours after transfection for protein analysis. Please see the sequences of siRNA in Table S1.
Plasmid construction and transfection
The GATA4 gene was cloned into the vector pcDNA3.1 (TranSheep Bio Co., Shanghai, China). Lipofectamine® 2000 Reagent (Invitrogen, Carlsbad, CA, USA) was used to transfect plasmids at different concentrations (1 μL/0.75 μg, 1 μL/1 μg, and 1 μL/1.25 μg) as referenced to plasmid DNA into mouse C2C12 cells following the manufacturer's instructions.
Muscle strength testing
The forelimb grips of the animals were evaluated using a mouse grip instrument (SANS, Jiangsu, China). The front paws of the mice were placed on the gripping device in the grip test, the mouse was lifted by the tail and hind limbs suspended in the air, and then slowly pulled until the mouse's forelimb grip disappeared. Three replicates for every mouse were carried out on the grip measurement of the forelimbs. The recorded maximum value (in Grams) obtained by the software analysis was considered to be the muscle grip strength and then was measured in Newtons according to the formula 1 gram of force equals 9.8 × 10−3 Newtons, as previously described.(32)
Preparation of TA muscle sections
TA muscular samples from 8-week-old mice were removed from the lower limbs, frozen and embedded in Optimal Cutting Temperature (OCT) compound (No. 4583; SAKURA Finetek USA, Torrance, CA, USA), and sectioned (7 μm) using a freezing microtome (Thermo Scientific Cryotome FSE Cryostats, Loughborough, Leicestershire, UK) for immunohistochemical or immunofluorescence staining.(32)
Immunohistochemical staining
Staining was performed as previously described.(4, 33) Primary antibodies against NF-κB-p65 (No. 8242; Cell Signaling Technology, Beverly, MA, USA), IL-1β (sc-52012; Santa Cruz Biotechnology, Dallas, TX, USA), IL-6 (sc-1265; Santa Cruz Biotechnology Inc.), TNF-α (sc-52746; Santa Cruz Biotechnology), ATM (No. 2873; Cell Signaling Technology), p-ATM(Ser1981) (sc-47739; Santa Cruz Biotechnology), p-ATR(Ser428) (ab178407; Abcam, Cambridge, MA, USA), γH2A.X (Ser139) (No. 80312 S; Cell Signaling Technology), 8-OHdG (ab62623; Abcam), GATA4 (No. 19530; Proteintech, Rosemont, IL, USA), p16 (ab211542; Abcam), and β-gal (ab616; Abcam). After washing, the sections were incubated with secondary antibody (biotinylated IgG; Sigma-Aldrich, Saint Louis, MO, USA), washed and processed using Vectastain ABC-HRP kits (Vector Laboratories, Burlingame, CA, USA).
Immunofluorescent staining
Primary antibodies against Pax7 (No. Pax7; DSHB, USA), Laminin (No. L9393; Sigma-Aldrich), MYHCIIA (No. SC-71; DSHB, Iowa, IA, USA) and MYHCIIB (No. BF-F3; DSHB), Dylight594-conjugated secondary antibody (goat anti-rabbit IgG, GAR5942; Multi Sciences Biotech, Co.), Dylight488-conjugated secondary antibody (goat anti-mouse IgG, GAR5942; Multi Sciences Biotech, Co.), Dylight488-conjugated secondary antibody (goat anti-mouse IgM, No. A21042; Invitrogen), and Alexa Fluor 568-conjugated goat anti-mouse IgG1 (No. A21124; Invitrogen) were used.
Please see Table S2 for the antibodies and dilution for immunohistochemical and immunofluorescent staining.
RNA extraction and real-time RT-PCR
RNA was extracted from TA muscular samples or C2C12 cells using TRIzol reagent (No. 15596; Invitrogen) according to the manufacturer's protocol. Levels of mRNA in muscle samples were quantified by real-time RT-PCR. See primers in Table S3.
