Hormone-Independent Sexual Dimorphism in the Regulation of Bone Resorption by Krox20
ABSTRACT
Krox20/EGR2 is a zinc finger transcription factor, implicated in the development of the hindbrain, nerve myelination, and tumor suppression. In skeletal biology, we have demonstrated that Krox20 also regulates adult bone metabolism. We and others have characterized several functions of Krox20 in the osteoclast lineage, namely, preosteoclast proliferation and differentiation, and mature osteoclast apoptosis. We have previously reported that systemically Krox20-haploinsufficient mice have a low bone mass with increased bone resorption. However, new data have now revealed that this phenotype is restricted to females. In addition, we discovered that conditional knockout of Krox20 (cKO) restricted to osteoclast progenitors is sufficient to induce the same female-specific bone loss observed in systemic mutants. To test whether this sexual dimorphism results from an interaction between Krox20 and sex hormones, we examined the sex- and hormone-dependent role of Krox20 deficiency on proliferation and apoptosis in osteoclastic cells. Our results indicate that male and female sex hormones (dihydrotestosterone [DHT] and estradiol [E2], respectively) as well as Krox20 inhibit preosteoclast proliferation and augment osteoclast apoptosis. The observation that Krox20 expression is inhibited by DHT and E2 negates the hypothesis that the effect of sex hormones is mediated by an increase in Krox20 expression. Interestingly, the effect of Krox20 deficiency was observed only with cells derived from female animals, regardless of any sex hormones added in vitro. In addition, we have identified sexual dimorphism in the expression of several Krox20-related genes, including NAB2. This sex-specific epigenetic profile was established at puberty, maintained in the absence of sex hormones, and explains the female-specific skeletal importance of Krox20. The findings described in this study emphasize the medical importance of sex differences, which may be determined at the epigenetic level. © 2019 American Society for Bone and Mineral Research.
Introduction
The zinc finger transcription factors of the early growth response family (EGR) are encoded by immediate early response genes and are important for the induction of cellular responses to environmental stimuli. These may involve differentiation, proliferation, or cell death. The most prominent members of the EGR family, EGR1, EGR2, and EGR3, share a highly conserved DNA-binding domain composed of three zinc fingers that recognize G:C-rich DNA motifs in the promoters of target genes.1-3
Krox20, the murine homologue of EGR2, has been implicated in hindbrain development, peripheral nerve myelination, tumor suppression, and the determination of the fate of monocyte/macrophages.4-6 The gene has also been associated with Charcot–Marie–Tooth disease, which, like amyotrophic lateral sclerosis (ALS), is a severe neurodegenerative disease.7 Other proposed functions include involvement in the pathophysiology of autoimmune disease, mediating allergen-induced mast cell production,8 and maintaining T-cell homeostasis.9
There have been a number of reports describing the important roles played by Krox20 in bone development and homeostasis. During embryonic development, Krox20 is expressed both in hypertrophic chondrocytes and osteoblasts/osteocytes and its deficiency results in impaired endochondral ossification in newborn mice.10 We and others have shown that in skeletally mature mice, Krox20 haploinsufficiency results in increased bone resorption and a low bone mass phenotype,11 whereas Krox20 expression in monocytic cells regulates preosteoclast proliferation, osteoclastogenesis, and apoptosis of mature osteoclasts.11-13
Sex steroids play key roles in the development and maintenance of the skeleton in virtually all species.14 The most common sex hormones are estradiol (E2), that binds to the estrogen receptors (ERα and ERβ), and testosterone (Te) and dihydrotestosterone (DHT) that bind to the androgen receptor (AR). Both estrogens and androgens exert a potent influence on the size and shape of the skeleton during growth, with ERα, ERβ, and AR all playing a role in bone cells.15 In addition, sex hormones contribute to skeletal homeostasis during adulthood, mainly by restraining the rate of bone remodeling.16 Importantly, the estrogen receptor (ER) is also activated in males through aromatization of Te to E2, whereas DHT is a nonaromatizable AR agonist.17, 18 In the monocytic lineage, estrogens and androgens have been shown to attenuate preosteoclast proliferation and differentiation and to induce apoptosis in mature osteoclasts.17, 19, 20
Sexual dimorphism represents one of the hallmarks of non-Mendelian biology and is evident across all species in physiological and behavioral traits, as well as in differences in the prevalence and course of many common diseases such as asthma, diabetes, lupus, autism, and major depression.21 Although it is generally accepted that endocrinological differences are involved in the sexual dimorphism of complex diseases, the molecular mechanisms responsible for such hormonal effects are still only partly understood. Here, we describe a sexual dimorphism in the skeletal roles of Krox20 and present data that portray a sex-specific epigenetic profile that is maintained independently of sex hormones.
