Effects of Zoledronate on Cancer, Cardiac Events, and Mortality in Osteopenic Older Women
Funding information: Health Research Council of New Zealand, Grant/Award Number: 15/576; Eli Lilly; Novartis; Merck; Amgen
ABSTRACT
We recently showed that zoledronate prevented fractures in older women with osteopenia (hip T-scores between −1.0 and −2.5). In addition to fewer fractures, this study also suggested that women randomized to zoledronate had fewer vascular events, a lower incidence of cancer, and a trend to lower mortality. The present analysis provides a more detailed presentation of the adverse event data from that study, a 6-year, double-blind trial of 2000 women aged >65 years recruited using electoral rolls. They were randomly assigned to receive four infusions of either zoledronate 5 mg or normal saline at 18-month intervals. Supplements of vitamin D, but not calcium, were provided. There were 1017 serious adverse events in 443 participants in the placebo group, and 820 events in 400 participants in those randomized to zoledronate (relative risk = 0.90; 95% CI, 0.81 to 1.00). These events included fractures resulting in hospital admission. Myocardial infarction occurred in 39 women (43 events) in the placebo group and in 24 women (25 events) in the zoledronate group (hazard ratio 0.60 [95% CI, 0.36 to 1.00]; rate ratio 0.58 [95% CI, 0.35 to 0.94]). For a prespecified composite cardiovascular endpoint (sudden death, myocardial infarction, coronary artery revascularization, or stroke) 69 women had 98 events in the placebo group, and 53 women had 71 events in the zoledronate group (hazard ratio 0.76 [95% CI, 0.53 to 1.08]; rate ratio 0.72 [95% CI, 0.53 to 0.98]). Total cancers were significantly reduced with zoledronate (hazard ratio 0.67 [95% CI, 0.51 to 0.89]; rate ratio 0.68 [95% CI, 0.52 to 0.89]), and this was significant for both breast cancers and for non-breast cancers. Eleven women had recurrent or second breast cancers during the study, all in the placebo group. The hazard ratio for death was 0.65 (95% CI, 0.40 to 1.06; p = 0.08), and 0.51 (95% CI, 0.30 to 0.87) in those without incident fragility fracture. These apparent beneficial effects justify further appropriately powered trials of zoledronate with these nonskeletal conditions as primary endpoints. © 2019 American Society for Bone and Mineral Research.
Introduction
Zoledronate (also known as zoledronic acid) is a long-acting, potent bisphosphonate used for treating osteoporosis and in the prevention of skeletal-related events in patients with disseminated malignancy. In addition to its established efficacy in osteoporosis,1, 2 we have recently showed that it is effective in preventing fractures in older women with less marked bone loss,3 known as osteopenia. In addition to fewer fractures, our recent study also suggested that women randomized to zoledronate had fewer vascular events, a lower incidence of cancer, and a trend to lower mortality. A similar reduction in mortality was reported in one of the osteoporosis phase 3 trials of zoledronate,2 and there is already a substantial but inconsistent literature relating to bisphosphonate effects on vascular disease4 and cancer.5 These findings are potentially of great significance in determining the impact of bisphosphonates on the health of older people. Therefore, the present work provides a detailed presentation of the adverse event data from our recent trial and discusses the potential mechanisms of these apparent bisphosphonate effects.
Methods
The details of this study's protocol have already been published,3 but are summarized here. The study was registered at the Australian New Zealand Clinical Trials Registry, number ACTRN12609000593235.
Participants
Participants were ambulant postmenopausal women aged >65 years, with T-score at the total hip or femoral neck in the range −1.0 to −2.5, who were able to give informed consent. Exclusion criteria were: lumbar spine T-score < −3.0, estimated glomerular filtration rate (eGFR) <30 mL/min, major systemic disease, malignant disease in the last 2 years, metabolic bone disease, or regular use of bone-active drugs in the previous year.