Western blots
Western blots were generated as previously described.(33) Primary antibodies against Bmi-1 (No. 66161–1; Proteintech), RING1B (No. 5694; Cell Signaling Technology), VDR (ab3508; Abcam), p16 (ab211542; Abcam), p19 (sc-1665; Santa Cruz Biotechnology), p53 (sc-126; Santa Cruz Biotechnology), p21 (sc-471; Santa Cruz Biotechnology), γH2A.X(Ser139) (No. 80312 S; Cell Signaling Technology), CHK2 (sc-5278; Santa Cruz Biotechnology), phospho-CHK2(Thr68) (No. PA5-104715; Invitrogen), GATA4 (No. 19530; Proteintech; sc-25310; Santa Cruz Biotechnology), p65 (No. 8242; Cell Signaling Technology), p-p65(Ser536) (ab76302; Abcam), IκB-α (AF1282; Beyotime Biotechnology, Shanghai, China), p-IκB-α(Ser32) (sc-8404; Santa Cruz Biotechnology), IL-1β (sc-52012; Santa Cruz Biotechnology), IL-6 (sc-1265; Santa Cruz Biotechnology), and TNF-α (sc-52746; Santa Cruz Biotechnology) were used. GAPDH (HRP-60004; Proteintech) was used as the loading control for the cytoplasmic fraction and total cellular protein. Please see Table S4 for the antibodies and dilution for Western blots.
Chromatin immunoprecipitation
ChIP was performed using a Magna ChIP™ Chromatin Immunoprecipitation A kit (2931149; Millipore, Billerica, MA, USA) using C2C12 cells following the manufacturer's instructions as previously described.(33) Antibodies against GATA4 (No. 19530; Proteintech) and rabbit IgG (No. PP64; Millipore) were used to incubate the chromatin samples. The primers for different Rela promoter regions are listed in Table S5.
Dual luciferase assay
The chimeric genes of the Rela promoter plasmids for the transfection experiments were constructed in a pGL4.1-basic vector (TranSheep Bio Co., Shanghai, China) by ligating the luciferase gene at the 5′-flanking regions upstream of the gene. Mouse C2C12 cells were plated into 24-well cell culture plates 24 hours before transfection. The mixture of 1 μg each of both pCDNA3.1-basic and pGL4.1-basic, GATA4 overexpression pcDNA3.1 and pGL4.1-basic, and GATA4 overexpression pcDNA3.1 and pGL4.1-deletion (deleting the binding sequence “ATTTATCTTTTATTTTATTTTATTATTTTTATTTTTG”) was successively co-transfected with Firefly luciferase (Fluc)-Renilla luciferase (Rluc) into mouse C2C12 cells using the Lipofectamine® 2000 Reagent (Invitrogen) following the manufacturer's instructions as previously described.(33) Two days later, a commercial kit (Promega Corporation, Madison, WI, USA) was used to measure the promoter-driven luciferase activity.
Statistical analysis
All data were analyzed by GraphPad Prism software version 6.07 (GraphPad Software, San Diego, CA, USA) as previously described.(33) Measurement data are described as the mean ± SEM fold-change over the vehicle group and were analyzed using Student's t test and one-way ANOVA to compare differences among groups. Qualitative data are described as percentages and were analyzed using chi-squared tests as indicated. P values were two-sided, and a p value <.05 was considered statistically significant.
Results
Muscle Bmi-1 and VDR decrease with physiological aging, activating DNA damage and GATA4-dependent SASP, and promoting sarcopenia
To gain insight into the mechanism by which aging causes sarcopenia progression, bioinformatics methods were used to analyze gene expression profiles in physiologically aged skeletal muscle. After searching the Gene Expression Omnibus (GEO) database, the mRNA expression data of skeletal muscle from physiologically aged (24 months) and young (6 months) mice were analyzed from the GSE184348 dataset. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis using differentially expressed gene (DEG) data was performed. The results showed that the p53 signaling pathway and NF-κB signaling pathway were activated in aging skeletal muscle, which was likely to be involved in the occurrence of sarcopenia (Fig. 1A).