Materials and Methods
Animals and husbandry
All animal research protocols and experiments were conducted in accordance with the guidelines and with the approval of the Institutional Animal Care and Use Committee of Tel Aviv University and the University of Southern California. The minimal number of mice were used for the study and all efforts were made to minimize potential suffering. Unless otherwise indicated, all mice were 8 weeks old and weighed 20 g on average. Mice were kept 5 to 6 per cage under a constant 12-hour light/dark cycle, at room temperature (22 ± 2°C). Food (Maintenance rodent chow, Altromin, Germany) and water were available ad libitum. Data from Krox20+/− female mice22 and their controls (wild type [WT]) were described previously,11 and we present here unpublished data obtained from their male littermates. To generate animals deficient for Krox20 in the monocytic lineage (conditional knockout [cKO]), we used Krox20flox/flox (Krox20 fl/fl) mice generated by Dr Charnay23, 24 crossed with the LysM:cre mice (Lyz2tm1(cre)Ifo, targeted mutation 125). Because LysM:cre+/− displayed incomplete recombination in murine myeloid cells,26 we employed the same strategy as described previously,27, 28 using homozygous LysM:cre+/+ to conditionally delete Krox20 in the Krox20 fl/fl alleles. To account for the possible skeletal phenotype of LysM:cre+/+ mice, the controls for the cKO LysM:cre+/+-Krox20 f/f mice are the LysM:cre+/+-Krox20 WT/WT littermates (controls carrying both alleles of the WT Krox20 gene, referred here as WT) obtained by breeding two LysM:cre+/+-Krox20 WT/fl parents. All mice were on a C57BL/6J-Rcc background (Envigo, Jerusalem, Israel).
Genotyping
To identify mice carrying the floxed allele of the Krox20 gene or the LysM:cre, DNA was prepared from tail biopsies (Kapa Biosystems, Wilmington, MA, USA) and subjected to PCR amplification with the primers listed in Table 1. {TBL 1} The PCR products were then separated on a 1.5% agarose gel stained with ethidium bromide.
| Genotyping | ||||
| Gene | References | Sequence (5′ ➔ 3′) | ||
| Krox20 | 11 | F | GCAGAAGGAACGGAAGAGCAG | |
| R | ATCAAGGTCCTTTGCCCAGATC | |||
| Krox20 Mutant | 11, 22 | F | GGCCGCTTTTCTGGATTCAT | |
| R | ATCAAGGTCCTTTGCCCAGATC | |||
| Flox Krox201 | 23, 24 | F | GTGTCGCGCGTCAGCATGCGTG | |
| R | GGGAGCGAAGCTACTCGGATACGG | |||
| LysM (WT) | JAX primers 004781(25) | F | CTTGGGCTGCCAGAATTTCTC | |
| R | TTACAGTCGGCCAGGCTGAC | |||
| LysM:cre | JAX primers 004781(25) | F | CTTGGGCTGCCAGAATTTCTC | |
| R | CCCAGA AATGCCAGATTACG | |||
| RT-qPCR | ||||
| Gene | GenBank ID | PrimerBank/Ref. | F/R Sequence (5′ ➔ 3′) | |
| Krox20 | NM_010118 | 11 | F | AGTTGGGTCTCCAGGTTGTG |
| R | GGAGATCCAGGGGTCTCTTC | |||
| cFMS (csf1r) | NM_001037859 | 11 | F | GGAGGTGGATTCCCTACCAT |
| R | GGCCCAGAACTGGTTGTAGA | |||
| Gapdh | NM_008084 | F | GAAGGTGAAGGTCGGAGT | |
| R | GAAGATGGTGATGGGATTTC | |||
| Nab1 | NM_008667 | 31543307a1 | F | GATGCCTTTATCCAACAAGGTGG |
| R | CCAGGATTTGTAACCCAGTCTC | |||
| Nab2 | NM_001122895 | 6679004a1 | F | TGGCAGAGGGGATAACACAC |
| R | CGTTGCAGGACCCGATACAG | |||
| Acp5 | NM_001102404 | 156151432c1 | F | CACTCCCACCCTGAGATTTGT |
| R | CATCGTCTGCACGGTTCTG | |||
| Bpgm | NM_007563 | 6680806a1 | F | GGACCAGAAACTTAACAACGACG |
| R | CAGGCTGTGTGAATGGACCT | |||
| Hcfc1 | NM_008224 | 34328130a1 | F | CGGCAACGAGGGGATAGTG |
