Skeletal Structure in Postmenopausal Women With Osteopenia and Fractures Is Characterized by Abnormal Trabecular Plates and Cortical Thinning
ABSTRACT
The majority of fragility fractures occur in women with osteopenia rather than osteoporosis as determined by dual-energy X-ray absorptiometry (DXA). However, it is difficult to identify which women with osteopenia are at greatest risk. We performed this study to determine whether osteopenic women with and without fractures had differences in trabecular morphology and biomechanical properties of bone. We hypothesized that women with fractures would have fewer trabecular plates, less trabecular connectivity, and lower stiffness. We enrolled 117 postmenopausal women with osteopenia by DXA (mean age 66 years; 58 with fragility fractures and 59 nonfractured controls). All had areal bone mineral density (aBMD) measured by DXA. Trabecular and cortical volumetric bone mineral density (vBMD), trabecular microarchitecture, and cortical porosity were measured by high-resolution peripheral computed tomography (HR-pQCT) of the distal radius and tibia. HR-pQCT scans were subjected to finite element analysis to estimate whole bone stiffness and individual trabecula segmentation (ITS) to evaluate trabecular type (as plate or rod), orientation, and connectivity. Groups had similar age, race, body mass index (BMI), and mean T-scores. Fracture subjects had lower cortical and trabecular vBMD, thinner cortices, and thinner, more widely separated trabeculae. By ITS, fracture subjects had fewer trabecular plates, less axially aligned trabeculae, and less trabecular connectivity. Whole bone stiffness was lower in women with fractures. Cortical porosity did not differ. Differences in cortical bone were found at both sites, whereas trabecular differences were more pronounced at the radius. In summary, postmenopausal women with osteopenia and fractures had lower cortical and trabecular vBMD; thinner, more widely separated and rodlike trabecular structure; less trabecular connectivity; and lower whole bone stiffness compared with controls, despite similar aBMD by DXA. Our results suggest that in addition to trabecular and cortical bone loss, changes in plate and rod structure may be important mechanisms of fracture in postmenopausal women with osteopenia. © 2014 American Society for Bone and Mineral Research.
Introduction
The majority of fragility fractures occur in women with osteopenia.1, 2 Within this population, however, it is often difficult to determine which patients are at greatest risk for fracture and require treatment. Dual-energy X-ray absorptiometry (DXA) measurements of areal bone mineral density (aBMD) alone are not able to distinguish between patients at low and high risk. Skeletal microarchitecture is an important determinant of bone strength and fracture susceptibility, independent of aBMD.3-5 Abnormal microarchitecture may contribute to skeletal fragility and fracture susceptibility in osteopenic women.
High-resolution peripheral quantitative computed tomography (HR-pQCT; Xtreme CT, Scanco Medical AG, Brüttisellen, Switzerland) is a noninvasive, three-dimensional imaging technique, which measures volumetric BMD of the distal radius and tibia, can distinguish between cortical and trabecular bone, and visualize fine details of trabecular microarchitecture. Several HR-pQCT studies have demonstrated differences in microarchitecture and stiffness in subjects with a history of osteoporotic fracture compared with controls.6-13 However, only one previous study used HR-pQCT to compare microarchitecture in osteopenic women with and without fractures.6 In that study, women with fractures had worse microarchitecture at the radius but not at the tibia.
Recently, techniques have been developed that provide greater insight into skeletal structure and strength. Data sets from individual HR-pQCT scans can be computationally modeled by microstructural finite element analysis (μFEA) to assess bone stiffness, a surrogate measure of strength. We have developed a novel technique based upon HR-pQCT data, individual trabecula segmentation (ITS)–based morphological analysis. This three-dimensional model independent technique directly measures individual trabecula, characterizing trabecular type (plate versus rod), orientation, and connectivity. Because trabecular plates and rods of different orientations have distinct roles in mechanical properties and failure mechanisms of trabecular bone,14-17 ITS increases mechanistic understanding of bone stiffness and fracture susceptibility. We have shown that disparities in plate and rod structure explain differences in bone strength between Chinese and white women18 and premenopausal women with idiopathic osteoporosis.19
We performed this study to determine whether osteopenic women with and without fracture have differences in trabecular morphology and biomechanical properties of bone. We sought to expand the current knowledge by using ITS to evaluate the plate and rod structure of bone in women with osteopenia. We hypothesized that these novel techniques would reveal that skeletal abnormalities in osteopenic women with fractures are far more pronounced than indicated by DXA. Specifically, we hypothesized that although aBMD measured by DXA might not differ between women with fractures and controls, HR-pQCT would demonstrate that women with fragility fractures have worse microarchitecture and lower stiffness compared with controls. We further hypothesized that women with osteopenia and fractures would have fewer trabecular plates and less connectivity between plates and rods.