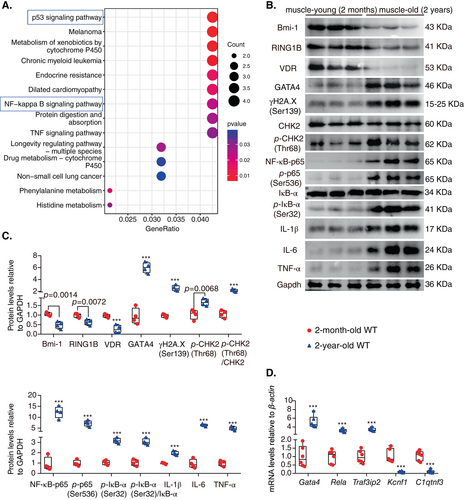
We observed muscles of young (2 months) and physiologically aged (24 months) mice and found that the size and ratio of muscle weight relative to body weight and the grip strength relative to body weight dramatically decreased in physiologically aged mice (Figure S1). To determine cell senescence, DNA damage, and GATA4-dependent NF-κB signaling pathway-mediated SASP in age-related sarcopenia, Western blotting was conducted to detect protein levels in the tibialis anterior (TA) muscles of young (2 months) and physiologically aged (24 months) mice. The results showed that VDR, Bmi-1, and RING1B protein levels were downregulated; however, GATA4, γH2A.X(Ser139), p-CHK2(Thr68)/CHK2, NF-κB-p65, p-p65(Ser536), pIκB-α(Ser32)/IκB-α, IL-1β, IL-6, and TNF-α protein levels were upregulated in muscles from aged mice when compared to young mice (Fig. 1B,C). We also showed that in comparison with young mice, the mRNA levels of Gata4, Rela, and TRAF3IP2 increased, while Kcnf1 and C1qtnf3 decreased in muscle tissues of physiologically aged mice (Fig. 1D).
Bmi-1 overexpression improves sarcopenia and muscle satellite cell reduction in 1,25(OH)2D3-deficient mice
To determine whether Bmi-1 overexpression improved sarcopenia caused by 1,25(OH)2D3 deficiency, changes in body weight, TA muscle weight, muscle weight/body weight ratio, and grip strength were examined. Not only body size but also the size of TA muscles decreased significantly in the Cyp27b1−/− mice, which were increased by Bmi-1 overexpression (Fig. 2A,B). The ratio of TA muscle weight to body weight and the ratio of grip strength to body weight were decreased dramatically in Cyp27b1−/− mice but increased by Bmi-1 overexpression (Fig. 2C,D and Figure S2). Protein levels of Bmi-1 and VDR all decreased in the TA muscles of Cyp27b1−/− mice compared to WT mice but upregulated in Bmi-1Tg and Bmi-1TgCyp27b1−/− mice (Fig. 2E,F). These results suggest that 1,25(OH)2D3 deficiency could lead to a sarcopenia phenotype but be ameliorated by Bmi-1 overexpression.
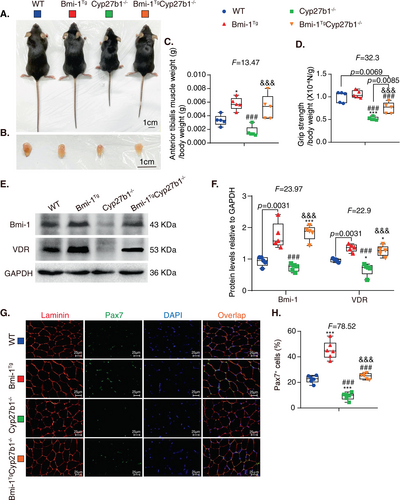
To further determine whether satellite cells were affected by 1,25(OH)2D3 deficiency, immunofluorescence of Pax7 was conducted using TA muscles, and revealed a reduction in satellite cells in TA muscles in Cyp27b1−/− mice compared to WT mice but an increase in Bmi-1TgCyp27b1−/− mice (Fig. 2G,H).
Bmi-1 overexpression improves myofiber atrophy and cell senescence in 1,25(OH)2D3-deficient mice
To determine whether Bmi-1 overexpression improved myofiber atrophy caused by 1,25(OH)2D3 deficiency, we compared the differences in number and cross-sectional area (CSA) of myofibers among the different mouse genotypes. Immunofluorescence of TA muscles showed that the percentage of myosin heavy chain (MYHC) IIA-positive myofibers in the total myofibers decreased, while the percentage of MYHCIIB-positive myofibers was upregulated (Fig. 3A,B). The CSA of both types of myofibers were reduced in Cyp27b1−/− mice compared to WT mice but improved by Bmi-1 overexpression (Fig. 3C). These results indicate that Bmi-1 overexpression could improve muscle atrophy induced by 1,25(OH)2D3 deficiency.
