Sodium/myo-inositol cotransporter 1 and myo-inositol are essential for osteogenesis and bone formation
Abstract
myo-Inositol (MI) plays an essential role in several important processes of cell physiology, is involved in the neural system, and provides an effective treatment for some psychiatric disorders. Its role in osteogenesis and bone formation nonetheless is unclear. Sodium/MI cotransporter 1 (SMIT1, the major cotransporter of MI) knockout (SMIT1−/−) mice with markedly reduced tissue MI levels were used to characterize the essential roles of MI and SMIT1 in osteogenesis. SMIT1−/− embryos had a dramatic delay in prenatal mineralization and died soon after birth owing to respiratory failure, but this could be rescued by maternal MI supplementation. The rescued SMIT1−/− mice had shorter limbs, decreased bone density, and abnormal bone architecture in adulthood. Deletion of SMIT1 resulted in retarded postnatal osteoblastic differentiation and bone formation in vivo and in vitro. Continuous MI supplementation partially restored the abnormal bone phenotypes in adult SMIT1−/− mice and strengthened bone structure in SMIT1+/+ mice. Although MI content was much lower in SMIT1−/− mesenchymal cells (MSCs), the I(1,4,5)P3 signaling pathway was excluded as the means by which SMIT1 and MI affected osteogenesis. PCR expression array revealed Fgf4, leptin, Sele, Selp, and Nos2 as novel target genes of SMIT1 and MI. SMIT1 was constitutively expressed in multipotential C3H10T1/2 and preosteoblastic MC3T3-E1 cells and could be upregulated during bone morphogenetic protein 2 (BMP-2)–induced osteogenesis. Collectively, this study demonstrated that deficiency in SMIT1 and MI has a detrimental impact on prenatal skeletal development and postnatal bone remodeling and confirmed their essential roles in osteogenesis, bone formation, and bone mineral density (BMD) determination. © 2011 American Society for Bone and Mineral Research.
Introduction
myo-Inositol (MI) is the most prominent naturally occurring form of inositol (hexahydroxycyclohexane, C6H12O6) and a crucial constituent and nutrient for living cells. MI and its derivatives, including MI polyphosphates (IPs), phosphatidylinositols (PIs), and phosphoinositides (PIPs), are involved in several important processes of cell physiology, including cell signaling, cell adhesion, and vesicular trafficking.1, 2 MI also acts as a “compatible osmolyte” in the control of intracellular osmolarity in various tissues.3 Most studies of MI have focused on the neural and renal systems, the sites of high concentrations of MI.4, 5 MI has been shown to be an effective treatment for several psychiatric disorders, such as panic disorder, obsessive-compulsive disorder, and depression,6-8 and to be useful in the management of the side effects of lithium therapy and in cancer prevention.9, 10 Previous studies also have proven that derivatives and the related pathways of MI are involved in bone metabolism. For example, I(1,4,5)P3-mediated Ca2+ signaling is a significant pathway that modulates mechanical sensing in osteoblasts.11 Nonetheless, there is no evidence of a direct relationship between MI and the bone system or disorders. Whether or not modulation of intracellular MI content can affect osteogenesis and be mediated through I(1,4,5)P3-mediated Ca2+ flux remains unclear.
Serum levels of MI in mammals vary between 30 and 70 µM,12 although tissue levels (ie, brain and kidney) can be much higher.4, 5 To achieve this MI accumulation in cells, cotransporters exchange MI and specific ions by the electrochemical gradient of coupled ion against the MI concentration gradient. To date, three different MI cotransporters have been identified in mammalian tissue; sodium/myo-inositol cotransporter 1 (SMIT1) is the major one.13 SMIT1, also named SLC5A3 (the third member of the sodium/glucose cotransporter family SLC5), is an integral membrane protein responsible for transporting one molecule of MI coupled with two Na+ molecules out of cells.14
SMIT1 knockout (SMIT1−/−) mice were generated to study the role of MI and SMIT1 in skeletal development and osteoblastogenesis. These mice show significantly decreased MI levels in many tissues, have nervous system defects, and die soon after birth owing to respiratory failure.15 The addition of MI prenatally to maternal drinking water prevented this neonatal mortality, and rescued SMIT1−/− mice could grow up and age just like SMIT1+/+ mice. In this article we characterize the skeletal abnormalities of SMIT1−/− mice and show that the defect is due to a shortage of osteoprogenitor cells and defective osteoblastic differentiation and maturation. This is associated with downregulation of several important osteogenesis-related transcription factors, including Runx2, osterix, and NFATc1. It is interesting to note that the action of MI and SMIT1 in osteogenesis is associated with altered expression of several novel mediators, not through osmotic changes or I(1,4,5)P3-mediated Ca2+ flux. By verifying the beneficial effect of MI supplementation on bone in both SMIT1−/− and SMIT1+/+ mice, we propose a potential anabolic action of MI on the skeleton and bone cells.