| R | TAGGCGAGTACCATCACACAC | |||
| Ndufs2 | NM_153064 | 23346461a1 | F | CAGCCAGATATTGAATGGGCA |
| R | TGTTGGTCACCGCTTTTTCCT | |||
| Rbm15b | NM_175402 | 30425110a1 | F | AGGGCGAAGGTGGCTATGT |
| R | GCGAGGTGTTAGGTCCGAG | |||
| Rimklb | NM_027664 | 12855934a1 | F | CGGATCAGTGGAGAGCTAATCT |
| R | GTGGCGCAAAACAGTAATATCAC | |||
| Rpl7l1 | NM_025433 | 27754134a1 | F | CGACTAGAATCTTTCGTGCATGA |
| R | GGGATGTTTATCAGGCACCTC | |||
| Ddx20 | NM_017397 | 31980857a1 | F | AATCAGCGCCTTGATGCTATG |
| R | ACGGCACATCGAGATTTACAAC | |||
- F = forward; R = reverse; Ref. = reference; WT = wild type.
- 1 The flox and WT alleles yield bands of different size.
Microcomputed tomography (μCT) analysis
Structural analysis of the trabecular bone in the distal femoral metaphysis was carried out using a μCT50 system (Scanco Medical AG, Bruttisellen, Switzerland). Briefly, scans were performed at a 10-μm resolution in all three spatial dimensions. The mineralized tissues were differentially segmented using a global thresholding procedure, with a different threshold for the cortical (224 permil) and trabecular (150 permil) bone compartments.29 All morphometric parameters were determined by a direct 3D approach.30 Trabecular bone in the distal femoral metaphysis was analyzed in a region spanning 1.5 mm and ending distally 1 mm proximal to the growth plate. Customized algorithms developed on proprietary Scanco IPL software were used to assess the morphometric parameters: trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), trabecular connectivity density (Conn.D), trabecular spacing (Tb.Sp), and volumetric bone mineral density (vBMD). All parameters were measured according to the official 2010 guidelines for bone morphometry.31
Histomorphometry
Osteoclasts were counted with the aid of Media Cybernetics (Rockville, MD, USA) ImagePro software (v6.0) as described previously.11 The terminology and units used for these measurements were according to the convention of standardized nomenclature.32
Cell cultures
Bone marrow cells were extracted and preosteoclasts were obtained after 3 days of culture in standard medium (α-MEM, 10% fetal calf serum [FCS]) supplemented with 100 ng/mL macrophage colony-stimulating factor (M-CSF). To measure proliferation, preosteoclasts were plated in CSS medium (phenol red free α-MEM, 10% charcoal-stripped FCS devoid of sex steroid hormones) supplemented with 100 ng/mL M-CSF at a density of 3000 cells per well in 96-well plates. The number of viable cells present after 1 and 3 days was assessed by the thiazolyl blue tetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO, USA) assay according to the manufacturer's instruction. To induce osteoclast differentiation, adherent preosteoclasts were plated at 5000 cells per well in 96-well plates with standard medium supplemented with 20 ng/mL M-CSF and 50 ng/mL receptor activator of NF-κB ligand (RANKL; R&D Systems, Minneapolis, MN, USA). After 3 to 4 days, as the first multinucleated osteoclasts appeared, the medium was changed to CSS medium supplemented with 20 ng/mL M-CSF, and 50 ng/mL RANKL (osteoclastogenic CSS medium) for 24 hours. Duplicate samples of cells were then treated with ethanol (EtOH), E2, DHT, or H2O2 as indicated and incubated for another 24 hours before being evaluated for apoptosis.