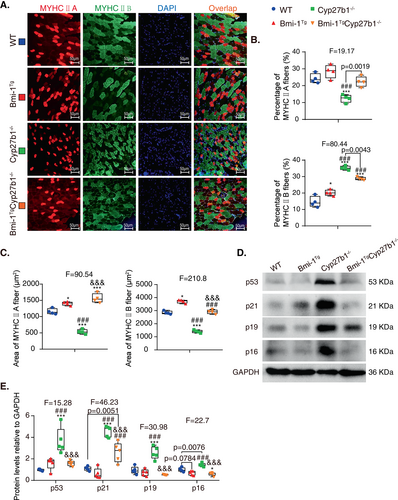
To determine whether Bmi-1 overexpression could prevent cell senescence caused by 1,25(OH)2D3 deficiency, the TA muscles were examined for markers of senescence. The protein expression of p53, p21, p19, and p16 were all upregulated in Cyp27b1−/− mice compared to WT mice but decreased in Bmi-1TgCyp27b1−/− mice (Fig. 3D,E). This suggests that Bmi-1 overexpression could ameliorate cell senescence caused by 1,25(OH)2D3 deficiency.
Bmi-1 overexpression improves SASP of muscle in 1,25(OH)2D3-deficient mice
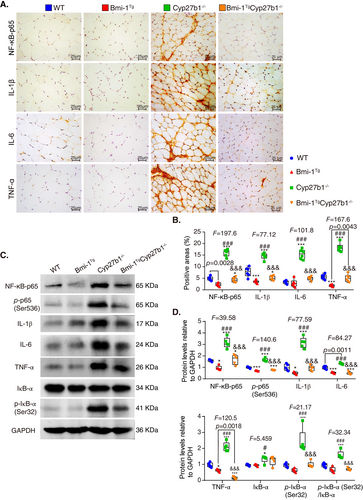
To investigate whether muscular aging is caused by SASP and ameliorated by Bmi-1 overexpression, TA muscles were examined for SASP-related markers. Obvious increases were observed in NF-κB-p65-positive proinflammatory cells and IL-1β-, IL-6-, and TNF-α-positive areas in Cyp27b1−/− mice. However, the percentage of NF-κB-p65-positive proinflammatory cells and IL-1β-, IL-6-, and TNF-α-positive areas were reduced by Bmi-1 overexpression (Fig. 4A,B). Protein expressions of NF-κB-p65, p-p65(Ser536), IL-1β, IL-6, TNFα, IκB-α, and p-IκB-α (Ser32) also increased because of 1,25(OH)2D3 deficiency. However, Bmi-1 overexpression could reduce the aforementioned SASP-related protein levels (Fig. 4C,D).
Bmi-1 overexpression improves DNA damage induced muscle damage in 1,25(OH)2D3-deficient mice
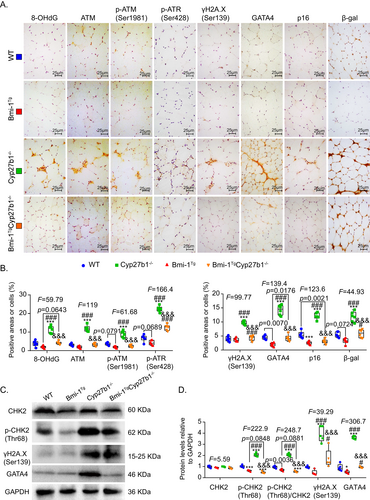
To determine whether 1,25(OH)2D3 deficiency caused DNA damage to induce senescence and SASP, the TA muscles were examined for DNA damage and senescence markers. Significant increases were observed in 8-OHdG-, ATM-, p-ATM(Ser1981)-, p-ATR(Ser428)-, γH2A.X(Ser139)-, GATA4-, p16-, and β-gal-positive cells in the TA muscles of Cyp27b1−/− mice compared to WT mice. However, 8-OHdG-, ATM-, p-ATM(Ser1981)-, p-ATR(Ser428)-, γH2A.X(Ser139)-, GATA4-, p16-, and β-gal-positive cells decreased in Bmi-1TgCyp27b1−/− mice compared to Cyp27b1−/− mice (Fig. 5A,B). The TA muscle protein levels of p-CHK2(Thr68), γH2A.X(Ser139), and GATA4 were obviously upregulated by 1,25(OH)2D3 deficiency. However, Bmi-1 overexpression could reduce the protein levels of γH2A.X(Ser139), p-CHK2(Thr68), and GATA4 in Cyp27b1−/− mice (Fig. 5C,D).