Materials and Methods
Mice
The generation of mice lacking SMIT1 in a C57BL/6/129Sv background has been reported previously.15 Mice were placed in an air-conditioned room with temperature at 20°C ± 1°C, relative humidity at 55% ± 5%, and 12-hour light/dark cycles. MI (Sigma-Aldrich, St Louis, MO, USA) supplement was administered by adding MI at 1% (wt/vol) to the drinking water. Embryonic mice were divided into nonrescued and rescued groups depending on whether there was prenatal maternal supplementation of 1% MI. In the rescued group, the lactating mothers continued to receive MI supplementation for 3 weeks to prevent death in the SMIT1−/− mice. After weaning, mice were divided into two groups. Group 1 continued to receive 1% MI supplementation in the drinking water (Cont); group 2 was given a normal diet with no supplementation (Discont). The animals were maintained in accordance with the guidelines for the care and use of laboratory animals of the University of Hong Kong, and the study was approved by the University of Hong Kong Committee on the Use of Live Animals in Teaching and Research.
Skeleton staining and histomorphometry
For skeletal evaluation, nonrescued and rescued P0 embryos (n = 5) were euthanized by asphyxia, skinned, and eviscerated. Their bone and cartilage, respectively, were stained with alizarin red and alcian blue (Sigma-Aldrich) as described previously.16 For histologic analysis, the right humeri from nonrescued E17.5 and E18.5 embryos and the right tibias from mice aged 2 weeks (n = 5 each) were dissected, stripped off soft tissues, and placed in 4% paraformaldehyde at 4°C for overnight fixing. Decalcification of 2-week-old bones was performed using 0.5 M EDTA (pH 7.4) at 4°C for 7 days. After dehydration with sequential changes of ascending concentrations of ethanol and xylene, both the undecalcified embryonic bones and decalcified 2-week-old bones were embedded in low-melting-point paraffin. Sections of the entire humerus and the proximal tibia were cut at 5 µm using a Leica RM2125 RT rotary microtome (Leica Microsystems GmbH, Wetzlar, Germany) for hematoxylin and eosin (H&E) staining, picrosirius red staining, and tartrate-resistant acid phosphatase (TRACP) staining as described previously with modification.17, 18
Computer-assisted image analysis
Images of the fields from histologic study were captured with a Leica digital camera, digitally recorded using a rectangular template, and processed using Image-Pro Plus Version 5.0 (Media Cybernetics, Inc., Bethesda, MD, USA). The positive area was determined as described previously19 and expressed as a percentage of the entire field. Green and red channels were employed to set thresholds, which were determined interactively and empirically on the basis of three different images. All recorded images from the same staining then were analyzed automatically with the same set threshold under the same conditions. For the growth plates of proximal tibias, all digital images were captured at ×100 magnification, which could include the entire growth plate from the epiphyseal side to the metaphyseal side. Primary spongiosa likewise were observed in the images taken at ×100 magnification from the boundary of the metaphyseal side of the growth plate toward the diaphysis. The cell number was counted per square millimeter of tissue area.
Micro–computed tomographic (µCT) analysis
The right humeri were harvested from 8-week-old SMIT1+/+ and SMIT1−/− mice (n = 5) for analysis of trabecular and cortical bone properties. Trabecular and cortical bone mineral density (BMD) and architecture were examined by a 3D µCT system (µCT 40, Scanco Medical, Brüttisellen, Switzerland) with a standard protocol.20 The scans were performed top down at a resolution of 12 µm in all three spatial dimensions. Images were stored in 3D arrays with an isotropic voxel size of 20 µm. Scanco software was used for the analysis. Tissue mineral density was derived from the linear attenuation coefficient of thresholded bone through precalibration of the apparatus for the acquisition voltage chosen. The bone volume fraction of trabecular metaphysis (Tb.BV/TV) was measured on a set of 160 chosen sections under the growth plate within the secondary spongiosa. Trabecular thicknesses (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) were calculated without assuming a constant model. Cortical thickness (Ct.Th) and tissue mineral density (Ct.BV/TV) were calculated by integration of the value on each transverse section of a set of 50 chosen in the midshaft area. Polar moment of inertia (pMOI) was evaluated based on the midshaft radius.
Cell culture
Primary mesenchymal cells (MSCs) were isolated from the bone marrow and cultured using a standard protocol.21 Cell proliferation was detected by cell counting. To induce their osteoblastic differentiation, cells were cultured in α-MEM containing 10% fetal bovine serum (FBS), 400 µM ascorbic acid, and 5 mM β-glycerophosphate (Sigma-Aldrich). The number of alkaline phosphatase (ALP)–positive colonies (ALP-CFU) was determined by ALP staining after 10 days of culture to determine the recruitment of MSC progenitors into the osteoblastic lineage. ALP activity assay and von Kossa staining were performed to examine osteoblastic differentiation and mineralized bone matrix.
Mouse embryonic multipotential fibroblast C3H10T1/2 cells (clone 8, American Type Culture Collection [ATCC], Manassas, VA, USA) were cultured in basal medium Eagle (Gibco BRL, Grand Island, NY, USA) with 2 mM L-glutamine containing 10% FBS. Mouse preosteoblastic MC3T3-E1 cells (subclone 4, ATCC) were maintained in α-MEM supplemented with 10% FBS. Bone morphogenetic protein 2 (PeproTech EC, Ltd., Rocky Hill, NJ, USA) was used to induce their osteogenic differentiation.