Materials and Methods
Patients
Postmenopausal women, older than 60 years or more than 10 years postmenopause, were recruited at Columbia University Medical Center (CUMC; New York, NY, USA) or Helen Hayes Hospital (HHH; West Haverstraw, NY, USA) by advertisement or self- or physician referral. Women were included who had osteopenia by DXA (T-score between −1.0 and −2.5, at lumbar spine [LS], total hip [TH], or femoral neck [FN]). Subjects were eligible for inclusion as fracture cases if they had a documented history of a low-trauma symptomatic vertebral or nonvertebral fracture that occurred after menopause or an asymptomatic vertebral fracture documented on spine radiograph. Fractures of the skull or digits were excluded. Low trauma was defined as equivalent to a fall from a standing height or less. Nonvertebral fractures were confirmed by review of radiographs or radiograph reports when possible. Vertebral fractures were identified by spine X-rays according to the semiquantitative method of Genant and colleagues.20 Control subjects had no history of low-trauma fractures at any site and no vertebral deformity on lateral radiographs, as dictated by prespecified exclusion criteria. Potential cases and controls were excluded if they had endocrinopathies (eg, untreated hyperthyroidism, Cushing's syndrome, prolactinoma), celiac or other gastrointestinal diseases, abnormal mineral metabolism (eg, osteomalacia, primary hyperparathyroidism), malignancy (except for skin cancer), and drug exposures that could affect bone metabolism (eg, glucocorticoids, anticonvulsants, anticoagulants, methotrexate, aromatase inhibitors, thiazolidinediones). Women using hormone replacement therapy or raloxifene were permitted to participate. Women who had ever used teriparatide or who had taken bisphosphonates for more than 1 year were excluded. At the study visit, past medical history, reproductive history, and medication use were assessed. A physical exam was performed including height by Harpenden stadiometer and weight, and body mass index (BMI) was calculated. All subjects provided written informed consent, and the Institutional Review Board of Columbia University Medical Center approved this study.
Areal bone mineral density
Areal BMD was measured by DXA (QDR-4500, Hologic Inc., Walton, MA, USA, at CUMC; Lunar Prodigy, GE, Madison, WI, USA, at HHH) at the LS (L1 to L4), TH, FN, 1/3 radius (1/3R), and ultradistal radius (UDR). Lumbar vertebrae with significant deformity, osteosclerosis, osteophytes, or degenerative disease were excluded from the analysis. T-scores compared subjects and controls with young-normal populations of the same race and sex, as provided by the manufacturer. Spine radiographs were performed to evaluate prevalent vertebral fractures.
HR-pQCT and image-based µFEA of the distal radius and tibia
HR-pQCT (XtremeCT) was performed by immobilizing the nondominant forearm and ipsilateral tibia (or nonfractured arm or leg in subjects with prior wrist or ankle fracture) in a carbon fiber shell and scanning as we have described in prior publications.9, 21-24 The European Forearm Phantom was scanned daily for quality control. All scans were acquired by the same technician.
HR-pQCT data were used to calculate whole bone stiffness, a measure of bone's resistance to force. The analysis methods have been described, validated,25-27 and applied in several recent clinical studies.6-8, 10-12, 28, 29
Cortical porosity
To evaluate the cortical bone structure, a validated auto-segmentation method30 was applied to separate the cortical and trabecular compartments and measure cortical porosity (Ct.Po, %). Ct.Po was calculated as the percentage of void space in the cortex. This method has been validated for accuracy31 and reproducibility32 and is distributed by the manufacturer (Scanco Medical).