Bmi-1-RING1B binds to GATA4 and promotes its ubiquitination and inhibits GATA4-dependent SASP in skeletal muscle during 1,25(OH)2D3 deficiency
To determine whether increased GATA4 in Cyp27b1−/− muscle could be ubiquitinated by Bmi-1-RING1B and then degraded, as seen in our recent report in cardiomyocyte,(28) protein immunoprecipitations of TA muscles from WT, Bmi-1Tg, Cyp27b1−/−, and Bmi-1TgCyp27b1−/− mice were performed, and the results showed that GATA4 bound to RING1B and Bmi-1. GATA4 ubiquitination levels and binding levels of GATA4 with Bmi-1 or RING1B in TA muscles of Cyp27b1−/− mice were lower than in TA muscles from Bmi-1TgCyp27b1−/− mice (Fig. 6A). Thus, Bmi-1-RING1B binds to GATA4 and promotes its ubiquitination in skeletal muscles of 1,25(OH)2D3-deficient mice.
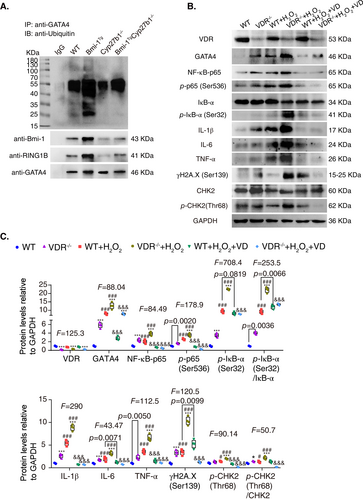
To clarify whether the increased GATA4 was induced by DNA damage in skeletal muscle after VD deficiency, we isolated fibroblasts from extensor digitorum longus (EDL) muscles in WT and VDR−/− mice, used hydrogen peroxide (H2O2) to induce senescence of muscular fibroblasts, and added 1,25(OH)2D3 to correct this. Compared to the vehicle group, the expression of proteins related to DNA damage increased significantly in the H2O2-treated group, especially in the VDR−/− group. GATA4 increased, and the expressions of NF-κB-p65, p-p65(Ser536), p-IκB-α(Ser32)/IκB-α, IL-1β, IL-6, and TNF-α were upregulated. However, the addition of 1,25(OH)2D3 reduced the expression of these proteins (Fig. 6B,C), revealing that increased GATA4 was induced by DNA damage in muscular fibroblasts during 1,25(OH)2D3 deficiency.
To investigate whether GATA4 mediated the SASP in skeletal muscle, we used siRNA to inhibit GATA4 expression in muscular fibroblasts from WT mice. The results showed that GATA4 expression was markedly reduced in Gata4 siRNA-treated cells. With H2O2 treatment, the expression of proteins involved in DNA damage, including γH2A.X(Ser139), p-CHK2(Thr68), and GATA4, as well as NF-κB-dependent SASP-related proteins, including NF-κB-p65, p-p65(Ser536), p-IκB-α(Ser32)/ IκB-α, IL-1β, IL-6, and TNF-α, was upregulated, but the knockdown of GATA4 decreased the expression of the aforementioned proteins (Figure S3A,B). These results demonstrated that DNA damage led to increased GATA4 expression, which further induced muscular SASP.
GATA4 positively regulates Rela expression through binding to promoter
To investigate the role of GATA4 in the regulation of muscular aging, we transfected C2C12 cells with or without a gradient concentration of GATA4 overexpression plasmid using ratios of Lipo2000 to plasmid of 1 μL/0.75 μg, 1 μL/1 μg, and 1 μL/1.25 μg. The NF-κB-p65 and p-p65(Ser536) protein levels of C2C12 were upregulated with the increase of GATA4 concentration (Fig. 7A,B). We then transfected C2C12 cells with or without GATA4 overexpression plasmid (1 μL/1.25 μg), and as a result, the Rela mRNA levels in C2C12 cells increased, suggesting that GATA4 could transcribe Rela (Fig. 7C).