Real-time quantitative PCR
Total RNA was extracted using the TRIzol reagent (Invitrogen, Grand Island, NY, USA) in accordance with the manufacturer's instructions. First-strand cDNA was synthesized using 1.5 µg of total RNA, random hexamer, Oligo(dT)12–18 primer, and dNTP with M-MLV reverse transcriptase (Invitrogen) in a 20-µL reaction. For quantitative real-time PCR, the total 20-µL reaction of each sample contained 4.5 µL of template cDNA, primers, and 10 µL of 2× Taqman Universal PCR master mix (Invitrogen). Samples were amplified in an ABI Prism 7000 sequence detector (Applied Biosystems, Inc., Foster City, CA, USA) with an initial melt at 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Data were analyzed using the comparative threshold cycle (Ct) method with signals normalized with 18S signal for each sample (ΔCt). The sample of SMIT1+/+ MSCs on day 0 was set as control (ΔCt,control), and the difference between other samples and the control was ΔΔCt. Finally, the relative mRNA expression is presented as fold changes calculated by 2−ΔΔCt.
Determination of MI, osmolality, and I(1,4,5)P3
Intracellular and medium MI content was determined by a DX500 high-performance liquid chromatography (HPLC) system (Dionex Corporation, Sunnyvale, CA, USA) using an MA-1 analytical column with a guard column and an ED40 electrochemical detector. Peak integration was performed using the Dionex Peaknet Software (Dionex Corporation). The MI level was calculated using a known standard and normalized by preadded xylitol (Sigma-Aldrich) as an internal control. Osmolality of the culture medium was measured indirectly by determining vapor pressure with the hygrometric method using Vapro Vapor Pressure Osmometer 5520 (Wescor, Inc., Logan, UT, USA). Cellular I(1,4,5)P3 content was determined based on competitive ligand binding with the Inostitol-1,4,5-trisphosphate [3H] Radioreceptor Assay Kit (PerkinElmer Life Sciences Inc., Boston, MA, USA).
PCR array analysis
Signal pathways were screened using the Mouse Signal Transduction PathwayFinder PCR Array (SABiosciences, Frederick, MD, USA). Using qPCR with cDNA samples from SMIT1+/+ and SMIT1−/− MSCs, a total of 84 key genes representative of 18 different pathways were profiled. Amplification was performed in an ABI Prism 7000 sequence detector using standard settings of 40 cycles with an annealing temperature of 60°C. Results were expressed as fold change of gene expression relative to wild-type (WT) controls by the comparative Ct method (2−ΔΔCt).
Statistics
Data are represented as mean ± SD. Statistical analysis was performed using SPSS Version 17.0 (SPSS, Inc., Somers, NY, USA). One-way ANOVA was used for multiple comparisons between groups and Student's t test for single comparisons between two groups. Post hoc test of Student-Newman-Keuls was applied to detect differences between two groups after ANOVA testing. A p value of less than .05 was considered statistically significant.
Results
Delayed embryonic bone formation and shortened adult long bones in SMIT1−/− mice
SMIT1−/− embryos had a small body size, short limbs, drooped forelimbs and skull, and a curved spine compared with SMIT1+/+ mice. Double staining with alizarin red and alcian blue for bone and cartilage, respectively, confirmed that the abnormalities were due to a marked reduction in the area of mineralized tissues (Fig. 1A, red signal). A significant reduction in mineralization was observed in the phalanges, sternum, and skull (a widening of cranial sutures and open fontanelles) at P0 in SMIT1−/− skeletal preparations (Fig. 1B–E, arrows). Histologic analysis further confirmed that SMIT1−/− embryos had abnormal formation of primary spongiosa, leading to a reduced ossified region and much less trabecular bone at both E17.5 and E18.5, whereas the periarticular, columnar, and hypertrophic chondrocyte regions of the growth plate were normal (Fig. 2). Prenatal maternal supplementation of MI (1% in drinking water since E0.5) rescued the neonatal lethality and normalized skeletogenesis and physical appearance of SMIT1−/− embryos but had no effect on SMIT1+/+ mice (Fig. 1F–J).
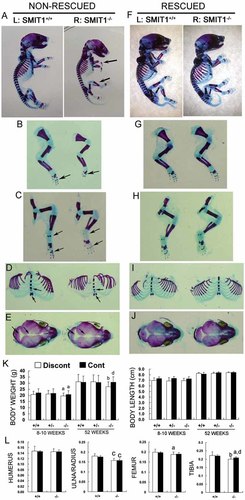
Delayed osteogenesis and retarded growth in SMIT1−/− mice. SMIT1+/+ and SMIT1−/− embryos at P0 without (nonrescued, A–E) or with MI supplementation (rescued, F–J) were killed, and their whole skeletons were prepared using alizarin red/alcian blue staining for bone/cartilage (n = 5). Arrows indicate the bent wrist and short limbs (A) and delayed bone formation (alizarin red staining) in the forelimbs (B), hind limbs (C), sternum (D), and skull (E) in nonrescued SMIT1−/− embryos. Maternal MI supplementation completely restored the retarded osteogenesis in SMIT1−/− embryos (F–J). Both young and old SMIT1−/− mice had decreased adult body weight (K, left) but not body length (K, right) (n = 12). Also, 8- to 10-week-old SMIT1−/− mice had short ulna/radius, femur, and tibia but not humerus relative to the body length by radiography (L). MI supplementation partially restored the retarded growth in SMIT1−/− mice. ap < .05; bp < .01; cp < .001 versus SMIT1+/+ mice with the same diet; dp < .05 versus Discont SMIT1−/− mice.