ITS-based morphological analyses of HR-pQCT images
The trabecular bone compartment of each HR-pQCT image was manually extracted from cortex.27 All trabecular bone images were then subjected to ITS-based morphological analyses. A complete volumetric decomposition technique was applied to segment the trabecular network into individual plates and rods.15 Briefly, digital topological analysis (DTA)–based skeletonization33 was applied first to transform a trabecular bone image into a representation composed of surfaces and curves skeleton while preserving the topology (ie, connectivity, tunnels, and cavities)34, 35 as well as the rod and plate morphology of the trabecular microarchitecture. Then, digital topological classification was applied in which each skeletal voxel was uniquely classified as either a surface or a curve type.36 Using an iterative reconstruction method, each voxel of the original image was classified as belonging to either an individual plate or rod. Based on the 3D evaluations of each individual trabecular plate and rod, bone volume and plate and rod number were evaluated by plate and rod bone volume fraction (pBV/TV and rBV/TV), as well as plate and rod number densities (pTb.N and rTb.N, 1/mm). Plate-to-rod ratio (P-R ratio), a parameter of plate versus rod characteristics of trabecular bone, was defined as plate bone volume divided by rod bone volume. The average size of plates and rods was quantified by plate and rod thickness (pTb.Th and rTb.Th, mm), plate surface area (pTb.S, mm2), and rod length (rTb.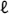 , mm). Intactness of trabecular network was characterized by plate-plate, plate-rod, and rod-rod junction density (P-P, P-R, and R-R Junc.D, 1/mm3), calculated as the total junctions between trabecular plates and rods normalized by the bulk volume. Orientation of trabecular bone network was characterized by axial bone volume fraction (aBV/TV), defined as axially aligned bone volume divided by the bulk volume. Detailed methods describing the complete volumetric decomposition technique and ITS-based measurements can be found in our prior publications.15, 16
, mm). Intactness of trabecular network was characterized by plate-plate, plate-rod, and rod-rod junction density (P-P, P-R, and R-R Junc.D, 1/mm3), calculated as the total junctions between trabecular plates and rods normalized by the bulk volume. Orientation of trabecular bone network was characterized by axial bone volume fraction (aBV/TV), defined as axially aligned bone volume divided by the bulk volume. Detailed methods describing the complete volumetric decomposition technique and ITS-based measurements can be found in our prior publications.15, 16
Statistical methods
Analyses were conducted with STATA version 9.0 (Stata Corp., College Station, TX, USA) and SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Two-sided p values < 0.05 were considered to indicate statistical significance. Descriptive data are presented as mean ± standard deviation (SD) and group comparisons as mean ± standard error of the mean (SEM). Differences between fracture and control subjects were assessed by Student's t test or chi-square test. Normality testing (Kolmogorov-Smirnov) was performed, and variables that were not normally distributed were logarithmically transformed before group comparisons. Satterthwaite adjustment was performed in the case of unequal variance between the groups.
Results
Of 117 postmenopausal women with osteopenia enrolled (mean age 66 ± 6 years), 58 had a history of low-trauma fracture after menopause, or prevalent vertebral fracture by X-ray. The 59 controls had no fracture history and normal spine radiographs. Women enrolled were ambulatory and generally in good health. The most common sites of fracture were wrist (28%), vertebrae (26%), and ankle (21%). One subject had a hip fracture. The average time between symptomatic fracture and study evaluation was 4 ± 6 years.
Women with fractures and controls did not differ on the basis of age, race, height, weight, or time since menopause (Table 1). Family history of osteoporosis by BMD or fractures and alcohol and tobacco use did not differ. Women with fractures were somewhat more likely to be using bisphosphonates and selective serotonin reuptake inhibitors, although the differences were not significant. Use of other medications, including hormone replacement therapy and raloxifene, did not differ. Calcium intake from supplements was similar between groups. Vitamin D intake appeared to be greater in controls but was not significantly different, likely because of the wide range of intakes among the subjects. Estimations of the 10-year risk by the FRAX algorithm in both groups were well below the treatment thresholds for major osteoporotic fracture and hip fracture. However, FRAX scores were greater in women with fractures for both risk estimates. Only 4 fracture subjects and no controls had FRAX scores for any major osteoporotic fracture above 20%. Nine fracture subjects and 2 controls had estimates of hip fracture risk greater than 3%.