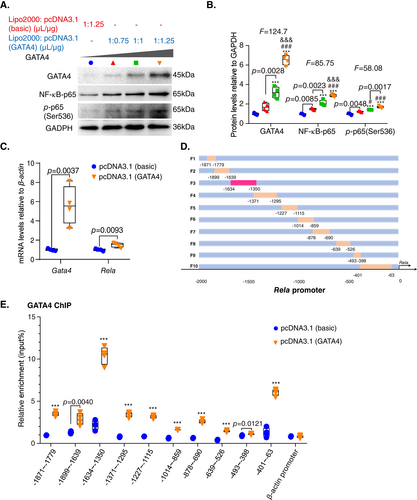
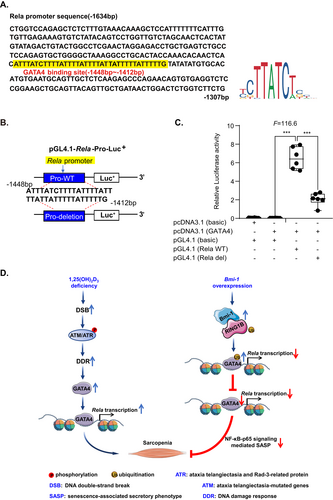
Next, to explore whether GATA4 bound to the Rela promoter, a series of mouse Rela promoter primers were constructed (Fig. 7D). Chromatin immunoprecipitation (ChIP) using C2C12 cells transfected with or without GATA4 was conducted, and an anti-GATA4 primary antibody was used to capture chromatin. The ChIP results showed that GATA4 bound significantly to the Rela promoter at the region from −1634 to −1350 bp compared with the control group (Fig. 7E). These results demonstrated that GATA4 could bind to the Rela promoter at the region indicated and cause upregulation of Rela expression.
GATA4 regulates Rela expression at transcriptional level
To further confirm whether Rela expression was regulated by GATA4 at the transcriptional level, GATA4 overexpression plasmid was transfected into C2C12 cells. A GATA4-like sequence was identified at −1634 to −1307 bp upstream of the transcriptional start site of the Rela gene (JASPAR CORE database; http://jaspar.genereg.net/) (Fig. 8A). Therefore, mouse Rela promoter luciferase reporter plasmids with or without a GATA4-like sequence (−1448 to −1412 bp) deletion were constructed (Fig. 8B). The results from luciferase activity assays demonstrated that luciferase activity increased significantly in C2C12 cells transfected with a GATA4 binding sequencing plasmid compared to the vehicle group. In contrast, luciferase activity decreased obviously in C2C12 cells transfected with a pGL4.1-Rela deletion plasmid compared to transfection with a GATA4 binding sequencing plasmid (Fig. 8C). The results suggested that GATA4 regulated Rela expression at the transcriptional level.
Discussion
In this study, we found that muscle-derived Bmi-1 and VDR decreased with physiological aging, activated DNA damage and GATA4-dependent SASP, and promoted sarcopenia. 1,25(OH)2D3 deficiency promoted DNA damage-induced GATA4 accumulation in muscles and GATA4 upregulated Rela at the transcriptional level to cause NF-κB-dependent SASP affecting cell senescence, muscular dysfunction, and sarcopenia. Bmi-1 promoted the ubiquitination and degradation of GATA4 by binding RING1B, which prevented cell senescence, SASP and dysfunction of the muscle, and improved sarcopenia induced by 1,25(OH)2D3 deficiency (Fig. 8D).
It has been demonstrated that 1,25(OH)2D3 deficiency results in increased oxidative stress by inhibiting the transcription of Nrf2 and enhancing DNA damage, causing cellular senescence and SASP to promote the aging process.(34) Our recent study found that serum 1,25(OH)2D3 levels decreased in physiologically aged mice compared with young mice, and VD deficiency induced senescence-associated pulmonary fibrosis.(33) Several lines of evidence suggest that 1,25(OH)2D3 deficiency results in the development of age-related sarcopenia by inducing oxidative stress, muscle cell senescence, and SASP and by inhibiting skeletal muscle regeneration.(32, 35-37) Low 1,25(OH)2D3 status is associated with decreased muscle strength and mass, which is improved by 1,25(OH)2D3 supplementation, especially for an older population at high risk of sarcopenia.(15) In this study, we found that 1,25(OH)2D3-deficient mice exhibited a sarcopenia phenotype, including muscular atrophy and decreased grip strength, which was caused by increased DNA damage, cell senescence, and SASP of muscle cells. Our data also demonstrated that Bmi-1 overexpression improved sarcopenia in 1,25(OH)2D3-deficient mice by inhibiting muscular cell senescence and SASP. However, whether Bmi-1 overexpression could promote skeletal muscle regeneration in 1,25(OH)2D3-deficient mice requires further study.