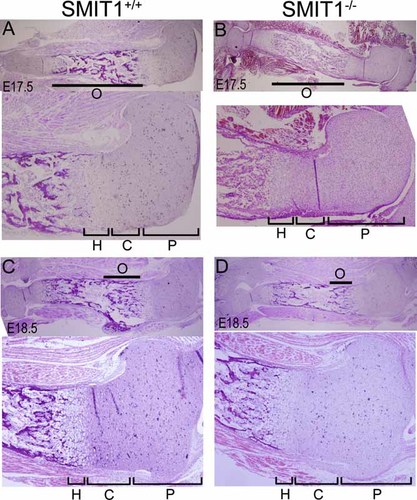
Histologic analysis showed delayed prenatal bone formation in SMIT1−/− embryos. Humerus sections were isolated from nonrescued SMIT1+/+ and SMIT1−/− mice at E17.5 (A, B) and E18.5 (C, D) for histologic H&E staining (n = 5). The lower image of each panel shows the proximal epiphysis of the humerus with higher power accordingly. SMIT1−/− embryos showed much less trabecular bone at both E17.5 and E18.5 but comparatively normal chondrocyte regions. O = ossification region and trabecular bone; P = periarticular region; C = columnar region; H = hypertrophic region.
Moreover, Discont SMIT1−/− mice had a lower body weight but normal body length at both young and old age (Fig. 1K). Continuous MI supplementation until 52 weeks but not 8 to 10 weeks could successfully restore the body weight of SMIT1−/− mice to normal (Fig. 1K). Radiography revealed normal vertebral height and spine curvature in adult Discont SMIT1−/− mice, although the long bones (ie, ulna/radius, femur, and tibia) remained shorter (Fig. 1L). Continuous MI supplementation partially restored the lengths of femurs and tibias in young SMIT1−/− mice but did not affect limb lengths of SMIT1+/+ mice.
SMIT1 deficiency resulted in reduced bone mass, decreased osteoblast number, and osteoporosis-like microarchitecture
To investigate the possible changes in bone histologic structure in SMIT1−/− mice, histomorphometric study of tibia sections from 2-week-old mice was performed followed by quantitative image analysis. H&E staining revealed normal width of the growth plate and proliferating or hypertrophic zones (Fig. 3A, B), which was consistent with the previous findings in E17.5 and E18.5 mice (Fig. 2), indicating unaltered chondrogenesis. The metaphyseal bone volume (Fig. 3A) and osteoblast cell number (360 ± 38 versus 447 ± 83 cells/mm2 T.Ar, p < .01; Fig. 3C) were decreased in the primary spongiosa of SMIT1−/− mice. Together with reduced collagen in the trabeculae bone (30.4% ± 4.4% versus 42.9% ± 5.0% T.Ar, p < .01; Fig. 3D), these results suggested decreased trabecular bone volume in these animals. Nevertheless, TRACP staining demonstrated a normal number and size of osteoclasts in SMIT1−/− mice (67 ± 8 versus 70 ± 9 cells per mm2 T.Ar, p > .05; Fig. 3E), suggesting a lack of osteoclastic involvement in the SMIT1−/− mice.
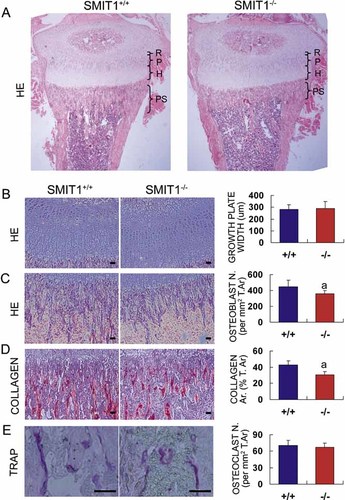
Deletion of SMIT1 delays postnatal bone formation with decreased bone mass and osteoblasts. Tibia sections were isolated from 2-week-old SMIT1+/+ and SMIT1−/− mice (n = 5) for histomorphometric analysis with different staining: (A) H&E staining of the whole proximal tibia (R = resting zone; P = proliferating zone; H = hypertrophic zone; PS = primary spongiosa and trabecular bone); (B) H&E staining of the growth plate; (C) H&E staining of the primary spongiosa; (D) picrosirius red staining for total collagen (red); and (E) TRACP staining for osteoclasts (purple). (B–E, left panel) Representative images (bars = 100 µm). (Right panel) Respective quantitative data from sections of three different SMIT1+/+ and SMIT1−/− mice. ap < .01 versus SMIT1+/+ mice.
