Effects of Thickness and Calcium Phosphate Ceramic Inclusion on PTMC/PLGA Blend Membranes for Supporting Bone Regeneration
Funding: This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—Research Productivity Fellowship to Elizabeth Ferreira Martinez, Grant/Award Number: #307169/2021-9.
ABSTRACT
The present study assessed the physical and biological properties of membranes obtained by the electrospinning of a polymeric blend (70:30) of poly(lactic-co-glycolic acid) (PLGA) and poly(trimethylene carbonate) (PTMC) in two different thicknesses. Membranes were formed with or without incorporating ceramic particles containing 60% hydroxyapatite (HA) and 40% β-tricalcium phosphate (TCP). The following parameters were assessed: (i) ultrastructural characterization, (ii) measurement of surface area and total pore volume, (iii) tear resistance, (iv) dissolution pH evaluation, (iv) in vivo assessment of potential new bone formation in critical defects. The specimens were divided into the following sample groups: G1 = 300 μm, G2 = 300 μm + HA/TCP, G3 = 600 μm, G4 = 600 μm + HA/TCP. The findings revealed fibrillar structure in various orientations in all the groups. Surface area, total pore volume, and dissolution pH were higher in G2 and G4, compared with G1 and G3. Tear resistance was higher for thicker membranes. Furthermore, G4 showed a greater percentage of new bone volume and density at 30 days than the other groups. The results suggest that the polymeric blend of PLGA and PTMC is a promising material for use as a membrane for clinical guided bone regeneration procedures, and represents a potential alternative to the currently available products.
1 Introduction
The regeneration of bone defects remains a significant challenge in dental implant rehabilitation, compromising predictability and prognosis. Successful rehabilitation treatment depends on the presence of healthy alveolar bone with adequate volume and characteristics [1]. Inadequate bone compromises predictability in implant placement and long-term prognosis. As a result, various strategies have been established to stimulate new bone formation, while preserving both esthetics and function [2]. Among the viable strategies, guided bone regeneration (GBR) stands out as one of the most commonly applied methods for reconstructing alveolar bone and treating peri-implant bone deficiencies [3]. One of the principles of GBR is the mechanical exclusion of undesirable tissues within the defect, such as connective and epithelial tissues, so that the volume needed for new bone tissue formation can be maintained [4]. The use of biomaterials, including membranes, has proven effective to this end.
Collagen has emerged as the most widely used material in resorbable membranes due to its potential to enhance cellular adhesion and its intrinsic properties, such as homeostasis, biocompatibility, ease of use, and minimal immunogenicity [5]. However, it lacks desirable mechanical properties, and its degradation may result in failure to maintain the space to be repaired [6].
In response to the limitations of xenograft membranes, resorbable polymeric membranes based on aliphatic polyethers and copolymers were developed for clinical use [7]. Among these, poly(lactic-co-glycolic acid) (PLGA) and poly(trimethylene carbonate) (PTMC) stand out as promising options. PLGA is a biodegradable and bioresorbable copolymer with favorable characteristics for GBR [8]. However, a challenge associated with PLGA is the potential release of acidic byproducts during degradation, which can induce an inflammatory response [9], in addition to reduced osteoconductivity [10]. PTMC is also recognized for its biocompatibility but degrades at a slower rate than PLGA, thus potentially providing longer support for tissue regeneration [11].
An additional strategy involves the incorporation of ceramic nanoparticles, such as hydroxyapatite (HA) and β-tricalcium phosphate (TCP) [12, 13], to enhance the recruitment of undifferentiated mesenchymal cells and promote osteogenesis [14, 15]. Not only are materials similar to bone tissues, but they should contain a homogeneous distribution of HA and TCP within each particle and exhibit interconnected microporosity, thus facilitating cell proliferation [16]. An ideal ratio for biphasic bioceramics is between 65:35 and 55:45 of HA and β-TCP, respectively, for simultaneous material resorption and bone formation by replacement [15].