Satellite cells are stem cells with myogenic potential and are located under the basal lamina of muscle fibers; further, they can be activated after muscle injury and are responsible for muscle regeneration.(38-40) It is widely accepted that satellite cells are crucial for the remodeling of muscle fibers and contribute to the maintenance of healthy muscle mass throughout life.(40) Promoting decreased proliferation of muscle satellite cells raises the incidence of sarcopenia.(41, 42) A recent study showed that satellite cell content and function decline with aging, and this plays a major role in impaired muscle regeneration and loss of muscle mass.(43) In this study, the reduction of Pax7-positive cells indicated that the proportion of satellite cells decreased significantly in 1,25(OH)2D3-deficient mice. Therefore, 1,25(OH)2D3 deficiency might contribute to the reduction of muscle regeneration, which is a major cause of sarcopenia. However, the mechanism of 1,25(OH)2D3 deficiency-induced satellite cell decrease remains to be further clarified. Our previous report found that Pax7-positive muscle satellite cells decrease in Bmi-1-deficient mice compared to WT littermates.(44) Here, we found that Bmi-1 overexpression could increase Pax7-positive muscle satellite cells in 1,25(OH)2D3-deficient mice. Whether Bmi-1 overexpression could promote skeletal muscle regeneration in these mice by maintaining Pax7-positive cells, however, needs further study.
Sarcopenia is characterized by myofiber atrophy, and the incidence increases with age.(45) The type of myofiber is clarified by different MYHC, and composition directly affects skeletal muscle fitness and function.(46, 47) Type I is characterized as a slow oxidative myofiber, type IIA is considered a fast oxidative myofiber, and type IIB is identified as a fast glycolytic myofiber.(48, 49) It has been shown that type I myofibers reveal type II-like features among groups of type I fibers in the elderly,(50) and aging leads to a decline in type IIA muscle fibers and a significant increase of type IIB fibers in muscle.(51-53) In this study, we found a predominance of type IIB fibers in the TA muscle from 1,25(OH)2D3-deficient mice, together with a marked reduction in the proportion of type IIA fibers, implying conversion toward fast glycolytic fibers. This metabolic change may be caused by 1,25(OH)2D3, but the exact mechanism needs to be further identified. We also found that the cross-sectional areas of both types IIA and IIB fibers decreased in 1,25(OH)2D3-deficient mice, but whether type I fibers could be regulated by 1,25(OH)2D3 remains unclear. Our data further showed that Bmi-1 overexpression increased the proportion of type IIA fibers, but the cross-sectional areas of both types IIA and IIB fibers, however, reduced the proportion of type IIB fibers in 1,25(OH)2D3-deficient mice. Whether this metabolic change in myofibers could be regulated by Bmi-1 overexpression needs further investigation.
A functional relationship between the VD/VDR axis and the expression of DNA repair factors has been found in the context of oncogene-induced senescence.(54) Here, DNA damage contributes to fiber atrophy and loss that cause sarcopenia,(55) and we found that 1,25(OH)2D3 deficiency promoted DNA strand break and an ATM-CHK2-γH2A.X-mediated DNA damage response in muscle cells. As previously reported, USP28 is activated by ATM/ATR for the deubiquitinating and stabilizing GATA4 after DNA damage.(56) This study found that 1,25(OH)2D3 deficiency promoted DNA damage in muscle cells, and as a result, the expression of GATA4 increased significantly. A previous study and our recent report showed that GATA4 induced NF-κB-p65 signaling-mediated SASP through downregulation of TRAF3IP2 expression, which causes cell senescence acceleration.(28, 57) This study demonstrated that NF-κB-p65 signaling-mediated SASP was upregulated by 1,25(OH)2D3 deficiency in skeletal muscle cells, which was dependent on GATA4. Our recent report also showed that GATA4 elevation in the myocardium from physiologically aged mice was accompanied by Rela upregulation in comparison to young mice. Therefore, we further determined whether GATA4 regulated Rela expression at the translational level and triggered NF-κB-p65 signaling-mediated SASP in this study. Here, we first reported that GATA4 transcriptionally upregulated Rela expression, which further promoted NF-κB-p65 signaling-mediated SASP. It has also been reported that VDR interacts directly with NF-κB-p65, and the VDR-p65 interaction blocks the nuclear translocation of p65/p50, which demonstrates the inhibition of NF-κB by VD.(58) Therefore, whether the physical interaction between VDR and NF-κB-p65 plays a critical role in sarcopenia requires further study.
