Syntaxin 4a Regulates Matrix Vesicle-Mediated Bone Matrix Production by Osteoblasts
ABSTRACT
Osteoblasts secrete matrix vesicles and proteins to bone surfaces, but the molecular mechanisms of this secretion system remain unclear. The present findings reveal the roles of important genes in osteoblasts involved in regulation of extracellular matrix secretion. We especially focused on “soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor” (SNARE) genes and identified notable Syntaxin 4a (Stx4a) expression on the basolateral side of the plasma membrane of osteoblasts. Furthermore, Stx4a overexpression was found to increase mineralization by osteoblastic cells, whereas Stx4a knockdown reduced levels of mineralization. Also, BMP-4 and IGF-1 induced the localization of Stx4a to the basolateral side of the cells. To examine the function of Stx4a in osteoblasts, we generated osteoblast-specific Stx4a conditional knockout mice, which demonstrated an osteopenic phenotype due to reduced matrix secretion. Bone mineral density, shown by peripheral quantitative computed tomography (pQCT), was reduced in the femur metaphyseal and diaphyseal regions of Stx4a osteoblast-specific deficient mice, whereas bone parameters, shown by micro–computed tomography (μCT) and bone histomorphometric analysis, were also decreased in trabecular bone. In addition, primary calvarial cells from those mice showed decreased mineralization and lower secretion of matrix vesicles. Our findings indicate that Stx4a plays a critical role in bone matrix production by osteoblasts. © 2016 American Society for Bone and Mineral Research.
Introduction
Osteoblasts are differentiated from undifferentiated mesenchymal cells and gradually show increased liver/bone/kidney alkaline phosphatase (ALP) activity to produce phosphate (Pi) from pyrophosphate. In addition, osteoblasts secrete matrix vesicles (20 to 200 nm in diameter) into the bone matrix to form hydroxyapatite crystals by concentrating calcium (Ca2+) and Pi. Osteoblast matrix vesicles contain Ca2+, Pi,1 ALP,2 Annexin A5 Ca2+ channel (ANXA5),3 SLC20A1 phosphate transporter,4 and phosphatidylserine.5 Furthermore, matrix proteins such as collagens are also secreted by osteoblasts, then hydroxyapatite crystallization spreads into proximal collagen fibers and the contiguous matrix is calcified. Thereafter, osteoblasts continue their activity of additive bone matrix production and ossification is produced at the mineralization front. Thus, osteoblasts first secrete matrix vesicles to form crystal nuclei, followed by secretion of matrix proteins to serve as a bridge for crystal propagation.6 Finally, lamellar bone mineralization accompanies the transition of late osteoblasts to osteocytes.7
Eukaryotic cells are known to have two secretion systems; a constitutive secretory pathway that functions as signal-independent default pathway in various cell types and a regulated secretory pathway that only functions with specific signaling in specialized cells. These specialized cells possess secretory vesicles or granules where secretory materials are concentrated and stored, which are secreted via exocytosis by a specific extracellular signal. The regulated secretory pathway has been well studied in regard to neuronal synaptic vesicle secretion8, 9 and in yeast.10, 11 Those findings revealed that vesicles are released via the steps of vesicle formation, vesicle trafficking, vesicle tethering, docking, membrane fusion, and endocytosis9, 12 in the regulated secretory pathway. Among those steps, “soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor” (SNARE) proteins mediate membrane fusion, in which the vesicle membrane is fused with the plasma membrane (secretory vesicle) or an intracellular compartment, such as the Golgi apparatus (transport vesicle).9-13 SNARE proteins are classified into two groups; vesicle-SNARE (v-SNARE), localized in the membranes of vesicles, and target-SNARE (t-SNARE), found in the membranes of target compartments. Of those, SNARE proteins are composed of three groups of proteins: syntaxin,14 synaptosomal associated protein (SNAP),13 and vesicle-associated membrane protein (VAMP).15 Of those, syntaxin and SNAP are t-SNARE proteins. Furthermore, syntaxin family proteins are comprised of 16 members, with syntaxin 1 (STX1), STX2, STX3, and STX4 localized in the plasma membrane, and SNAP23 and SNAP25 positioned on the plasma membrane, whereas others are localized in the Golgi compartment, endosomes, or endoplasmic reticulum. VAMP proteins are part of the v-SNARE family, and VAMP1 and VAMP2 are associated with the membranes of neuronal synaptic vesicles.16, 17
It is not clear whether osteoblasts utilize a constitutive secretory or regulated secretory pathway. Bhangu and colleagues18 reported that SNARE proteins, such as SNAP25, STX4, VAMP1, SYN1, STXBP2, EXOC4, DAB2, STX6, SYP, SYT1, and RAB3A, are localized in vesicular glutamate exocytosis sites of osteoblasts, whereas Prele and colleagues19 noted that they are positioned in cell-to-cell contact sites and on the leading edges of migratory cells. Those results confirmed the cellular localization of SNARE proteins in osteoblasts, though analysis of their functions in those cells has yet to be reported.