Apoptosis assay
Annexin V (AnnV) (green)-propidium iodide (PI) (red) staining (MBL Medical & Biological Laboratories, Ltd., Nagoya, Japan) was used to identify apoptotic osteoclasts according to the established definition of late apoptotic cells (AnnV+/PI+).33 Apoptotic cells were counted under an EVOS FLc fluorescent microscope (Thermo Fisher Scientific, Waltham, MA, USA).
RNA extraction and quantitative real-time PCR
Bone marrow-derived preosteoclasts were cultured at a density of 1 × 106/well in 6-well plates with either vehicle, E2, or DHT. After 24 hours, preosteoclasts were rinsed with phosphate-buffered saline, and RNA was purified using TRIzol reagent (Invitrogen, Grand Island, NY, USA) and phenol/chloroform according to the manufacturer's instructions. An aliquot containing 1 μg of RNA was then reverse-transcribed using oligo (dT) primers and the High Capacity cDNA Reverse Transcription Kit (Invitrogen). The resulting cDNA samples were processed for real-time RT-qPCR analysis on a StepOne real-time PCR machine (Applied Biosystems, Grand Island, NY, USA). RNA gene expression was normalized to Gapdh transcript levels for each sample and presented as fold changes from control. Most new primers were validated sequences from PrimerBank.34 The primers used in this study are listed in Table 1.
Statistical analysis
Values are expressed as mean ± SD. For paired comparisons, we used either a Student t test for n ≥ 5 or a nonparametric Mann–Whitney test for n ≤ 4. For multiple group comparisons, we used parametric (n ≥ 5) or nonparametric (n ≥ 5) ANOVA with a Newman–Keuls post hoc correction. Differences between groups were considered significant when p < 0.05. Pairwise comparison was used for experiments conducted in batches. All analyses were performed using Prism v7.0 (GraphPad Software, La Jolla, CA, USA).
Results
Systemic Krox20-haploinsufficiency results in bone loss only in females
We have previously reported that Krox20 haploinsufficiency in 8-week-old female mice results in increased osteoclast number, resulting in increased bone resorption and significant bone loss.11 Here, analysis of the bone phenotype of their male littermates (WT and Krox20+/−) at the same age revealed that the skeletal effect of Krox20 haploinsufficiency is sex dependent (Fig. 1) {FIG1} as Krox20+/− male mice displayed no noticeable Krox20 haploinsufficiency-related skeletal phenotype with respect to any of the analyzed parameters (Fig. 1 A, B). There were no differences between WT and Krox20-haploinsufficient male mice in bone mass or the number of osteoclasts per bone surface (Oc.N/BS) (Fig. 1 C). These results indicate that the bone loss induced by systemic Krox20 haploinsufficiency is restricted to female mice.
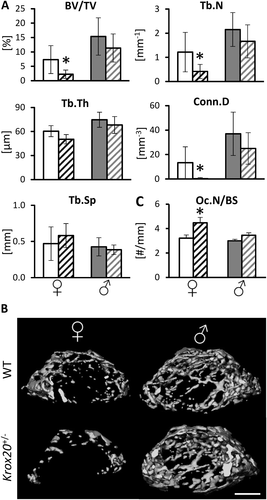
Conditional knockout of Krox20 in the monocytic lineage recapitulates the bone phenotype observed in systemically Krox20-deficient mice
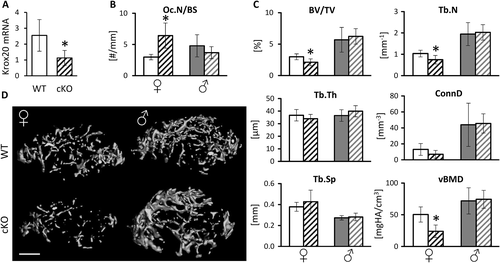
To determine whether Krox20 expression in osteoclasts and their precursors drives the skeletal phenotype observed in systemically Krox20-deficient mice, we generated conditional knockout mice (cKO) by crossing Krox20fl/fl with LysM:cre expressing mice. Preosteoclasts isolated from the femur of the cKO mice exhibited a significant reduction in the expression levels of Krox20 (Fig. 2 A). {FIG2} Our histomorphometric and microcomputed tomographic (μCT) analyses of the distal femur of 8-week-old cKO female mice revealed a significant increase in osteoclast number (Fig. 2 B) and bone loss (Fig. 2 C, D) relative to female controls. This bone loss was evidenced by a lower BV/TV and lower trabecular number in the cKO mice (Fig. 2) and was similar to that we had previously observed in systemically Krox20-deficient female mice.11 We also found a ~50% lower vBMD in female cKO mice compared with WT (Fig. 2 C). As expected, the bone morphometric parameters in control male animals revealed generally higher values than in females (Fig. 2 C), but there were no significant differences between cKO and WT control male mice (Fig. 2). These results indicate that deletion of Krox20 in the osteoclastic lineage is sufficient to recapitulate, at least in part, the same sex-specific bone loss observed in female mice carrying a systemic heterozygous deletion of Krox20.