Study design
This is a prospective, randomized, placebo-controlled, double-blind trial in osteopenic postmenopausal women aged ≥65 years, to determine the antifracture efficacy of zoledronate. The primary study endpoint was the time to first fragility fracture. Two thousand women were recruited using electoral rolls and randomized (1:1) to receive four infusions of either zoledronate 5 mg or normal saline at 18-month intervals. Each participant was followed for 6 years. Women who were not already taking vitamin D supplements were given a single oral dose of cholecalciferol (2.5 mg [100,000 IU]) at least 1 week before their first infusion, and subsequently received cholecalciferol at a dose of 1.25 mg per month for the duration of the trial. Calcium supplements were not supplied. Trial procedures took place at the Clinical Research Center, University of Auckland, with participant visits every 18 months. The trial was approved by the regional Health and Disability Ethics Committee, and all participants provided written informed consent.
Participants recorded fractures, adverse events, and changes in medications in a quarterly questionnaire. For hospital admissions, an investigator (AMH) confirmed diagnoses directly from the participants' medical records and reviewed these with another investigator (IRR), if in doubt. Serious adverse events were coded to the high-level terms of the Medical Dictionary for Regulatory Activities (MedDRA) by an experienced coder who had no other involvement in the study. The vital status of all participants was determined at the study's conclusion from a national database of deaths.
In light of previous findings with zoledronate, the following adverse events were prespecified for analysis: (i) death; (ii) sudden death; (iii) myocardial infarction; (iv) coronary artery revascularization; (v) stroke; (vi) transient ischemic attack; (vii) cancer; (viii) osteonecrosis of the jaw; (ix) atrial fibrillation; and (x) a composite of vascular events (any of events ii–v).
Statistical analysis
Data are fully described as total number of events, events per patient-year, women with at least one event, and time to first event. A complementary group of statistical analyses were used to assess these parameters. Relative risks were used to compare risks of events between treatment arms, and were calculated assuming a binomial distribution with a log-link function. Time to first event was modeled using the Cox proportional hazards approach for both treatment groups and the proportionality assumption was verified for each model. This analysis was used for prespecified adverse events, since event dates were precisely known. SAS v 9.4 (SAS Institute Inc, Cary, NC, USA) was used for these analyses. The confidence interval for the number of events per patient-year was calculated using the Mid-P exact method. Exact rate ratios were used to compare these rates between treatment arms, and were calculated using a Taylor series method using Open Source Epidemiologic Statistics for Public Health (www.openepi.com).
Analyses were based on the intention-to-treat principle. No imputation for missing data was made. All tests were two-tailed, and p < 0.05 was considered significant. No adjustment for multiplicity was performed. Data are presented with 95% confidence intervals (CIs).
Results
Baseline characteristics are shown in Table 1. None were significantly different between the two groups. There was a slight excess of participants with a history of cancer in the placebo group, the main imbalance being for breast cancers, but this was not significant.
| Placebo | Zoledronate | |
|---|---|---|
| n | 1000 | 1000 |
| Age (years), mean ± SD | 71 ± 5.1 | 71 ± 5.0 |
| European ethnicity, n (%) | 940 (94) | 954 (95) |
| BMI (kg/m2), mean ± SD | 26.9 ± 4.7 | 26.8 ± 4.6 |
| Dietary calcium intake (mg/day), mean ± SD | 882 ± 388 | 871 ± 360 |
| History of nonvertebral fracture after age 45 years, n (%) | 238 (24) | 237 (24) |
| Prevalent vertebral fracture, n (%) | 126 (13) | 137 (14) |
| FRAX 10-year hip fracture risk (%)a | 2.3 (1.5, 3.8) | 2.4 (1.5, 3.9) |
| Total hip bone density T-score, mean ± SD | −1.24 ± 0.60 | −1.27 ± 0.59 |
| eGFR (mL/min), mean ± SD | 72.4 ± 13.1 | 72.9 ± 12.9 |
| Current smokers, n (%) | 33 (3.3) | 23 (2.3) |
| Pretrial history, n (%)b | ||
| Cancer | 152 (15.2) | 127 (12.7) |
| Breast cancerc | 84 (8.4) | 61 (6.1) |
| Hypertension | 406 (40.6) | 409 (40.9) |
| Hyperlipidemia | 339 (33.9) | 328 (32.8) |
| Heart disease (including IHD and CHF) | 124 (12.4) | 132 (13.2) |
| Myocardial infarction or coronary revascularizationc | 42 (4.2) | 46 (4.6) |
| Stroke or TIA | 49 (4.9) | 42 (4.2) |
| Diabetes | 60 (6.0) | 58 (5.8) |
- Data are mean ± SD, or n (%), unless indicated otherwise. There were no significant differences between the trial groups in any of these characteristics.