For the present study, we generated osteoblast-specific Stx4a knockout mice, which exhibit an osteopenic phenotype caused by a decrease of bone matrix production, to investigate the role of Stx4a in the mechanism of matrix vesicle-mediated bone matrix production by osteoblasts. Our findings establish important roles of Stx4a in osteoblast bone matrix production.
Materials and Methods
Generation of Stx4a conditional knockout mice
Conditional knockout mice were constructed by deletion of exon 1 upon cre-mediated excision. The detailed targeting construct and screening strategies utilized can be found in the Extended Experimental Procedures section. After deletion of the neomycin cassette by flp, Stx4aflox/flox mice were crossed with 2.3-kb type I collagen promoter-derived cre recombinase mice (Col1-cre) to excise the floxed exon 1 containing the first ATG codon, resulting in osteoblast-specific Stx4a-deficient mice. All experiments were performed according to protocols approved by the Dentistry Animal Care Committee of Osaka University Graduate School.
Skeletal analysis
In situ hybridization was performed as described.20 Newborn mice were dissected and stained with Alcian blue 8GX (cartilage) and Alizarin red S (bone) for examining skeletal preparations. Eight-week-old mice were labeled with calcein at 4 days and tetracycline at 1 day before euthanasia. Following radiological analyses of the skeletons, femurs were used for peripheral quantitative computed tomography (pQCT) and high-resolution microfocus X-ray micro–computed tomography (μCT) examinations, according to the ASBMR guidelines.21 Tibias were dissected and nondecalcified bones were embedded in glycol methacrylate, then sections were prepared using a rotation microtome and stained with toluidine blue, Villanueva Goldner's trichrome, and tartrate-resistant acid phosphatase (TRAP)/ALP. Bone histomorphometric analysis was performed using the tibia proximal metaphysis, according to the report of the ASBMR histomorphometry nomenclature committee.22
Additional details can be found in the online Supplemental Materials and Methods.
Results
Stx4a expressed in osteoblasts
We speculated that osteoblasts utilize the same regulated secretory pathway as neurons to secrete matrix vesicles. To identify genes that regulate bone matrix production, we performed gene expression analysis using mouse primary calvarial cells after 3, 7, and 14 days of culture in differentiation medium using qRT-PCR, and identified several SNARE genes (Fig. 1A), of which Stx4a and Stx5 were preferentially expressed. A previous study found that Stx4a is localized in the plasma membrane and Stx5 in the Golgi apparatus,23 whereas our results showed that the expression level of Snap23 was higher than that of Snap25. Expression of Vamp2, Vamp5, and Vamp8 was also found in calvarial cells. It has been reported that Vamp2 is localized in synaptic vesicles, whereas Vamp5 and Vamp8 are localized in the plasma membrane and endosomes, respectively.23 In our experiments, expressions of SNARE proteins were also confirmed in mouse osteoblastic MC3T3-E1 cells during osteoblast differentiation (days 2, 7, and 14) by qRT-PCR (Supplemental Fig. 1A). Consequently, Stx4a, Snap23, and Vamp2 were shown to be preferentially expressed in osteoblast cells, suggesting that these proteins function together in osteoblasts. The present findings elucidated the function of Stx4a in secretion of matrix vesicles from osteoblasts, whereas we hope to clarify the roles of Stx4a, Snap23, and Vamp2 in osteoblasts in a future study.