Sex hormones inhibit preosteoclast proliferation
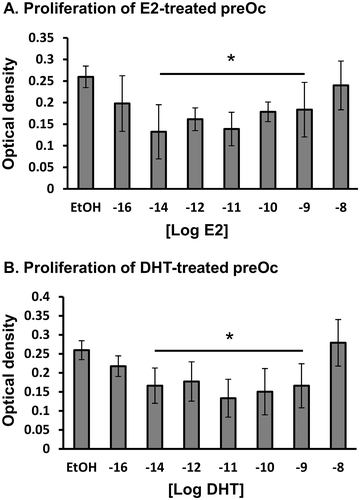
We originally hypothesized that the sex specificity of the bone response to Krox20 deficiency relates to the actions of sex hormones. First, we carefully titrated the effect of sex hormones on preosteoclast proliferation. Because a dose range commonly reported in the literature (≥10 nM35, 36) did not reveal any effect of estrogens or androgens, we undertook a dose-response experiment over a larger dose range. Interestingly, we found that the effective dose of E2 was lower than 10 nM and a suppressive effect of E2 on preosteoclast proliferation was found at 10−9 to 10−14 M (Fig. 3 A). {FIG3} With respect to androgens, DHT similarly inhibited preosteoclast proliferation at the same dose range (Fig. 3 B). Overall, we found no statistically significant differences between the effects of E2 versus DHT on preosteoclast proliferation.
Sex hormones induce apoptosis of mature osteoclasts
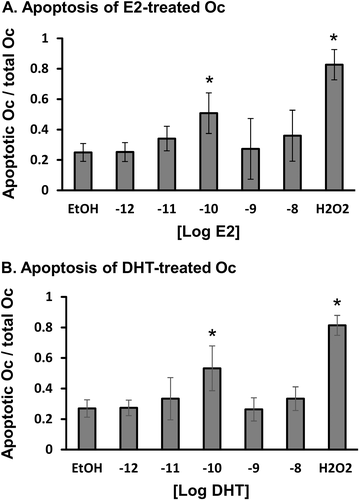
There have been a number of previous reports of the ability of sex hormones to induce apoptosis in mature osteoclasts.17 To determine the optimal conditions, we exposed differentiated mature osteoclasts to different concentrations of DHT and E2 (0.001–10 nM) for 24 hours before staining the culture with annexin-propidium to identify apoptotic osteoclasts. Exposure to 10 nM H2O2 for 24 hours served as a positive control. The results indicated the most efficient concentration for the induction of apoptosis was 0.1 nM for both E2 and DHT (Fig. 4 A, B). {FIG4} In addition, the pro-apoptotic effect and dose range of DHT were very similar to those of E2. In all subsequent experiments, we used E2 and DHT at a dose of 0.1 nM.