- FRAX = Fracture Risk Assessment Tool; IHD = ischemic heart disease; CHF = congestive heart failure; TIA = transient ischemic attack; eGFR = estimated glomerular filtration rate.
- a Median (interquartile range).
- b Based on participant report, unless indicated otherwise.
- c Confirmed from medical records.
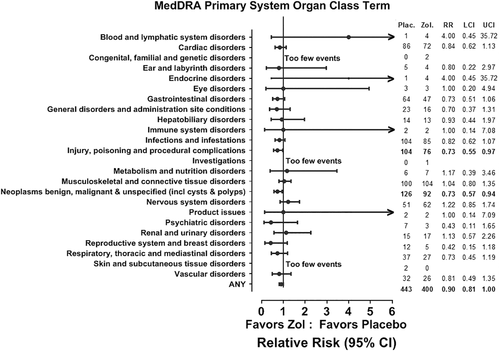
There were 1017 serious adverse events in 443 participants in the placebo group and 820 events in 400 participants in those randomized to zoledronate (relative risk = 0.90; 95% CI, 0.81 to 1.00). These events included fractures resulting in hospital admission. When grouped using high level MedDRA terms there were downward trends in many major classes, and for “Injuries” (which includes fractures) and “Neoplasms” the CIs did not overlap unity (Fig. 1). Relative risks for cardiac and vascular disorders were 0.84 (95% CI, 0.62 to 1.13) and 0.81 (95% CI, 0.49 to 1.35), respectively. The four women with events in the “Product issues” category all had problems with previously placed joint prostheses, the “General disorders” category was a miscellaneous group of complaints for many of which the diagnosis was uncertain, and the “Injury, poisoning, and procedural complications” group was mostly injuries or complications of surgery. There were no events related to trial procedures or infusions in any of these categories.
Several vascular endpoints were prespecified in the protocol (see Methods) as already reported.3 For myocardial infarction, 39 women had 43 events in the placebo group, and 24 women had 25 events in the zoledronate group (hazard ratio 0.60 [95% CI, 0.36 to 1.00]; rate ratio 0.58 [95% CI, 0.35 to 0.94]). Figure 2 shows time to first event, suggesting that a protective effect is developing within the first year, and is progressive throughout the study. For the prespecified composite cardiovascular endpoint (sudden death, myocardial infarction, coronary artery revascularization, or stroke) 69 women had 98 events in the placebo group, and 53 women had 71 events in the zoledronate group (hazard ratio 0.76 [95% CI, 0.53 to 1.08]; rate ratio 0.72 [95% CI, 0.53 to 0.98]), and the time-course was similar to that for myocardial infarction (Fig. 2). For stroke, 20 women had 22 events in the placebo group and 17 women had 20 events in the zoledronate group (relative risk 0.85 [95% CI, 0.45 to 1.61]; rate ratio 0.90 [95% CI, 0.49 to 1.66]), but fatal stroke was significantly reduced in the zoledronate group (one versus seven in the placebo group, exact mid-p = 0.04). The absolute reduction in vascular events was 4.7 (95% CI, 0.4 to 9.0) events/1000 person-years of treatment with zoledronate.