The proximal humerus and its midshaft were scanned with a µCT system to evaluate the trabecular and cortical bone structure, respectively (Fig. 4A, B). Discont SMIT1−/− mice showed reduced trabecular bone volume (Tb. BV/TV, 6.6% ± 1.5% versus 9.6% ± 2.2%, p < .05), Tb.N (2.9 ± 0.5 versus 3.6 ± 0.5/mm, p < .05), and Tb.Th (0.036 ± 0.003 versus 0.040 ± 0.003 mm, p < .05) with correspondingly increased Tb.Sp (0.36 ± 0.07 versus 0.28 ± 0.04 mm, p < .05; Fig. 4C–F). In the diaphysis, there was decreased cortical bone mineral density (Ct.BV/TV, 40.2% ± 1.1% versus 44.9% ± 1.8%, p < .01; Fig. 4G) but not Ct.Th (0.126 ± 0.007 versus 0.131 ± 0.005 mm, p > .05; Fig. 4H). Most interestingly, the radius in the humeral midshaft was enlarged in SMIT1−/− mice compared with SMIT1+/+ mice (0.89 ± 0.06 versus 0.71 ± 0.04 mm, p < .001; Fig. 4J). This resulted in a markedly enhanced pMOI (0.177 ± 0.034 versus 0.121 ± 0.016 mm4, 46% increase, p < .01; Fig. 4I), a measure of bone stiffness that depends on the radius of the long bone shaft.22 The findings in the cortical bone suggested that young SMIT1−/− mice had disturbed bone remodeling of the humerus during periods of growth that resulted in altered bone geometry.
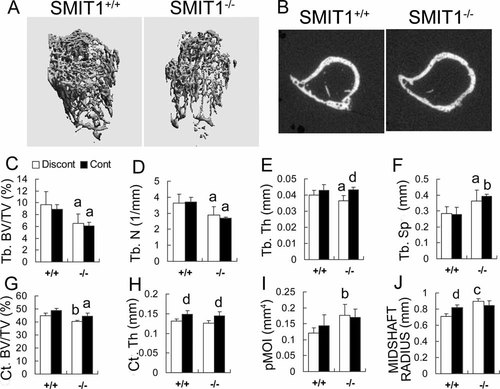
Deletion of SMIT1 resulted in osteoporosis-like bone microstructure. Humeri were isolated from 8-week-old SMIT1+/+ and SMIT1−/− mice (n = 5) for µCT scanning: (A) 3D images of proximal humerus; (B) transverse 2D images of the midshaft. SMIT1−/− mice had reduced Tb.BV/TV (C), Tb.N (D), and Tb.Th (E) with increased Tb.Sp (F). There also was reduced Ct.BV/TV (G) but not Ct.Th (H) in SMIT1−/− mice. pMOI was increased significantly in SMIT1−/− mice (I) owing to an enlarged radius at the midshaft (J). MI supplementation could benefit bone microstructure in both SMIT1+/+ and SMIT1−/− mice (E, H, J). ap < .05; bp < .01; cp < .001 versus SMIT1+/+ mice with the same diet; dp < .05 versus Discont mice of the same genotype.
Continuous MI supplementation for 8 weeks normalized trabecular thickness and increased cortical thickness in SMIT1−/− mice (Fig. 4E, H). It also enlarged humerus size in SMIT1+/+ mice by increasing thickness and radius (Fig. 4H, J). These findings indicate that MI supplementation partially restored the abnormal bone microstructure of SMIT1−/− mice and had a strengthening effect on the long bones of SMIT1+/+ mice.
Deletion of SMIT1 impaired osteoblastic recruitment, differentiation, and maturation of primary MSCs
To further delineate the bone abnormalities of SMIT1−/− mice, we examined primary MSCs from SMIT1+/+ and SMIT1−/− mice. There was no difference in the number of MSCs collected per SMIT1+/+ or SMIT1−/− mouse. Nonetheless, following culture for 3 and 5 days, the total number of SMIT1−/− MSCs was reduced by 61% (1.8 ± 0.3 ×104 versus 3.0 ± 0.3 ×104 cells, p < .001) and 64% (3.2 ± 0.4 ×104 versus 5.0 ± 0.3 ×104 cells, p < .001), respectively, compared with SMIT1+/+ MSCs, indicating a slower proliferation caused by SMIT1 deficiency (Fig. 5A). Recruitment of MSCs into the osteoblastic lineage also was decreased in SMIT1−/− mice, as evidenced by fewer MSC-derived ALP-CFUs (34.8 ± 4.1 versus 44.3 ± 4.9 clones per well, p < .001; Fig. 5B). Secretion of ALP, a differentiation marker for osteoblast maturation, was delayed and much reduced in SMIT1−/− MSCs during osteogenesis, with the maximum level being 59% that of SMIT1+/+ MSCs (2.69 ± 0.26 versus 4.60 ± 0.11 U/mg of protein, p < .001; Fig. 5C). Von Kossa staining of bone nodules revealed no staining in SMIT1−/− MSCs even on day 10 compared with positive staining achieved on day 7 in SMIT1+/+ MSCs (Fig. 5D). We also examined the expression of key regulating transcription factors Runx2, osterix, and NFATc1, as well as osteoblast differentiation markers type IAI collagen (ColIAI) and the OCN gene (Fig. 5E). In SMIT1−/− MSCs, Runx2 expression was suppressed throughout osteogenesis, with the peak level reduced to 41% that of SMIT1+/+ MSCs (1.08- ± 0.07- versus 2.61- ± 0.13-fold, p < 0.001); osterix expression was reduced at the commitment and differentiation phase (from days 0 to 7), whereas NFATc1 expression was reduced by 30% to 40% (1.33- ± 0.11- versus 2.00- ± 0.02-fold on day 5, 1.33- ± 0.08- versus 1.71- ± 0.27-fold on day 10, and 1.30- ± 0.06- versus 1.73- ± 0.42-fold on day 15, all p < .05) following commencement of osteogenesis for 15 days (Fig. 5F).