Krox20 deficiency stimulates proliferation of preosteoclasts derived from female but not male animals
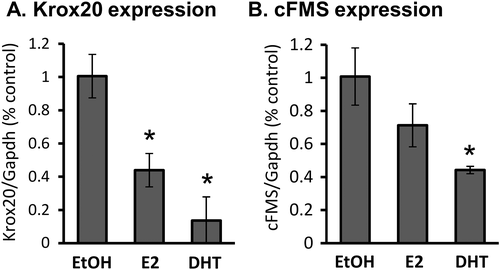
Because Krox20 and sex hormones exhibit very similar effects in the osteoclastic lineage, we asked whether Krox20 mediates the response to sex hormones. To answer this question, we treated bone marrow–derived preosteoclasts with 0.1 nM E2 or DHT and examined the expression of cFMS and Krox20. The results indicated that both Krox20 and cFMS mRNA levels were reduced by E2 and DHT (Fig. 5), {FIG5} thereby nullifying the hypothesis that the effect of sex hormones is mediated by an increase in Krox20 expression.
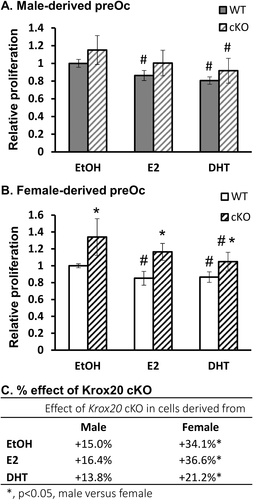
To obtain a better understanding of the interaction between Krox20 and sex hormones in osteoclasts and their progenitors, we next treated cells derived from either cKO or WT mice with E2 and DHT. The results indicated that although the sex hormones inhibited the proliferation of cells from either sex, the effect of Krox20 deficiency was restricted to cells derived from female mice. Indeed, Krox20 deficiency led to an increased proliferation in preosteoclasts derived from female but not from male animals (cKO versus WT, Fig. 6 A, B). {FIG6} In general, we found that the proliferative effect of Krox20 deficiency was ~2-fold higher in female- than in male-derived cells (p < 0.05, Fig. 6 C), without reference to the hormonal milieu.
Krox20 deficiency attenuates osteoclast apoptosis in female- but not in male-derived cells
We compared the apoptotic rate in osteoclasts derived from WT versus Krox20 cKO mice in the presence of 0.1 nM E2 or DHT. Parallel cultures were treated with H2O2 and ethanol (positive and negative controls, respectively).
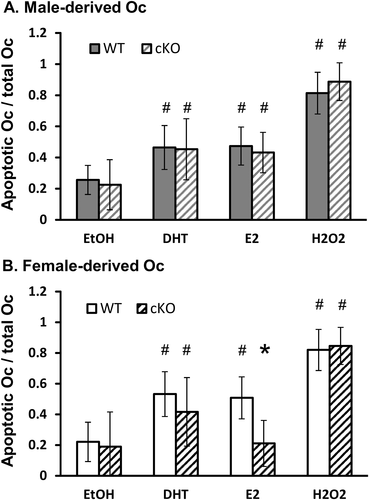
In the complete absence of sex hormones (ethanol and CSS), the rate of apoptosis in cKO cultures was similar to that of WT osteoclasts. In DHT-treated cultures also, Krox20 deficiency had no effect on osteoclast apoptosis. These two observations were independent of the sex of the donor animal; namely, a similar trend was found in male- and female-derived cells (Fig. 7 A, B). {FIG7} However, in E2-treated cultures, we found that Krox20 deficiency significantly reduced the apoptosis rate in osteoclasts when the cells were derived from female (Fig. 7 B) but not from male mice (Fig. 7 A).
Differential expression profile of Krox20-related genes in preosteoclasts derived from female and male mice
One explanation for the lack of response to Krox20 deficiency in osteoclasts derived from male animals could be that Krox20 expression and/or activity is inhibited in WT male-derived cells, so that Krox20 knockout has no noticeable effect. In addition, because this sexual bias persisted independently of E2 or DHT treatment, we proposed that osteoclast progenitors have a distinct sex-associated epigenetic profile that persists independently of the hormonal milieu. Krox20 activity may be regulated by a number of co-factors, such as Nab1, Nab2, and Ddx20, which are known to have a suppressive effect on Krox20 activity,37-39 or by Hcfc1, which is a Krox20 coactivator.40 The IntAct Database was screened for other genes related to Krox20 or to its human homolog EGR2. Overall, we examined the expression of Krox20, Nab1, Nab2, Acp5, Bpgm, Hcfc1, Ndufs2, Rbm15b, Rimklb, Rpl7l1, and Ddx20 in bone marrow–derived preosteoclasts obtained from 5 male and 5 female 8-week-old mice. The cells were cultured for 10 days in the absence of sex hormones, with charcoal-stripped serum and M-CSF only (no further treatment). Remarkably, we found several differences in the expression levels of Krox20-related genes between cells from male and female animals (Fig. 8 A). {FIG8} Notably, male-derived preosteoclasts exhibited a significant increase in the expression of Nab2, a repressor of Krox20,38 and a significant decrease in Bpgm and Rimklb expression.