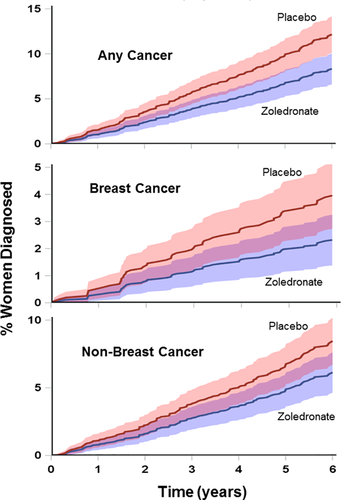
Cancer data are shown in Table 2 and Fig. 3. Only total cancers were prespecified, but data on individual cancers are also presented. Total cancers were significantly reduced (hazard ratio 0.67 [95% CI, 0.51 to 0.89], rate ratio 0.68 [95% CI, 0.52 to 0.89]). This was independent of baseline smoking status and history of cancer, and remained significant after adjustment for those variables. Breast cancer was the most common malignancy, accounting for about one-third of cancer diagnoses, and both breast cancer and the sum of all the non-breast cancers were less common in the zoledronate group. Trends to fewer events in the zoledronate group were apparent within the first year, and were progressive subsequently (Fig. 3). Of the 62 women with a diagnosis of breast cancer during the study, 11 had previously had breast cancer, all of them in the placebo group. Of these 11, six were thought to be recurrences of the previous tumor, three were judged to be second tumors, and for two this was not able to be determined. None of the 61 women in the zoledronate group with a history of breast cancer had breast cancer diagnosed during the study. The distribution of estrogen and progesterone receptor status and of HER2 positive tumors was similar in the two treatment groups (data not shown). Modeling incidence of breast cancer after adjustment for prestudy breast cancer, the hazard ratio for zoledronate compared with placebo is 0.60 (95% CI, 0.36 to 1.00). Prestudy breast cancer is associated with an increased hazard of incident breast cancer (hazard ratio 1.67 [95% CI, 1.42 to 5.49]) across the whole cohort. In those randomized to zoledronate, there was no significant difference in the number of women developing breast cancer in those with or without a prior history of breast cancer (0/61, 0% [95% CI, 0% to 5.9%] versus 23/939, 2.4% [95% CI, 1.6% to 3.7%] respectively, p = 0.23). The absolute reduction in cancer diagnoses was 6.9 (95% CI, 2.0 to 11.7) events/1000 person-years of treatment with zoledronate.
| Placebo | Zoledronate | |||||||
|---|---|---|---|---|---|---|---|---|
| Adverse event | Events | Events/1000 woman-years (95% CI) | Women with ≥1 event | Events | Events/1000 woman-years (95% CI) | Women with ≥1 event | Relative risk (95% CI) | Rate ratio (95% CI) |
| Cancera | 127 | 21.5 (18.0, 18.1) | 121 | 87 | 14.7 (11.7, 18.1) | 84 | 0.69 (0.53–0.90) | 0.68 (0.52–0.89) |
| Breast | 39 | 6.6 (4.7, 9.0) | 39 | 23 | 3.9 (2.5, 5.8) | 23 | 0.59 (0.35–0.98) | 0.59 (0.35–0.98) |
| Gastrointestinal | 28 | 4.7 (3.2, 6.9) | 28 | 21 | 3.5 (2.2, 5.4) | 20 | 0.71 (0.41–1.26) | 0.74 (0.42–1.31) |
| Melanoma | 16 | 2.7 (1.6, 4.4) | 15 | 13 | 2.2 (1.2, 3.7) | 13 | 0.87 (0.41–1.81) | 0.81 (0.38–1.69) |
| Hematologic/lymphoma | 15 | 2.5 (1.4, 4.2) | 15 | 12 | 2.0 (1.0, 3.5) | 12 | 0.80 (0.38–1.70) | 0.79 (0.36–1.71) |
| Respiratory | 12 | 2.0 (1.1, 3.6) | 12 | 7 | 1.2 (0.5, 2.4) | 7 | 0.58 (0.23–1.48) | 0.58 (0.21–1.47) |
| Gynecologic | 6 | 1.0 (0.4, 2.2) | 6 | 3 | 0.5 (0.1, 1.5) | 3 | 0.50 (0.13–1.99) | 0.50 (0.10–1.99) |
| Urinary tract | 3 | 0.5 (0.1, 1.5) | 3 | 4 | 0.7 (0.2, 1.7) | 4 | 1.33 (0.30–5.94) | 1.32 (0.27–7.10) |
| Meningioma | 3 | 0.5 (0.1,1.5) | 3 | 3 | 0.5 (0.1,1.5) | 3 | 1 .00 (0.20–4.94) | 0.99 (0.17–5.78) |
| Other | 5 | 0.8 (0.3, 2.0) | 5 | 1 | 0.2 (0.0, 0.9) | 1 | 0.20 (0.02–1.71) | 0.20 (0.01–1.43) |
| All non-breast cancers | 88 | 14.9 (12.0, 18.3) | 84 | 64 | 10.8 (8.4, 13.7) | 62 | 0.74 (0.54–1.01) | 0.72 (0.52–0.995) |
| Total deaths | 41 | 7.0 (5.4, 9.4) | – | 27 | 4.5 (3.0, 6.6) | – | 0.66 (0.41–1.06) | – |
| Neoplasm deaths | 25 | – | 16 | – | – | 0.64 (0.34–1.19) | – | |
| Nervous system deaths | 8 | – | 1 | – | – | 0.13 (0.02–1.00) | – | |
| Cardiac deaths | 3 | – | 4 | – | – | 1.33 (0.30–5.94) | – | |
| Infection deaths | 3 | – | 1 | – | – | 0.33 (0.03–3.20) | – | |
| Vascular deaths | 2 | – | 2 | – | – | 1.00 (0.14–7.09) | – | |
| Deaths from injury | 0 | – | 1 | – | – | – | – | |
| Urinary tract deaths | 0 | – | 1 | – | – | – | – | |
| Respiratory deaths | 0 | – | 1 | – | – | – | – | |
- Bold values are nominally significant.