Numerous types of membranes have been developed for GBR procedures, but there are still issues about the suitable membrane with respect to unpredictable bone formation and inadequate mechanical strength for space maintenance. Recently, Sartoretto et al. demonstrated that the combination of PLGA and PTMC can optimize the advantages of both polymers in terms of their mechanical and biological properties [17]. Therefore, in this study, we present a novel approach in GBR utilizing electrospun membranes composed of a polymeric blend of PLGA and PTMC varying thicknesses (300 and 600 μm), and combined with particles consisting of 60% HA and 40% TCP. It was assessed the morphological and physicochemical characteristics of fibers obtained and biological properties in terms of its osteogenic potential when used for GBR, using a critical size calvarial defect in rats.
2 Materials and Methods
2.1 Sample Groups
A polymeric blend of PLGA and PLLA/PTMC (ratio of 20:80) was used to obtain electrospun membranes. The polymers were dissolved in chloroform. This solution was then combined with particles composed of 60% hydroxyapatite (HA) and 40% tricalcium phosphate (TCP) (Nanosynt, FGM, Brazil). The HA/TCP particles were sifted in a 100 μm sieve to guarantee that all particles were ≤ 100 μm. The polymeric blend to ceramic blend ratio was maintained at 80:20.
The solution was transferred to an electrospinning machine with needleless technology, connected to a voltage supply. Subsequently, the solution underwent exposure to an intense electric field. When the electric field exceeds the surface tension and viscosity of the polymer solution, a charged stream of the solution is expelled toward the opposing pole (collector), which is also electrically charged, leading to the formation of fibers. The flow of the solution was continuously delivered to the nozzle, which had a diameter of 0.60 mm, under high voltage. Along this process, from the polymer solution to the fiber collection surface, the flow encounters unstable zones, resulting in solvent evaporation and solid fiber deposition on the collector. The resulting membrane is then detached from the collector and trimmed to meet the specific requirements and intended use [17]. The thickness of the membranes was determined by the speed of the spinning substrate (spin rate).
Subsequently, they were divided into four different sample groups according to their thickness and treatment: G1: 300 μm membrane; G2: 300 μm membrane associated with HA/TCP; G3: 600 μm membrane; G4: 600 μm membrane associated with HA/TCP. All the physical characterization assays were conducted in triplicate to test technical duplicates. For the in vitro and in vivo analysis, an a priori sample size calculation was performed by using an analysis software program (G*Power 3.1.9.4; Heinrich-Heine-Universität Düsseldorf) to estimate the number of specimens needed to detect differences among the groups. The effect size was identified as 0.830 based on a pilot study carried out with 3 specimens from each group. Such effect sizes would require at least 4 specimens per group to detect differences among the treatments at α = 0.05 and β = 0.80. In the present study, 5 specimens per group were used.
All membranes were sterilized by gamma radiation at a dose of 10 kGy (Sterigenics, São Paulo) prior to use.
2.2 Morphological Characterization and Membrane Porosity
The ultrastructural analysis of the membranes obtained was performed using scanning electron microscopy with a field emission gun (FEG ZEISS Auriga model and FEI SEM Magellan 400 L). Prior to examination, the sample surface was sputter-coated with gold to produce an approximately 20-nm-thick gold conductive film. The membrane surface area and total pore volume were characterized using the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively. The specimens were heated initially at 100°C for 24 h and then immersed in liquid nitrogen at 196°C under vacuum conditions. Data collection involved the adsorption of high-purity nitrogen gas on the tested specimens, and the specific surface area was determined using the BET theory. Thereafter, nitrogen desorption on isotherms was analyzed based on the adsorption curve of the material, and the average pore volume was determined using the BJH method.
2.3 Tear Resistance
A universal testing machine (EMIC DL 200, São José dos Campos, SP, Brazil) was used to perform the tear resistance assay. Membrane segments measuring 30 mm × 10 mm were clamped 5 mm from the edges of the membranes, in the direction of the longer length. The tensile strength in newtons (N) was measured using a 200 Kgf load cell at a constant speed of 1 mm/min, until membrane rupture.