It has been confirmed that Bmi-1 is indispensable for increased ubiquitination by interacting with E3 ubiquitin-ligase RING1B.(25, 27) Our recent report found that Bmi-1-RING1B mediated the ubiquitination of GATA4 and prevented senescence-associated pathological cardiac hypertrophy (SA-PCH) by promoting p62-mediated selective autophagy for the degradation of GATA4.(28) Here, we overexpressed Bmi-1 at the Prx1 promoter in 1,25(OH)2D3-deficient mice and found that GATA4 was ubiquitinated by Bmi-1-RING1B and downregulated, thereby improving GATA4-dependent SASP and sarcopenia. In a previous study, ATM and ATR increased GATA4 protein levels during cellular senescence.(20) We found that DNA damage in skeletal muscle cells caused by 1,25(OH)2D3 deficiency was quenched by Bmi-1 overexpression. Our recent report also found that adeno-associated viral vector (AAV) serotype 9-CMV-Bmi-1-RING1B could be used for translational gene therapy and cause ubiquitination of GATA4 and prevent GATA4-dependent SA-PCH.(28) The use of AAV9-mediated gene therapy in individuals as a potential therapy for Duchenne muscular dystrophy is ongoing in some clinical trials,(59) and a recent report found that a novel MyoAAV could transduce primate muscles with a higher efficiency than AAV9.(60) Furthermore, further investigations are required to understand whether AAV-Bmi-1-RING1B can effectively treat sarcopenia by inhibiting GATA4-dependent SASP.
Lastly, our results showed that Bmi-1 overexpression ameliorated sarcopenia induced by 1,25(OH)2D3 deficiency through downregulation of GATA4-dependent Rela transcription and sequentially inhibiting GATA4-dependent SASP in muscle cells. Therefore, Bmi-1-RING1B as well as 1,25(OH)2D3 or GATA4 siRNA administration could be used as a transgenic therapy to ubiquitinate GATA4 and prevent sarcopenia.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (82271614, 81871097, and 81571371 to JJ, 81730066 to DM), the Qinglan Project of Jiangsu Province of China (2020-10 to JJ), and the Natural Science Foundation of Jiangsu Province (BK20151554 to JJ).
Author Contributions
Conceptualization: Jianliang Jin and Dengshun Miao. Methodology: Qiuyi Wang, Jingyu Zhao, Haiyun Chen, Jiawen Zhou, Ao Chen, Jin'ge Zhang, Yue Wang, Zhiyuan Mao, Jiachen Wang, Xuehan Qiu, Yutong Chen, Rong Wang, Yongjie Zhang, Dengshun Miao, and Jianliang Jin. Software: Qiuyi Wang, Jingyu Zhao, Haiyun Chen, Jiawen Zhou, Ao Chen, and Jianliang Jin. Validation: Qiuyi Wang, Jin’ge Zhang, Haiyun Chen, Jiawen Zhou, Ao Chen, Rong Wang, and Jianliang Jin. Data analysis: Qiuyi Wang, Jin’ge Zhang, Haiyun Chen, Jiawen Zhou, Ao Chen, Jingyu Zhao, Yue Wang, Zhiyuan Mao, Jiachen Wang, Xuehan Qiu, Yutong Chen, Rong Wang, Yongjie Zhang, and Jianliang Jin. Writing—original draft: Qiuyi Wang, Haiyun Chen, and Jianliang Jin, with help from the other authors. Writing—review and editing: Yongjie Zhang and Dengshun Miao, with help from the other authors. Project administration and supervision: Rong Wang, Jianliang Jin, and Dengshun Miao. Funding acquisition: Jianliang Jin and Dengshun Miao.
Conflict of Interest
The authors declare no competing interests.
Open Research
Data Availability Statement
All data and materials used in the analysis are available to any researcher for purposes of reproduction or extending the analysis.