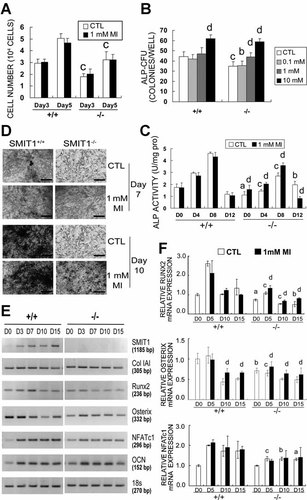
SMIT1 and MI regulate osteoblastic differentiation of primary MSCs. SMIT1−/− MSCs in vitro displayed decreased proliferation (A, cell count), reduced recruitment into osteoblasts (B, ALP-positive colony-forming-unit assay), decelerated differentiation, as evidenced by reduced ALP enzyme activity (C), retarded mineralization (D, von Kossa staining, bars = 100 µm), and downregulated gene expression of Runx2, osterix, and NFATc1 (E, RT-PCR; F, qPCR). The dysfunction of SMIT1−/− MSCs was partially restored by additional MI in the culture medium. Results are from three independent repeats in triplicate. ap < .05; bp < .01; cp < .001 versus SMIT1+/+ MSCs with the same treatment; dp < .05 versus control (CTL) of the same genotype.
Similar to its in vivo effect, MI supplementation increased the ALP-CFU number and enhanced ALP activity by 33% to 42% from day 0 to day 8 in SMIT1−/− MSCs in a dose-dependent manner (Fig. 5B, C). MI treatment also markedly accelerated mineralization (Fig. 5D) and partially reversed the reduced Runx2 and osterix but not NFATc1 mRNA levels in SMIT1−/− MSCs (Fig. 5F). Interestingly, MI supplementation also enhanced osterix expression in SMIT1+/+ MSCs at days 10 and 15 by 53% (0.66- ± 0.09- versus 0.43- ± 0.07-fold versus day 0, p < .05) and 30% (0.66- ± 0.03- versus 0.51- ± 0.03-fold versus day 0, p < .05), respectively.
In addition, SMIT1 was found to be constitutively expressed in a murine C3H10T1/2 MSC and murine MC3T3-E1 preosteoblast cell line (Supplemental Fig. S1A, B). In addition, BMP-2 further enhanced SMIT1 gene expression, indicating that SMIT1 expression is involved in osteogenic differentiation of these cell lines.
Deletion of SMIT1 led to reduced intracellular MI content but did not affect I(1,4,5)P3 level in primary MSCs
Since SMIT1 is the major cellular transporter of MI, we postulated that SMIT1−/− mice would have low cellular MI content that contributes to the abnormal osteogenesis of SMIT1−/− MSCs. As shown in Fig. 6, SMIT1−/− MSCs had significantly lower intracellular MI content (66.5 ± 12.0 versus 134.5 ± 40.9 nmol/g cells on day 0, 51% reduction, p < .05; Fig. 6A) than SMIT1+/+ cells. No difference was found in the MI level of the culture medium (Fig. 6B), indicating that the reduced intracellular MI content in SMIT1−/− MSCs was due to reduced MI transport caused by depletion of the SMIT1 transporter, not a difference in MI content of the culture medium.
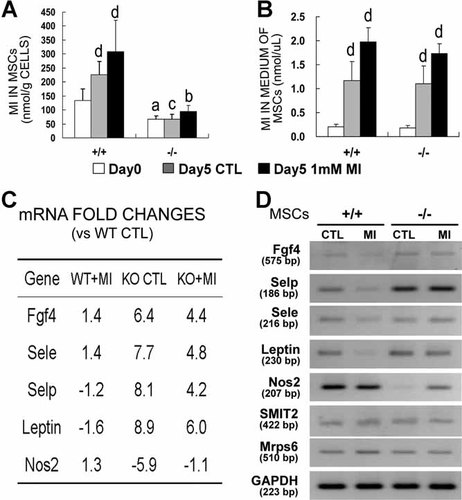
Reduced intracellular MI content and changed gene expression in SMIT1−/− MSCs. Intracellular (A) but not medium (B) MI content was markedly decreased in SMIT1−/− MSCs. Results are from three independent repeats in triplicate. ap < .05; bp < .01; cp < .001 versus SMIT1+/+ with respective time and treatment; dp < .01 versus day 0 of the same genotype. Expression array study was performed using qPCR in SMIT1+/+ (wild type) and SMIT1−/− (knockout) MSCs without or with MI treatment. Genes whose expression changed by more than fivefold compared with WT controls without MI supplementation are shown in panel C. The results represented the mean of three independent experiments. The expression-array results were confirmed by reverse-transcription PCR mRNA study (D).