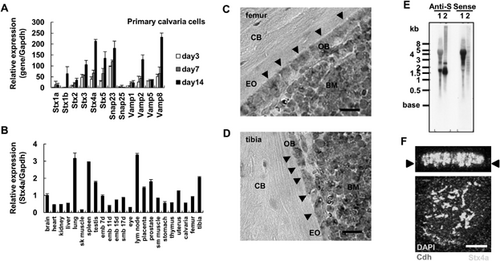
Next, we analyzed tissue distribution of the Stx4a gene by qRT-PCR analysis, and found that it was ubiquitously expressed in various tissues and had high levels of expression in lymph node, lung, and spleen specimens, whereas its expression was also observed in tibias, femurs, and calvaria (Fig. 1B). To examine the histological expression of Stx4a, we performed in situ hybridization. Sections from 3-week-old wild-type mice were hybridized with an Stx4a antisense cRNA probe. Osteoblast cells from both femurs and tibias were preferentially stained, and bone marrow cells also displayed Stx4a expression (Fig. 1C, D; Supplemental Fig. 2A–P). However, no in situ hybridization signals were found with a plant-derived sequence probe (negative control), confirming that the Stx4a antisense cRNA probe was adequate (Supplemental Fig. 2M–P). We also used an Stx4a sense cRNA probe to confirm the specificity of hybridization, which revealed hybridization signals (Supplemental Fig. 2I–L). In an online database, we found the antisense mRNA sequence for Stx4a (accession number: AK087072, 1729 bp). Using northern blotting analysis, the size of Stx4a mRNA with the antisense probe was shown to be about 1.5 kb, whereas the Stx4a sense probe was hybridized with about 4 kb of total RNA (Fig. 1E). Thus, Stx4a gene expression seems to be regulated by antisense RNA. In confocal microscopy findings, endogenous Stx4a expression was shown on the basolateral side of the plasma membrane of MC3T3-E1 cells (Fig. 1F; Supplemental Fig. 3). Together, these findings suggest that Stx4a is expressed by and localized within osteoblasts.
Stx4a involved in production of bone matrix
To further analyze its effects on bone matrix production, Stx4a was overexpressed or knocked down in osteoblastic cells. An adenovirus with Stx4a was transduced into mouse osteoblastic MC3T3-E1 cells, which were then cultured for 7 days and stained with liver/bone/kidney ALP, and cultured for an additional 21 days, then subjected to Alizarin red staining for Ca2+ deposits and von Kossa staining for Pi. We noted that staining for ALP, Alizarin red, and von Kossa was enhanced by overexpression of Stx4a, whereas each was reduced by Stx4a knockdown siRNA (Fig. 2A). Furthermore, ALP activity at 7 days was increased by Stx4a overexpression, whereas it was decreased by Stx4a siRNA knockdown (Fig. 2B). The level of Stx4a overexpression as well as knockdown was confirmed by qRT-PCR findings (Supplemental Fig. 1B–E).
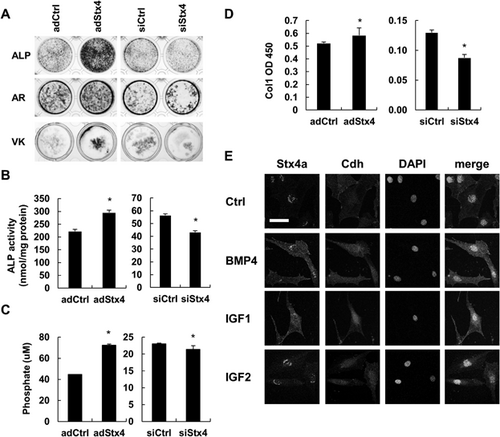
To quantify the amount of matrix vesicles produced by osteoblasts, purification was performed by ultracentrifugation, then analysis was performed using by Pi and Ca2+ assays, as well as ELISA. One day after Stx4a adenovirus transduction or siRNA knockdown, the cells were cultured in FBS-depleted medium for 1 day. Osteoblast matrix vesicles were found to contain Pi, type I collagen alpha 1 (COL1A1), Ca2+, ALP, ANXA5, bone sialoprotein (BSP), and osteocalcin (OCN) proteins. Also, bone matrix production was shown to be increased by Stx4a overexpression, whereas it was decreased by Stx4a knockdown (Fig. 2C, D; Supplemental Fig. 1F-H, 4A). Next, we analyzed the effects of several osteogenic factors, including BMP, IGF, ascorbic acid (AsA), and beta-glycerophosphate (bGP).24, 25 Basolateral localization of Stx4a was noted throughout the cells following exposure to BMP-4 and IGF-1, but not to IGF-2, AsA, or bGP (Fig. 2E; Supplemental Fig. 4B). Furthermore, the amount of purified matrix vesicles containing Pi was increased by addition of IGF-1, BMP-4, AsA, and bGP (Supplemental Fig. 4C, D), with the increase induced by BMP-4 and IGF-1 shown to be inhibited by exposure to Stx4a siRNA (Supplemental Fig. 4E). Together, these results suggest that Stx4a participates in bone matrix production, and BMP and IGF function as signals of bone matrix production.