| Fracture (n = 58) | Control (n = 59) | p Value | |
|---|---|---|---|
| Age (years) | 66 ± 6 | 67 ± 6 | 0.44 |
| Race (% white) | 80 | 74 | 0.54 |
| Height (cm) | 159 ± 11.9 | 160 ± 6.4 | 0.72 |
| Weight (kg) | 73 ± 17 | 70 ± 15 | 0.20 |
| BMI (kg/m2) | 30 ± 10 | 27 ± 6 | 0.20 |
| Years since menopause | 17 ± 7 | 18 ± 8 | 0.62 |
| Family history of osteoporosis by BMD (%) | 40 | 37 | 0.71 |
| Family history of fracture (%) | 56 | 43 | 0.21 |
| Tobacco use | |||
| Never (%) | 45 | 42 | 0.18 |
| Former (%) | 50 | 58 | |
| Current (%) | 5 | 0 | |
| Alcohol use (beverages per day) | 1 ± 1 | 1 ± 1 | 0.39 |
| Calcium supplements (total daily dose [mg]) | 600 ± 654 | 579 ± 538 | 0.86 |
| Vitamin D supplements (total daily dose [IU]) | 692 ± 770 | 1066 ± 1605 | 0.18 |
| Hormone replacement therapy | |||
| Past (%) | 37 | 43 | 0.54 |
| Current (%) | 5 | 13 | 0.16 |
| Bisphosphonatesa | |||
| Past (%) | 7 | 7 | 0.98 |
| Current (%) | 5 | 0 | 0.08 |
| Raloxifene (%) | 7 | 4 | 0.45 |
| Thyroxine (%) | 14 | 24 | 0.16 |
| SSRIs (%) | 28 | 15 | 0.10 |
| Inhaled glucocorticoids (%) | 4 | 2 | 0.59 |
| FRAX score | |||
| Major osteoporotic fracture | 13.2 ± 4.5 | 8.9 ± 3.4 | <0.001 |
| % with score ≥20 | 7 | 0 | <0.05 |
| Hip fracture | 1.8 ± 1.4 | ± 0.8 | <0.002 |
| % with score ≥3 | 16 | 3 | <0.03 |
- BMI = body mass index; BMD = bone mineral density; SSRI = selective serotonin reuptake inhibitor.
- a Bisphosphonate use limited to <1 year in past or present.
DXA T-scores were similar among women with and without fractures (Fig. 1) but tended to be lower at the LS (p = 0.09) and higher at the 1/3 radius (p = 0.07) among women with fractures compared with controls.
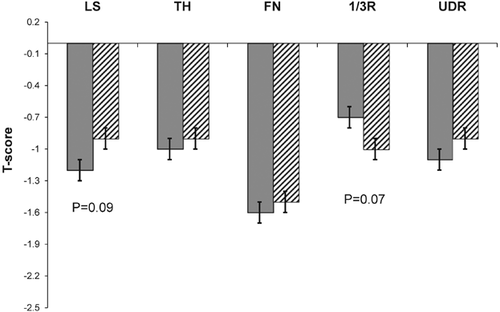
Volumetric BMD, cortical and trabecular microarchitecture by HR-pQCT, and whole bone stiffness by FEA differed markedly between fracture and control subjects (Fig. 2). At the radius, fracture subjects had significantly greater total cross-sectional area (6.3%; p < 0.05) and trabecular area (9.5%; p < 0.02) but smaller cortical area (−7.6%; p < 0.03) than controls. Total density was lower (−12.2%; p < 0.003). Cortical density (−3.2%; p < 0.03) and cortical thickness (−11.2%; p < 0.01) were significantly lower in fracture subjects. Trabecular density was significantly lower (−11.7%; p < 0.02) and trabecular microarchitecture tended to be worse, with a trend toward lower trabecular number (−5.3%; p = 0.10) and thickness (−5.5%; p = 0.10) and greater trabecular separation (6.5%; p = 0.08) in fracture subjects. Trabecular network heterogeneity, a measure of how irregularly spaced the trabeculae are, did not differ. Whole bone stiffness was significantly lower among fracture subjects (−10.0%; p < 0.01).