- a Excluding non-melanoma skin cancers. Total cancers were prespecified but the subcategories were not.
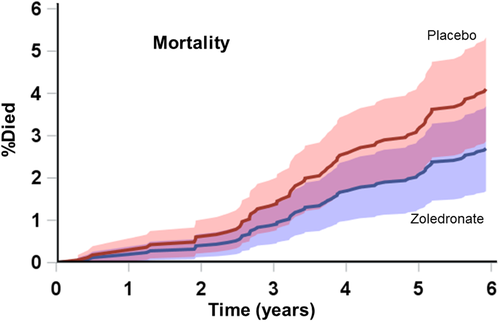
Mortality data are presented in Table 2 and Fig. 4. Forty-one women from the placebo group died compared with 27 assigned to zoledronate, hazard ratio 0.65 (95% CI, 0.40 to 1.06; p = 0.08). An imbalance in deaths was apparent for cancers and nervous system disorders (eight of those nine being strokes). As with the other adverse events, trends to fewer deaths were present in year 1, but became more apparent in the second half of the study as death rates increased (Fig. 4). To determine whether the trend in mortality was related to the effects of zoledronate on fracture, we looked at deaths according to incident fracture status. Of those with fragility fractures during the study, only seven died, two in the placebo group and five in the zoledronate group (p = 0.12). In the 1688 women without an incident fragility fracture, there were 39 deaths in the placebo group and 22 in the zoledronate group, hazard ratio 0.51 (95% CI, 0.30 to 0.87), p = 0.01.
Discussion
The data in this work are suggestive of beneficial effects of zoledronate on cardiovascular and cancer events and, possibly, mortality. None of these were efficacy endpoints of the study, and the study was, therefore, not powered to address them. Because this is the safety analysis of data from a published trial, there has been no adjustment to preserve an overall significance level. Including the prespecified adverse events, it is possible that three statistically significant results could arise by chance alone from these analyses. However, the size of the possible benefits is such that were these effects real, they could have as great an impact as that of fracture prevention, because for each 1000 person-years of treatment with zoledronate 16.5 fragility fractures (95% CI, 10.2 to 22.8), 4.7 vascular events (95% CI, 0.4 to 9.0), and 6.9 cancers (95% CI, 2.0 to 11.7) were prevented, and mortality appeared to be reduced by about one-third in the zoledronate group. The difference in deaths was mainly contributed to by fewer deaths from cancer and from stroke. In isolation these findings could be regarded as intriguing, but there are a number of other studies that contain similar suggestions of benefit from anti-resorptive drugs on these outcomes. However, these findings are inconsistent across trials.