Insufficient mineralization in Stx4a conditional knockout mice
Next, we constructed and analyzed Stx4a conditional knockout (cKO) (Stx4aflox/flox;Col1-cre) mice (Supplemental Fig. 5A–E), as well as their littermates as a control (Stx4a+/+;Col1-cre or Stx4a+/+; wild-type). Stx4a-deficient mice have been reported to die prior to embryonic day (E) 7.5.26 Indeed, the birth rate of the present osteoblast-specific Stx4a cKO mice was 7.3% (normal 25%) and birth number was 5.2 pups per mother (normal 10), indicating that osteoblast-specific deletion had an influence on embryonic or perinatal development. Although prenatal dead pups may have had a strong phenotype of defective mineralization, we first performed analysis of living pups. The gross appearance of the Stx4a cKO mice showed that they were smaller than their control littermates on E19.5 (Fig. 3A) and at the age of 8 weeks (Fig. 3E; Supplemental Fig. 6A), whereas body weight from 3 to 8 weeks was lower than that of the control mice (Supplemental Fig. 7A). Soft X-ray radiographic analysis of skeletal preparations of Stx4a cKO mice revealed an overall shorter body and shorter long bones as compared with the control mice (Fig. 3B–D, F–H; Supplemental Fig. 6B–D). qRT-PCR analysis was also performed to confirm Stx4a expression in tissues, which showed that the expression level of Stx4a in bone tissues (calvaria, femur, tibia) was much lower than the level of endogenous expression (Supplemental Fig. 7B). Together, these results indicate that Stx4a cKO mice develop body composition abnormalities.
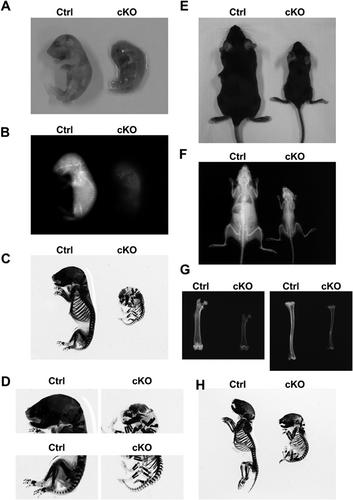
BMD in the femur metaphyseal and diaphyseal regions was analyzed by pQCT in both control and Stx4a cKO female mice at the age of 8 weeks. The Stx4a cKO mice displayed lower levels of BMD in the femur metaphyseal and diaphyseal regions (Fig. 4A, B), indicating impaired bone formation. In three-dimensional μCT analysis (Fig. 4C–F; Supplemental Fig. 7C, D) of the femur metaphyseal region, the values for bone volume to tissue volume ratio (BV/TV), trabecular thickness (Tb.Th), and trabecular number (Tb.N) were significantly lower, whereas that for trabecular separation (Tb.Sp) was higher in the Stx4a cKO mice (Fig. 4F). Other bone parameters, such as bone surface to bone volume ratio (BS/BV), marrow space star volume (V* m.space), node number to tissue volume ratio (N.Nd/TV), and total strut length to tissue volume ratio (TSL/TV), were also different in the Stx4a cKO mice (Supplemental Fig. 7C, D). Similar tendencies were also found in the 8-week-old male Stx4a cKO mice (Supplemental Figs. 6E, F, 8A–F), though females showed a more severe phenotype than males. The levels of Stx4a expression in females were slightly lower as compared to male wild-type mice (Supplemental Fig. 9A). Although these findings cannot be fully explained, we speculated that they were due to sex hormone differences or the differences between genders in regard to original bone mass.