At the tibia, fracture subjects tended to have greater total cross-sectional area (5.8%; p = 0.06), with significantly greater trabecular area (8.0%; p < 0.04) and smaller cortical area (−9.2%; p < 0.03). Total density was lower (−9.2%; p < 0.01). Cortical density (−3.2%; p < 0.04) and cortical thickness (−11.2%; p < 0.02) were lower among fracture subjects and of the same magnitude as radial differences. There was no significant difference in trabecular density, number, thickness, or separation. Trabecular network heterogeneity was similar between groups. Whole bone stiffness tended to be lower among fracture subjects, but the difference was not significant (−6.1%; p = 0.07). Cortical porosity did not differ at either site (radius: fracture 2.8 ± 0.1% versus control 2.5 ± 0.2%, p = 0.27; tibia: fracture 7.0 ± 0.2 versus 6.9 ± 0.2, p = 0.82). Representative HR-pQCT scans of the radius and tibia in a fracture subject and a control are shown in Fig. 3.
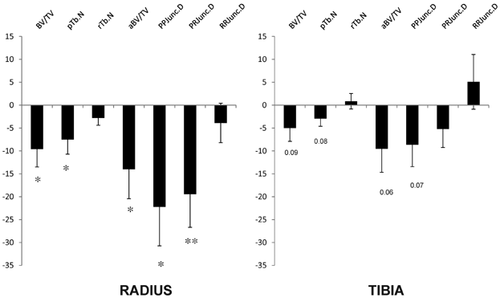
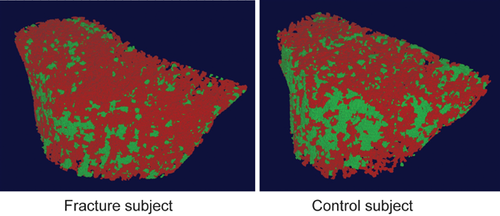
Differences in trabecular structure were further analyzed using ITS (Fig. 4). At the radius, women with fractures had significantly lower bone volume fraction (−9.6; p < 0.02), fewer trabecular plates (−7.5%; p < 0.03) and tended to have fewer trabecular rods (−2.8%; p = 0.08). They had significantly less axially aligned trabeculae (−14.0%; p < 0.04). They had lower connectivity between plates (−22.2%; p < 0.01) and between plates and rods (−19.5%; p < 0.01). There was no difference in connectivity between rods. At the tibia, differences were less pronounced and not statistically significant. Women with fractures tended to have lower bone volume fraction (−5.0%; p = 0.09), fewer trabecular plates (−2.9%; p = 0.08), fewer axially aligned trabeculae (−9.5; p = 0.06), and less connectivity between plates (−8.7%; p = 0.07). There was no difference in rod number or connectivity between plates, plates and rods, or rods. ITS images from a fracture and control subject are shown in Fig. 5, illustrating fewer trabecular plates (green) in the fracture subject.
We performed a multivariate regression to evaluate whether differences between groups were primarily associated with differences in bone geometry and density. We found that after adjusting for total area of the bone at the radius, differences in cortical thickness (p = 0.08) and trabecular density (p = 0.06) became trends. Differences in cortical density were no longer significant. Among ITS measures, plate trabecular number, plate-rod junction density, and plate-plate junction density remained significant. Rod trabecular number (p = 0.08) and axial bone volume fraction became a trend (p = 0.07). After further controlling for total density, none of the differences remained significant. At the tibia, after adjusting for total area, the difference in cortical thickness (p = 0.09) became a trend. Among ITS parameters, no differences remained significant after adjusting for area or density.
Discussion
In this study, HR-pQCT, ITS, and µFEA revealed many abnormalities in osteopenic women with fractures compared with osteopenic controls. We extend previous findings of microarchitectural abnormalities at the radius in osteopenic women with fractures by demonstrating cortical and stiffness deficits at the tibia as well. Using the novel technique of ITS, we show that women with osteopenia and fractures have differences in the type, number, orientation, and connectivity of their trabeculae compared with women without fractures and in this way highlight a new potential mechanism for fragility in this population.
Several studies by our group and others have reported microarchitectural abnormalities in women with fragility fractures compared with controls.6, 9-11, 22, 23, 29, 37 Although the majority of these studies evaluated women with bone densities that ranged from normal to osteoporotic, Boutroy and colleagues also compared microarchitecture in osteopenic women with and without fractures.6 They found that women with fractures had lower total, cortical, and trabecular volumetric BMD, lower trabecular number, and greater trabecular separation and heterogeneity. However, these differences were only observed at the radius, not at the tibia.