We also examined the role of intracellular I(1,4,5)P3, an important metabolite of MI that acts as a secondary messenger molecule.23, 24 Intracellular I(1,4,5)P3 increased dramatically and to the same extent from days 5 to 10 in SMIT1+/+ and SMIT1−/− MSC culture (Supplemental Fig. S1C), indicating that I(1,4,5)P3 was similarly involved in osteogenic differentiation of MSCs regardless of the SMIT1 deletion. Additional MI supplementation had no effect on the intracellular I(1,4,5)P3 content of SMIT1+/+ and SMIT1−/− MSCs. Thus I(1,4,5)P3 is unlikely the signaling pathway through which SMIT1 and MI affect osteogenesis and bone formation. MI also acts as an important osmolyte to control the osmolality in many tissues, although there was no difference in the medium osmolality of SMIT1−/− or SMIT1+/+ MSC cultures, even after treatment with additional MI (Supplemental Fig. S1D). Thus a change in medium osmolality does not contribute to the cellular abnormalities of SMIT1−/− mice and does not explain the rescue effect of MI supplementation.
Identification of transcriptional targets of SMIT1 deficiency on bone cells
To identify specific target genes and pathways related to SMIT1 deficiency and MI manipulation, we used a QRT-PCR array approach to profile the expression of 84 genes representing 18 different signal-transduction pathways. The genes whose transcription was significantly altered by more than fivefold in SMIT1−/− mice were identified (Fig. 6C, D). Compared with SMIT1+/+ MSCs, four genes including fibroblast growth factor 4 (Fgf4), leptin, E-selectin (Sele), and P-selectin (Selp), were upregulated in SMIT1−/− MSCs, whereas inducible nitric oxide synthase (Nos2) was downregulated. Additional MI treatment partially reversed the altered expression of these genes in SMIT1−/− MSCs.
SMIT2 mRNA expression in MSC cultures also was examined and revealed no change in SMIT1−/− cells with or without MI treatment compared with SMIT1+/+ cells (Fig. 6D). This suggests no compensating effect of SMIT2 in SMIT1-deleted MSCs. The SMIT1 gene is embedded within the mitochondrial ribosomal protein subunit 6 (Mrps6) gene in the same chromosome.25 These two genes share exon 1, and the entire coding region of the SMIT1 gene is contained in intron 1 of Mrps6. To exclude the possibility that the defects in SMIT1−/− mice are mediated by changes in the Mrps6 gene, we examined Mrps6 expression in SMIT1−/− primary cell cultures with primers designed to amplify the whole coding region and confirmed unaltered Mrps6 expression in SMIT1−/− MSCs (Fig. 6D).
Discussion
This study reported the novel finding of the important role of SMIT1 and MI in bone development and remodeling. SMIT1 regulates embryonic osteogenesis and postnatal bone formation by affecting osteoblast recruitment, differentiation, and mineralization. More important, we have demonstrated the beneficial effect of MI on normal bone as well as its rescue effect on bone in the presence of SMIT1 deficiency.
Two lines of SMIT1−/− mice are described in the literature. Besides ours, the other line of SMIT1−/− mice, generated and characterized by Berry and colleagues,26 also had markedly decreased MI tissue content, unaltered SMIT2 and Mrps6 expression, and expired shortly after birth owing to respiratory failure.26, 27 Unlike ours, these SMIT1−/− mice had normal external features and unaltered soft tissue organs. The discrepancy between the two SMIT1−/− mice lines may be explained by the different construct of targeted SMIT1 gene deletion. Furthermore, Berry and colleagues examined the external features and organs of their mice only by gross pathologic and light microscopic examinations with H&E staining. They failed to evaluate the skeletal system. It has to be noted that gross pathologic examination with H&E staining is unlikely to detect altered calcified bone mass, bone structure, or bone cell differentiation.
In addition to limb deformity, SMIT1−/− mice had reduced BMD and abnormal bone microarchitecture, which included reduced bone volume and thin and sparse bony trabeculae. Nonetheless, SMIT1−/− mice also had an enlarged cortical diameter but not cortical thickness of the long bones, which resulted in an increased pMOI, an index of bone strength and resistance to fracture.22 Bone quality is now acknowledged to be as important as bone quantity in determining fracture risk.28 Beyond BMD, the resistance of cortical bone to microdamage (ie, thickness, cross-sectional area, and moment of inertia), together with trabecular connectivity and orientation, helps to determine the bone's fragility, mechanical competence, and resistance to failure.29 The increased pMOI together with reduced BV/TV in SMIT1−/− mice indicates that SMIT1 deficiency leads to abnormal bone geometry, allowing these mice to withstand comparative stress in the presence of generally lesser bone mass. The altered bone geometry may be a direct effect of SMIT1 deficiency or an adaption to reduced bone strength in the long bones secondary to low BMD.