A beneficial effect on mortality was seen in the Lyles phase 3 trial of zoledronate for osteoporosis, and appeared to arise from fewer cardiac, respiratory, and neoplastic deaths.2, 6 That study was carried out in men and women with a hip fracture within 3 months prior to recruitment. In contrast, the other phase 3 osteoporosis study of zoledronate reported by Black and colleagues1 found a relative risk of death of 1.16 (95% CI, 0.90 to 1.48). The Black and colleagues1 study recruited women with osteoporotic bone densities, and participants were more ethnically diverse, but neither of those differences seems an adequate explanation for the apparent differences in mortality outcome. Beneficial trends in mortality were seen in the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) study of another anti-resorptive drug, denosumab,7 and one meta-analysis suggested that reduced mortality is a general property of effective osteoporosis treatments.8 However, there is no suggestion of reduced mortality in the recent very large studies of odanacatib9 and romosozumab,10 suggesting that this is not a general property of agents that reduce fracture frequency. Some observational studies have reported lower mortality in those taking bisphosphonates,11-14 though biases inherent in this study design might confound these findings. However, the study of Bliuc and colleagues15 is notable in that it finds a reduction in mortality in those using alendronate or risedronate in comparison with those using the non-nitrogen bisphosphonate, etidronate. This study design should reduce the risk of confounding. Fractures in elderly individuals are associated with increased mortality, but because the reduction in mortality in the present study is as large as that in fractures, and because there are also imbalances in adverse events (such as cancer) not related to fracture, it is unlikely that fracture prevention is the principal contributor to these mortality trends. This is supported by the analysis of those without incident fragility fractures during the study. The changes in vascular events and cancers appear to be the genesis of the downward trend in deaths.
There are a number of independent strands of evidence already available suggesting that bisphosphonates might have an anti-cancer effect. First, bisphosphonates inhibit the growth of neoplastic cells in vitro.16 However, similar effects are seen in non-neoplastic cells, so this probably represents a nonspecific toxicity from high doses of these drugs.16 Second, preclinical studies show antitumor effects of bisphosphonates in animals, reducing tumor burden in bone and in non-osseous tissues.17, 18 Third, observational studies have reported that oral bisphosphonate use is associated with fewer first breast cancers19-21; reduced risk of contralateral breast cancer (OR = 0.4)22; reduced risk of a combined endpoint of recurrence, second primary breast cancer, and breast cancer mortality23; and reduced risk of skeletal metastases in early breast cancer.24 The UK General Practice Database showed reduced risk of all cancers, and of breast and colorectal cancers specifically, in bisphosphonate users,25 and a nationwide Danish database suggested protection against colon cancer with alendronate use.26 A meta-analysis of observational studies of colon cancer also found a protective effect.27 However, some observational studies of oral bisphosphonate use and cancer are negative.28, 29 Fourth, there are clinical trial data suggesting bisphosphonates reduce incidence, progression, and mortality from breast cancer. A meta-analysis of trials giving bisphosphonates to women with early breast cancer found no benefit in premenopausal women but, among 11,767 postmenopausal women, bisphosphonates significantly reduced recurrence (relative risk 0.86; 95% CI, 0.78 to 0.94), distant recurrence (0.82; 95% CI, 0.74 to 0.92), bone recurrence (0.72; 95% CI, 0.60 to 0.86), and breast cancer mortality (0.82; 95% CI, 0.73 to 0.93).5 Follow-up to 10 years in the adjuvant zoledronic acid to reduce recurrence (AZURE) trial showed that benefits were sustained.30 However, breast cancer incidence was not reduced in randomized trials of alendronate or zoledronate in osteoporotic women.31 The present findings are the first trial evidence of reduced cancer risk with zoledronate in women not selected for having a history of breast cancer. The reduced risk of breast cancer in osteoporosis32 might have reduced the power of the previous zoledronate trials in osteoporosis to detect this effect.