We also performed histological analysis of tibia bone sections obtained from 8-week-old Stx4a cKO female mice. Metaphyseal and diaphyseal sections were stained with Villanueva Goldner's trichrome (Fig. 5A, C), which revealed that the amounts of calcified trabecular and cortical bone (blue stain), and numbers of noncalcified sections (red stain) were decreased in the Stx4a cKO mice as compared with the control mice (Fig. 5B, D). A similar tendencies were seen in 8-week-old male Stx4a cKO mice (Supplemental Fig. 10A–C).
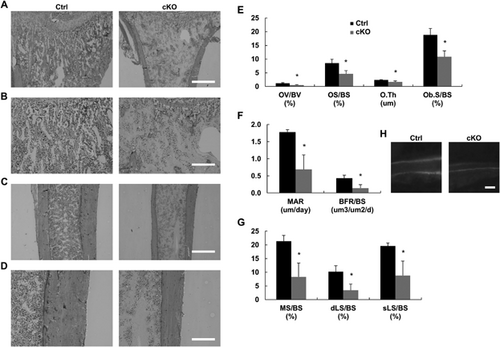
Bone histomorphometric analysis of 8-week-old Stx4a cKO female mice was performed using calcein/tetracycline double labeling. We found that the bone structure parameters BV/TV, Tb.Th, and Tb.N were significantly decreased in Stx4a cKO mice, which was similar to the findings of μCT analysis, whereas Tb.Sp was increased (Supplemental Fig. 7E). Also, bone formation parameters, such as osteoid volume (OV/BV), osteoid surface (OS/BS), osteoid thickness (O.Th), osteoblast surface (Ob.S/BS), mineral apposition rate (MAR), bone formation rate to bone surface (BFR/BS), mineralizing surface to bone surface (MS/BS), double-labeled surface to bone surface (dLS/BS), and single-labeled surface (sLS/BS), were decreased in the Stx4a cKO mice (Fig. 5E–H), whereas bone resorption parameters, including eroded surface (ES/BS), osteoclast number (N.Oc/BS), and osteoclast surface (Oc.S/BS), were decreased in the Stx4a cKO mice (Supplemental Fig. 7F). Similar results were also obtained in examinations of Stx4a cKO male mice (Supplemental Fig. 10E, F). However, the other parameters showed no statistically significant differences between the control and Stx4a cKO male mice (Supplemental Fig. 10D, G–J). In female Stx4a cKO mice, bone strength parameters, such as maximum breaking strength, fracture distance, stiffness, and breaking energy, were decreased (Supplemental Fig. 9B). In all Stx4a cKO mice, the number of matrix vesicles in the tibia section, determined by microscopy,27 was decreased (Supplemental Fig. 9C), whereas that number was also decreased in primary calvarial cells, as determined using nanoparticle analysis (Supplemental Fig. 9E). These findings indicate impaired bone formation in Stx4a cKO mice.
Reduced bone matrix production by calvarial cells
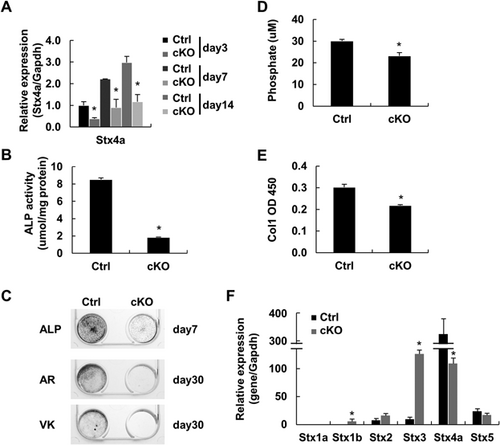
To confirm that the phenotype of the Stx4a cKO mice is caused by an intrinsic decrease in matrix vesicle secretion, we analyzed the production of bone matrix by primary cells from the calvaria of control and Stx4a cKO mice. qRT-PCR findings showed that Stx4a mRNA was decreased in the primary calvarial cells of Stx4a cKO mice (Fig. 6A). In addition, STX4A protein was overlapped with the ALP osteoblast marker in confocal microscopy findings, and showed complete ablation in primary calvarial cells from Stx4a cKO mice of both genders (Supplemental Fig. 9D). Primary calvarial cells were cultured for 7 days, then subjected to ALP activity analysis and ALP staining, which showed that ALP activity was clearly lower in Stx4a cKO calvarial cells (Fig. 6B), whereas ALP staining of cells from Stx4a cKO mice was slight as compared with the control cells (Fig. 6C). Other primary calvarial cells were cultured for 30 days and subjected to Alizarin red and von Kossa staining. We found that the extracellular matrix surrounding the nodules was poorly mineralized, whereas the control cell cultures displayed abundant mineralized nodules (Fig. 6C). Matrix vesicles were purified, then vesicles and proteins were examined using a Pi/Ca assay and ELISA, which indicated that secretion of matrix vesicles was diminished in the primary calvarial cells from Stx4a cKO mice (Fig. 6D, E). Next, we analyzed other syntaxin expressions in primary calvarial cells from Stx4a cKO mice using qRT-PCR. We found that Stx1b and Stx3 were increased in Stx4a cKO cells, though their expression levels were significantly lower as compared to those from Stx4a mice (Fig. 6F), indicating that compensation by other than syntaxin had occurred. Together, our results suggest that Stx4a is involved in the secretion of matrix vesicle by osteoblasts. Furthermore, ex vivo assay findings indicated reduced bone matrix production by osteoblasts in those mice.