2.4 pH Measurement of the Solution
The membranes were cut and weighed, and the specimens were subsequently immersed in phosphate buffered saline (PBS, Sigma, USA). A concentration of 0.2 g/mL of the membrane was used for this assay following the International Organization for Standardization guidelines (ISO 10993:2012) [18]. The specimens were maintained at 37°C to simulate an in vivo environment. The pH of the supernatant was measured using a pH meter (MS Tecnopon Instrumentação, Brazil) after 3, 7, 14, 21, 40, 60, and 90 days.
2.5 In Vivo Assessment of the Osteopromotive Potential of the Membranes
2.5.1 Animals
A total of 60 Wistar rats of the Rattus norvegicus albinus species with 3-month-old male rats weighing 300 g were used for this study [15, 19]. The rats were acquired from Anilab (Paulinia, SP, Brazil).
They were randomly divided into four groups (n = 15/each), according to the membrane. Random numbers were generated using the standard = RAND() function in Microsoft Excel. All the groups were analyzed on days 7, 30, and 60. Allocation of the procedures was revealed (P.G.M.) to the investigator (E.E.P.B) according to the sequence generated.
The research was conducted following the ethical principles adopted by the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiment) and in compliance with the US National Research Council's Guide for the Care and Use of Laboratory Animals, the US Public Health Service's Policy on Humane Care and Use of Laboratory Animals, and Guide for the Care and Use of Laboratory Animals. Prior to starting, the study was approved by the Research Ethics Committee for Animal Experimentation from Faculdade São Leopoldo Mandic (Protocol #2020/01). The animals were housed in cages under controlled lighting conditions (12-h light–dark cycle), regulated humidity and temperature, and balanced food and water, ad libitum. No inclusion or exclusion criteria were set in this study.
2.5.2 Critical Size Calvarial Defect Experiment
The animals underwent trichotomy in the calvaria region, followed by thorough antisepsis using PVPI alcohol containing 1% active iodine (Riodeine, Rioquímica, SP, Brazil). Next, a 2 cm longitudinal incision was made in the posterior portion of the calvaria along the sagittal suture, extending from the external occipital protuberance to the nasofrontal region. The periosteum was incised and carefully separated to fully expose the occipital, frontal, and right parietal bones. A bicortical critical bone defect, involving both the outer and inner cortical layers of the calvaria, was created in the parietal bone of each animal. A 6-mm-diameter trephine drill (reference #103207, Neodent, Curitiba, PR, Brazil) was used, fitted to a 20:1 contra-angle reducer attached to a controlled rotation motor. The procedure was conducted at a speed of 800 rpm, applying ample external irrigation with a 0.9% saline solution [15, 19]. The internal cortical layer was removed and meticulously separated from the dura mater, which remained intact.
Critical defects of both groups were randomly covered with the respective resorbable membranes under analysis. The periosteum and skin were placed together in layers and sutured with absorbable 4–0 polyglactin 910 thread (Vycril, Ethicon, Johnson & Johnson, USA). Following the surgery, the animals were administered tramadol hydrochloride (50 mg/mL, 5 mg/kg/day for 3 days, intraperitoneally), and a dose of flunixin meglumine (50 mg/mL, 1.1 mg/kg). Then, they were maintained for 7–60 days on a standard diet. The animals were euthanized using an overdose of 2% anesthetic isoflurane until respiratory arrest and cessation of heartbeat were confirmed. The soft tissue was removed using scissors, and the areas of interest were collected and immersed in a 10% buffered formalin solution.
2.5.3 Histological Processing and Analysis
The calvaria were demineralized in 20% formic acid, dehydrated, embedded in histological paraffin, and sectioned in the central region of the defects, parallel to the sagittal suture, in 4-μm-thick sections. The specimens were stained with hematoxylin–eosin, and then mounted on resin slides for photomicrography. Slide images were captured in a computerized imaging system (AxioVision rel 4.8, Carl Zeiss), integrated with an Axioskop 2 Plus light microscope (Carl Zeiss, Oberkochen, Germany).
The osteogenic potential of the investigated materials was assessed for qualitative and quantitative histological analyses to describe new bone formation at different analysis time points (7, 30 and 60 days). Bone maturation was evaluated descriptively using Masson's Trichrome [19]. A classification score was adopted to measure the inflammation, attributing the extent of the inflammatory process in the defect area with a score of 0–3, where 0 was absent, 1 was mild (up to 25%), 2 was moderate (from 25% to 50%), and 3 was intense (above 50%) [15, 19]. Histomorphometric evaluations were conducted using ImageJ 152r image analysis software (National Institute of Health, Maryland, USA).