The abnormal bone phenotype seen in SMIT1-deficient mice is the result of impaired bone formation but not bone resorption. The major defect of osteogenesis in SMIT1−/− mice lies with impaired osteoblastogenesis as a result of decreased recruitment of MSCs into osteoblast lineage and impaired osteoblastic differentiation and maturation. The key regulating transcription factors for osteoblastogenesis, that is, Runx2, osterix, and NFATc1, were downregulated in SMIT1−/− MSCs. Runx2, a crucial transcriptional factor necessary for osteoblast recruitment of MSCs, is involved in osteoblast maturation, embryonic bone development, and both endochondral and intramembranous ossification.30, 31 NFATc1, traditionally evidenced as a crucial factor for osteoclastogenesis, has been recognized recently to regulate osteoblastogenesis and accelerate bone formation by forming a complex with osterix that modulates osterix's transcriptional activity when binding to DNA.32 Osterix, the downstream transcriptional factor of Runx2, converts preosteoblasts to functional osteoblasts, is critical to the late stage of osteoblast differentiation, and modulates expression of most mature osteoblastic genes (eg, OCN).33 Down-regulation of these three key transcription factors contributes to altered prenatal osteogenesis and postnatal bone formation in SMIT1−/− mice.
Another important part of our study was to evaluate the effect of MI supplementation on rescuing the skeletal defects and osteogenic impairment in SMIT1−/− mice. MI supplementation completely restored the skeletal malformation in SMIT1−/− embryos and partially reversed the malformed skeletal structure and dysfunctional MSCs in adult SMIT1−/− mice. Continuous dietary MI supplementation also enlarged the bone size in young SMIT1+/+ mice by increasing cortical thickness and diameter of the long bones in vivo (Fig. 4H, J). At high concentration, MI supplementation enhanced osterix expression and markedly encouraged the recruitment of MSCs into osteoblastic linage in both SMIT1+/+ and SMIT1−/− MSCs. Collectively, these data confirm that MI deficiency contributes to skeletal defects and impaired osteogenesis in SMIT1−/− mice and that supplementation also may strengthen bone structure in normal mice.
SMIT1−/− MSCs had a markedly reduced amount of intracellular MI and significantly less MI enhancement by MI supplementation despite a normal MI content of the culture medium. This confirms that SMIT1 is the major cotransporter of MI in bone tissue, similar to other soft tissues.15 SMIT2 expression in MSCs nonetheless was not affected by SMIT1 depletion or MI supplementation, indicating the involvement of other compensating mechanisms. Normalization of MI in SMIT1−/− MSCs could not restore the ability to proliferate, suggesting that apart from regulating intracellular MI concentration, the SMIT1 gene may exert a direct effect on osteoblastogenesis and bone formation through other mechanisms.
The I(1,4,5)P3 pathway, recognized as a Ca2+ mobilization inducer,34 was excluded as the reason for the defects observed in SMIT1−/− MSCs because intracellular I(1,4,5)P3 content was similar in SMIT1−/− and SMIT1+/+ cells. The effect of MI on MSCs is also not mediated by osmotic changes in the medium. Instead, several novel transcriptional targets of the SMIT1 gene and MI in MSCs were identified. Genes upregulated in SMIT1−/− MSCs include Fgf4, leptin, Sele, and Selp. Fgf4 is reported to be involved in bone morphogenesis and limb development.35 It stimulates transcriptional activities and expression of Runx236 and regulates MSC proliferation,37 effects similar to those observed in SMIT1−/− mice. Leptin, a hormone secreted by adipose tissue, also has a dual effect on bone to modulate osteogenesis to directly stimulate osteoblasts through the mediation of Runx2 or to indirectly inhibit bone formation through action on the hypothalamus.38 Sele and Selp are critical molecules involved in the inflammatory process, whereas many inflammatory key factors [eg, receptor activator of nuclear factor κB ligand (RANKL) and interferon-γ (IFN-γ)] play important roles in osteogenesis and osteoporosis known as inflammatory bone loss and osteoimmunology.39 Similarly, downregulated Nos2 was found in SMIT1−/− MSCs and generates nitric oxide to mediate bone remodeling and adjust bone turnover.40, 41 Although we were able to show that the abnormal bone phenotype and altered expression of Runx2, osterix, and NFATc1 in SMIT1-deficient mice are a direct effect of SMIT1 deficiency, we were not able to establish a direct causal relationship between SMIT1 deficiency and the transcription of Fgf4, leptin, Sele, Selp, and Nos2 genes, and further studies are needed.
In conclusion, our study described the novel observation of the involvement of MI and SMIT1 in the skeletal system and bone cells. These findings also present a novel potential for MI or its analogue(s) as anabolic therapy for osteoporosis.
Disclosures
All the authors state that they have no conflicts of interest.
Acknowledgements
ZD was supported by the KC Wong Education Foundation. This project was supported by the Osteoporosis and Endocrine Research, the University of Hong Kong.