Discussion
The present findings revealed the mechanism of bone matrix production on the bone surface by osteoblasts and identified the Stx4a gene as one of the regulators of that production. These results provide the first evidence of a mechanism of osteoblast matrix secretion regulated by Stx4a.
It is important to elucidate the entire picture of the osteoblast secretion system. In neuronal synaptic vesicle secretion, several different genes participate in the delivery system steps.8, 9 Notably, syntaxin, as well as Snap and Vamp family members are major players in membrane fusion between secretory vesicles and the plasma membrane.13-15 In addition to Stx4a expression, Snap23 and Vamp2 are preferentially expressed in osteoblasts, thus they may also be involved in osteoblast matrix secretion. Other genes, such as syntaxin binding protein (Stxbp) and exocyst complex component (Exoc), have been implicated in the process of neuronal synaptic secretory vesicle docking and recycling.28, 29 Additional studies of other genes are needed to clarify the overall matrix vesicle secretion system in osteoblasts. It would also be interesting to clarify the position of secretory vesicle formation in the cytoplasm.
Osteoblast matrix vesicles serve as nuclei during crystallization and initiate the initial crystallization of hydroxyapatite. We confirmed that osteoblast matrix vesicles contain ALP, ANXA5, and type I collagen. Collagen fibrils function as scaffolding for matrix vesicle deposits and our findings showed that type I collagen is purified in matrix vesicles. It is also likely that matrix vesicles and collagen fibers are packed into the same secretory vesicles, or that matrix vesicles associate with collagen before secretion to facilitate formation of complexes. Based on our findings, we speculate that secretory vesicles contain other extracellular matrixes as well as matrix vesicles, whereas the secretory system may affect the production and formation of other extracellular matrixes. Precise mineralization at a precise position and moment is important to maintain bone homeostasis, though some regulation systems may require Stx4a activity. For example, extracellular signals such as BMP-4 and IGF-1 induce osteoblast differentiation and mineralization, and change Stx4a cellular localization. Although the mechanism underlying Stx4a mRNA expression regulation remains unclear, identification of proteins or small molecules that induce or reduce Stx4a expression will be helpful for understanding the regulatory mechanism of Stx4a expression.
In conclusion, we discovered a novel regulator of osteoblast matrix vesicle secretion in osteoblasts. Our results demonstrate that osteoblasts utilize a regulated secretory pathway to produce bone matrix via specific BMP and IGF signaling. Stx4a plays an important role in bone matrix production by osteoblasts, because osteoblast-specific deletion of Stx4a was found to result in low BMD due to reduced secretion of matrix vesicles and proteins. These findings contribute to a better understanding of bone mineralization, as well as development of therapeutic options for bone diseases and other types of ectopic calcification.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (KAKENHI No. C24592796) and by special funds for the Challenge to Intractable Oral Diseases. We thank the members of the Challenge to Intractable Oral Diseases and Center for Frontier Oral Science for their assistance and encouragement, and M. Benton for comments regarding our manuscript. We thank Japan SLC, Kureha Special Laboratory, and Genostaff for their technical assistance.
Authors’ roles: SK developed the study ideas, designed the experiments, and wrote the manuscript. SW, AA, JK, and KH wrote the manuscript. SK performed most of the experiments described in the manuscript. HK and TA constructed the floxed mice. IM performed some of the animal experiments.