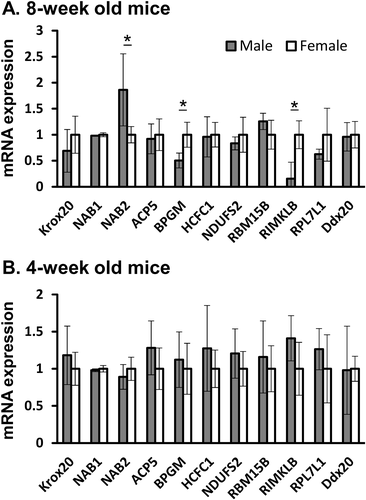
To examine whether this sex-specific epigenetic profile is determined at puberty, during which both sexes experience a surge in sex hormones, we compared osteoclast progenitors from 4-week-old prepubertal male and female mice. No sex-associated differences were observed in the expression levels of any of the Krox20-related genes when cells were extracted at the age of 4 weeks (Fig. 8 B). This suggests that the sex-specific gene expression profile in preosteoclasts results from epigenetic changes imprinted during puberty.
Discussion
The present study provides evidence for a sexual dimorphism in the response to Krox20 deficiency where the skeletal response to systemic Krox20 haploinsufficiency is mediated by a cell-autonomous role of Krox20 in the osteoclastic lineage and is restricted to females.
We demonstrated here that a conditional deletion of Krox20 in the monocyte lineage leads to the same female-restricted low bone mass phenotype observed in mice carrying a systemic heterozygous deletion of Krox20. These data indicate that Krox20 expression in the osteoclastic lineage has a determinant sex-specific role in bone mass regulation. Previous studies showed that Krox24 and Krox20 may have redundant roles in monocytic maturation,5 which may explain why Krox20 is not critical for M-CSF-related differentiation of liver monocytes into macrophages.41 We also reported that the number of bone marrow monocytes was not affected by Krox20 haploinsufficiency.11 In that same report, however, we noted for the first time that Krox20 deficiency affects preosteoclast proliferation and here we found that this gene also has a critical role in osteoclast survival. It therefore appears that Krox24 cannot compensate for the lack of Krox20 in the osteoclast lineage. This may be because Krox20 deficiency does not result in elevated expression of Krox24 in preosteoclasts.11
Our current results indicate that AR and ER signaling are both involved in the regulation of osteoclast survival because sex hormones inhibit proliferation of preosteoclasts and induce apoptosis in mature osteoclasts. Interestingly, the optimal dose in our in vitro setting is 10- to 100-fold lower than the dosage reported in most studies,20, 35, 36, 42-44 and is much closer to the physiological serum levels of sex hormones in mammals, which ranges from 0.001 to 0.1 nM in mice.45 Not all publications report a dose response that includes these low concentrations, but the one that did differed from our findings in that they showed that sex hormones had a stronger effect at concentrations of 10 nM and above than at lower doses.20 These discrepancies may originate from differences in experimental conditions such as serum batch and sex hormone production, as well as the background strain of the mice from which the cells were extracted. Be that as it may, these observations advocate for a careful titration of the cellular response to a vast range of sex hormone concentrations for each particular experimental setting.
To explain the female-specific bone phenotype due to Krox20 deficiency in mice, our first hypothesis was that the deletion of Krox20 leads to an increase in the rate of osteoclast apoptosis in male but not in female animals. According to this original assumption, Krox20 deficiency in females would increase preosteoclast proliferation, leading to increased bone resorption and a low bone mass, but the decrease in osteoclast survival in males would offset the increase in proliferation to give no net difference in osteoclast number and bone resorption. However, we found that the deletion of Krox20 led to increased osteoclast survival (lower apoptotic rate) in female cells only, with no significant change in male cells. In addition, Krox20 deficiency in preosteoclasts was associated with increased proliferation only in female cells, with no change in male cells. Together, our data support the notion that the net effect in males remains unchanged because Krox20 deficiency does not affect proliferation or apoptosis in the osteoclast lineage. In contrast, Krox20 deficiency in female animals promotes both preosteoclast proliferation and osteoclast survival, which act together to increase the rate of bone resorption.