The body of data reviewed in the previous paragraph has led to work seeking the mechanism of an anti-cancer effect of bisphosphonates. Many types of tumor seed to bone, where dormant tumor cells can persist in niches, possibly on endosteal surfaces, for many years. The activation and growth of these dormant tumor cells is dependent on bone turnover and osteoclast activity.33 Osteoclasts, by remodeling the endosteal niche, can release dormant cells from the niche and reactivate them to form overt tumors. Therefore, osteoclast inhibitors, such as bisphosphonates and inhibitors of RANKL, inhibit reactivation of dormant micrometastases in animal models,33 and reduce persistence of tumor cells in bone marrow in women with breast cancer.34, 35 Also, bisphosphonates are found in tumor-associated macrophages outside of bone and, via this route, have been shown to be present in a resected human breast tumor where they might inhibit tumor growth.36
There is also a substantial body of preclinical and observational evidence that bisphosphonates reduce the risk of vascular disease.37 Longitudinal, nonrandomized studies have shown that bisphosphonate use is associated with 28% to 75% reductions in incident myocardial infarction, though these findings could be subject to bias.38-40 Alendronate reduced carotid artery intima-media thickness in a nonrandomized but controlled study of 72 women,41 and zoledronate has been shown to reduce both high-density lipoprotein (HDL) cholesterol and carotid artery intima-media thickness in a randomized trial of 60 postmenopausal women.42 A recent meta-analysis of 61 trials in patients with either malignant or benign conditions (the latter mainly osteoporosis) suggested bisphosphonate reduced arterial wall calcification (two trials), cardiovascular mortality (10 trials; risk ratio 0.81; 95% CI, 0.64 to 1.02), and all-cause mortality (48 trials; risk ratio 0.90; 95% CI, 0.84 to 0.98), but without a significant change in total cardiovascular events (risk ratio 1.03; 95% CI, 0.91 to 1.17), stroke (risk ratio 1.06; 95% CI, 0.82 to 1.35), or myocardial infarction (risk ratio 0.82; 95% CI, 0.57 to 1.17).4
The romosozumab development program might also have provided indirect evidence of possible cardioprotective effects of alendronate. In the Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) study,10 where romosozumab was compared with placebo in 7180 women with osteoporosis, there was no difference between-groups in cardiovascular events. In contrast, the Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH) study43 compared romosozumab with alendronate in 4093 women and found a significant excess of cardiovascular serious adverse events in the romosozumab group (2.5% versus 1.9% in the alendronate group at 12 months). Although this could represent a chance finding, it is also consistent with alendronate having reduced cardiovascular events in ARCH. However, a 12-month placebo-controlled study in 245 men also suggested an adverse effect of romosozumab on vascular events (4.9% versus 2.5%).44
Bisphosphonates might act directly on arteries. They have high affinity for hydroxyapatite, so are selectively taken up by calcified plaque. They impact on endothelial function, increasing circulating nitric oxide end-products and restoring impaired endothelium-dependent vasodilation in spontaneously hypertensive rats.45 This is likely to be mediated by increases in endothelial nitric oxide synthase (eNOS) and nitric oxide levels as a result of bisphosphonate-inhibition of prenylation of the GTPase signaling protein RhoA, which negatively regulates eNOS expression.45, 46 Thus, bisphosphonates might have a statin-like effect consistent with their acting downstream from the statins on the mevalonate pathway. Bisphosphonates also regulate gamma-delta T cells and macrophage function, both of which are present in human atherosclerotic lesions, and have been suggested to influence atherosclerotic lesion progression and plaque stability, respectively.47, 48
The present study suggests that zoledronate has clinically relevant effects on both vascular and cancer endpoints. Previous studies have suggested similar findings for both this and other bisphosphonates, though there are unexplained inconsistencies among the published findings. The long duration, high compliance, and high participant retention in the present study may have contributed to the positive findings here. A recent analysis showed zoledronate to be cost-effective (at £30,000 per quality-adjusted life-year [QALY]) in women over age 65 years whose 10-year risk of osteoporotic fracture was >6.4%, based solely on fracture prevention.49 Ninety-nine percent of the present study cohort meets this criterion. The present data potentially strengthen these findings, lower the cost per QALY, and make zoledronate cost-effective in jurisdictions where costs are higher than those used in those analyses. The possible beneficial effects in the present study for both vascular and cancer endpoints justify the initiation of further appropriately powered trials of zoledronate in which these nonskeletal conditions are the primary endpoints.
Disclosures
IRR has received fees or research funding from Amgen, Merck, Novartis, and Eli Lilly. Other authors have no interests to declare.
Acknowledgments
This work was supported by grants from the Health Research Council of New Zealand. Trial medication was supplied by Novartis. We thank Leanne Purvis for her contributions to the organization and conduct of the trial.
Authors’ roles: IRR, AMH, and GDG were involved in study design; AMH, BM, AS, EG, and SB were involved in data acquisition; GDG was involved in data analysis; IRR, GDG, AMH, BM, and SB were involved in interpretation; IRR drafted the manuscript, which was critically revised by all authors, who approved the final version and agree to be accountable for it. IRR accepts responsibility for the integrity of the data and its analysis.