Our study confirms and extends this work in that we also measured stiffness by finite element analysis and assessed trabecular plate and rod microstructure using the novel imaging technique ITS.
In contrast to Boutroy and colleagues, we found abnormalities at both the radius and tibia in women with fractures and reductions in stiffness at both sites. Interestingly, differences in cortical area, density, and thickness were comparable between radius and tibia, whereas differences in trabecular bone parameters were less pronounced at the tibia. The differences in our findings compared with those of Boutroy and colleagues may be because that study had fewer women with fractures, and more than half were at the wrist. Additionally, that study utilized different software to distinguish cortical and trabecular bone. Finally, our study excluded women with secondary causes of bone loss and those with significant exposure to bisphosphonates. These distinctions may have enabled us to detect the more subtle abnormalities at the tibia.
In this study, we used the novel technique of ITS to evaluate trabecular characteristics and connectivity. ITS can reveal differences in plate and rod structure that are associated with differences in biomechanical properties. This has been demonstrated in comparisons of Asian and white women,38, 39 premenopausal women with idiopathic osteoporosis,19 and postmenopausal women with fractures.40 This is the first study to use ITS to compare women with osteopenia by fracture status, and we found that this technique revealed many trabecular abnormalities among osteopenic women with fractures, although only a few trabecular abnormalities were detected by standard HR-pQCT analyses. At the radius, women with fractures had fewer trabecular plates, fewer axially aligned trabeculae, and less connectivity between trabeculae, all features that have been associated with decreased strength.14-17 Differences at the tibia were less pronounced and not statistically significant, likely reflecting apparent preservation of trabecular microstructure at this weight-bearing skeletal site. Although the precision of ITS parameters is similar to that of the standard microstructural measurements, by delineating plate- and rod-specific structural characteristics, ITS provided additional insight into structural deterioration mechanisms of postmenopausal bone loss, which may have been concealed by standard approaches. For example, bone loss via plate-to-rod conversion or via loss of trabecular connections can lead to an increase in trabecular number but a decrease in bone volume. However, through differentiation of rods and plates by ITS, these effects can be decomposed, enabling detection of subtle structural changes.
We found substantial differences in cortical bone among osteopenic women with fractures: lower cortical area, thickness, and density at both radius and tibia. The lower cortical area but increased trabecular area seen in fracture subjects is suggestive of endocortical resorption, which has been reported to be a common mechanism of bone loss in postmenopausal women.41 Despite these cortical abnormalities, we did not appreciate differences in cortical porosity between the groups. Similarly, Nishiyama and colleagues reported differences in cortical density and thickness at the radius and cortical area and thickness at the tibia in women with fragility fractures and controls but found no difference in cortical porosity.13 Other authors have reported increased cortical porosity measured by HR-pQCT in diabetic subjects with fractures compared with controls.42 Women with osteopenia and osteoporosis have been shown to have thinner and more porous cortices than women with normal aBMD;31 however, our subjects all had osteopenia and differed only according to whether they had sustained a fracture. There may have been differences in fine porosity between women with fractures and controls that were not detectable by the resolution of our technique. This idea is supported by the fact that we did detect differences in cortical density, a measure that would be affected by both macro- and micro-porosity. Images for our analyses were thresholded by the global threshold technique provided by the HR-pQCT manufacturer, which may overestimate trabecular bone parameters, particularly in patients who have cortical thinning and increased endosteal porosity.41 It is conceivable that cortical porosity in osteopenic individuals is less the result of coalesced osteons, or macro-pores, and more a reflection of fine porosity. Perhaps using different methodology would have yielded different results for cortical porosity.
We estimated 10-year fracture risk in our subjects according to the FRAX algorithm. We found that estimates were higher among women with fractures, as would be expected given that FRAX takes fracture history into account. Interestingly, however, estimates for both groups were well below the thresholds of 20% for any major osteoporotic fracture and 3% for a hip fracture currently used to guide treatment decisions.43 Only 19% of fracture subjects were above the FRAX threshold for treatment, suggesting that FRAX may not be sufficient for identifying those osteopenic women at greatest fracture risk, even after a low-trauma fracture has occurred. Most risk factors for osteoporosis were similar between the two groups. However, women with fractures tended to use more selective serotonin reuptake inhibitors, which are associated with greater fracture risk.44 Not surprisingly, women with fractures were more likely to be using bisphosphonates. Although it is possible that bisphosphonate use was associated with fewer density and microarchitectural abnormalities in fracture subjects than would otherwise have been observed, this would have biased against detecting between-groups differences. Also, bisphosphonate use was limited to less than 1 year, precluding development of any long-term effects.