A reasonable explanation for this sexual dimorphism would be to assume that Krox20 expression or activity is inhibited by AR signaling in males. Evidence against this proposal is provided by the observation that Krox20 deficiency had no significant effects on cells from male animals even in the absence of DHT. As an additional point, the sensitivity of female cells to Krox20 deficiency cannot be explained by ER signaling because in vivo, males have similar levels of ER signaling to females due to Te aromatization, and Krox20 deficiency had no significant effect on male cells treated with E2. From a consideration of the overall results, we may therefore conclude that the effect of Krox20 deficiency in osteoclasts depends on the sex of the donor animal rather than on the hormonal milieu.
Returning to the mechanism behind the sexual dimorphism found in the skeletal response to Krox20 deficiency, there may be two plausible explanations for the influence of the sex of the donor animals. We may assume that the Y chromosome expresses a gene that provides “skeletal immunization” against the loss of Krox20 or, alternatively, it may be that the difference is epigenetic. According to the second option, the different hormonal milieu in male and female mice imprints epigenetic activation/inactivation of genes, and these differences are preserved even in the absence of sex hormones. The results presented in this study demonstrated sex-specific expression profiles of several Krox20-related genes, even in the absence of sex hormones. The observation that the sex-specific differences were evident in cells from 8-week-old but not 4-week-old mice strengthens the epigenetic hypothesis because the genetic sequence of the Y chromosome does not change between the age of 4 and 8 weeks, while puberty takes place exactly at this age in mice (http://www.informatics.jax.org/silver/contents.shtml). Our results therefore suggest that the pubertal male and female hormonal surge leads to a sex-specific epigenetic imprint on the genome. This sex bias is thereafter maintained even in the absence of sex hormones. More specifically, our data suggest that the sexual dimorphism in the response to Krox20 deficiency is due to elevated expression of Nab2, a Krox20 co-repressor,38 specifically in male cells. Because Krox20 is constantly under the inhibitory action of Nab2, Krox20 plays no major skeletal role in males, which explains the lack of skeletal phenotype in Krox20-deficient male mice.
On a broader perspective, it is well established that sexual dimorphism results from exposure to male and female sex hormones. This exposure can lead to reversible and sex hormone–dependent effects, but it can also lead to long-term and irreversible effects, as exemplified here by the sex-specific skeletal role of Krox20 that is maintained even in the absence of sex hormones. Our results here are therefore in accordance with the notion that sex hormone actions are mediated by gene-specific epigenetic modifications of DNA and histones. A striking example of such sex-specific epigenetic is the well-documented brain sexual dimorphism.46, 47 In humans, males and females show differences in several neurological phenotypes, including the regulation of emotions and cognitive functions.48 In an analogous situation to the brain, our study advocates for a similar scenario, where early-life hormonal exposure culminates in long-term molecular effects.
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was carried out in partial fulfillment of the requirements for an MSc degree for ES and EH from the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. This work was supported by Israel Science Foundation (ISF) grants nos. 1822/12 and 1367/12 to YG. We acknowledge our deepest appreciation to Professor Ron Apte (Ben Gurion University) for sharing the LysM:cre mice, to Professor Patrick Charnay (Ecole Normale Supérieure) for sharing the Krox20 floxed mice, and Dr Eric Hay (U606 Hôpital Lariboisier) for his technical advices. We also want to express our gratitude to Professor Martina Rauner for her help in the preparation of histological slides.
Authors' Roles
ES, EH, TL, SH, BF, and YG contributed to the design of the research. ES, EH, SH, and TL performed research and contributed to the obtained results. YG and BF supervised the project. ES and YG wrote the article and prepared it for publication. YG takes responsibility for the integrity of the data analysis. All authors read and approved the final manuscript.