Our findings have several important implications. They demonstrate a structural basis, distinct from areal BMD, which may account for skeletal fragility among postmenopausal women with osteopenia. They show for the first time that women with osteopenia and fractures have abnormal trabecular type morphology. They highlight that both cortical and trabecular bone abnormalities are found in women with fractures, and that cortical abnormalities are comparable at both a weight-bearing and non-weight-bearing site. As we have reported in other cohorts,9, 24 trabecular structure was better at the tibia, suggesting that weight bearing may have disparate influences on trabecular and cortical bone. Better trabecular structure at the tibia may reflect the effects of weight bearing on acquisition and maintenance of bone as well as an effect of weight bearing to mitigate postmenopausal losses. The results of our multivariate analysis suggest that three-dimensional measures of bone geometry and density are the most critical characteristics in determining fracture susceptibility among osteopenic women. They suggest that the main limitation of DXA in this population may be its two-dimensional capability.
This study has both unique strengths and important limitations. To our knowledge, this is the first study to compare FEA and ITS in fractured and nonfractured women with osteopenia, and in doing so we extend previous work in this area. We limited a number of potential confounders by excluding women with a history of certain diseases and medication exposures that are known secondary causes of bone loss. By excluding women who had used bisphosphonates for longer than 1 year, we limited the possibility of artifact on the HR-pQCT scans from hypermineralization, which can occur after long-term bisphosphonate use.45 Limitations of this work include the cross-sectional study design, which precluded assessment of microarchitecture at the time of fracture occurrence or, more important, before fracture occurrence. In addition, many of the fractures took place several years before the study. Although we were able to detect many significant differences in microarchitecture and stiffness between groups, it is possible that the fractures may have antedated the microstructural changes that we observed. In addition, unmeasured circumstantial factors (ie, falls) unrelated to any structural parameter may have been crucial determinants of fracture. Although we observed these differences in the postmenopausal period, because of our cross-sectional study design, we cannot tell whether microarchitectural abnormalities reflect differences in the axial distribution of bone established during the premenopausal period, or whether they result from rapid postmenopausal losses. This is an important question that should be addressed in longitudinal studies. There are also several potential measurement limitations, summarized earlier in the discussion.
In conclusion, in this study, we demonstrated several novel structural abnormalities in osteopenic women with fractures. Using ITS, we found that women with fractures had fewer trabecular plates, a less axially aligned trabecular network and less trabecular connectivity, and worse biomechanical properties of bone. We also found that women with fractures had marked cortical abnormalities at both radius and tibia, whereas trabecular abnormalities were more pronounced at the radius. This disparity suggests that weight bearing may be protective for trabecular but not cortical bone. Despite lower cortical volumetric BMD and thinner cortices, we found no difference in cortical porosity, suggesting that cortical pores in these women may be smaller than can be detected with standard HR-pQCT techniques. Overall, our results provide increased understanding of the causes of biomechanical compromise in women with osteopenia and fractures. They provide the basis for future work that may help identify and treat those women with osteopenia who are at greatest risk for fracture.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
This work was supported by NIH U01 AR055968 (ES), NIH K24 AR 052661 (ES), NIH K23 DK084337 (EMS), NIH R01 AR058004 (XEG and ES), NIH R01 AR051376 (XEG), and the Thomas L Kempner and Katheryn C Patterson Foundation.
Authors' roles: Study design: EMS, TLN, and ES. Study conduct: EMS, AK, and ES. Data collection: EMS, TLN, MW, FC, JN, and AK. Data analysis: EMS, XSL, KN, SB, BZ, XEG, DJM, and CZ. Data interpretation: EMS, DJM, CZ, KN, XSL, BZ, XEG, and ES. Drafting manuscript: EMS and ES. Revising manuscript content and approving final version of manuscript: all authors. EMS takes responsibility for the integrity of the data analysis.