Measurements of total area (in μm2) and bone perimeter (in μm) were performed [20]. Morphometric parameters (BV/TV, bone volume fraction; BS/TV, bone surface density; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation) were calculated using the stereological method proposed by Parfitt et al. [21] following the nomenclature set by the American Society for Bone and Mineral Research [22].
The analyses were performed by a single examiner (E.F.M.) blinded to the treatment performed.
2.6 Statistical Analysis
First, descriptive analyses were performed for all the variables using mean, standard deviation, and median (minimum; maximum) values. Next, descriptive and exploratory analyses of all the data were performed. The variables of surface area (m2/g), total pore volume (cm3/g) × 10−2, and membrane tear resistance (N) were analyzed by one-way analysis of variance (ANOVA) and Tukey's test. The pH data of the membrane dissolution medium were analyzed by a mixed model (ANOVA) for repeated measures over time. The data for the histomorphometric evaluations did not meet the assumptions of analysis of variance (ANOVA), as indicated by the Shapiro–Wilk test for normality. Therefore, the data were analyzed using the non-parametric Kruskal-Wallis and Dunn tests. All the analyses were conducted using the R program, with a significance level of 5%.
3 Results
3.1 Topographical Characterization
SEM was performed to characterize the surface of the membrane. Figure 1 illustrates the ultrastructural morphology of the surfaces. All the surfaces displayed randomly intertwined bead-free fibers with irregular pores. Granules of HA/TCP were distributed throughout the entire surface of both membrane thicknesses (300 μm and 600 μm) when HA/TCP was incorporated (G2 and G4).
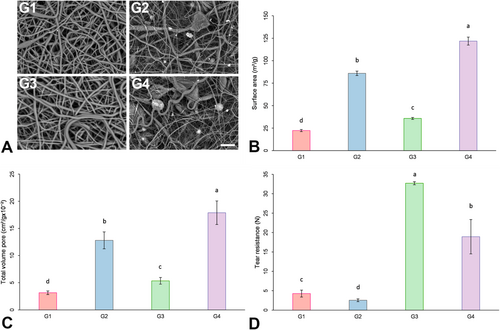
3.2 Surface Area and Pore Volume
The results for the specific surface area (m2/g) and total pore volume (cm3/g) are depicted in Figure 1B,C. The surface area was observed to be greater for G4, followed by G2, G3, and G1 (p < 0.05).
3.3 Tear Resistance
Figure 1D displays the tear resistance results. The tear resistance of the 600-μm-thick membrane was greater than that of the 300-μm-thick membranes (G1 and G2), regardless of HA/TCP incorporation (G3 and G4) (p < 0.05). Furthermore, HA/TCP incorporation resulted in a decrease in tear resistance for both thicknesses evaluated (G2 and G4), compared to G1 and G3 (p < 0.05).
3.4 pH Measurement of the Solution
Table 1 shows the pH changes in the solution after the membranes were immersed. The mean pH values of the membranes associated with HA/TCP (G2 and G4) were higher than those of the materials that were not associated (G1 and G3) at the time points (from 3 days up to 90 days), except for baseline. At all time points, the mean pH values in the solution for the pristine polymeric membrane, irrespective of their thickness (G1 and G3), were acidic.
| Time | Groups | |||
|---|---|---|---|---|
| G1 | G2 | G3 | G4 | |
| Mean (standard deviation) | Mean (standard deviation) | Mean (standard deviation) | Mean (standard deviation) | |
| Baseline | 7.00 (0.00) Aa | 7.00 (0.00) Aa | 7.00 (0.00) Aa | 7.00 (0.00) Aabc |
| 3 days | 2.91 (0.06) Bb | 7.24 (0.23) Aa | 3.27 (0.14) Bb | 7.40 (0.23) Aab |
| 7 days | 2.89 (0.01) Bb | 7.45 (0.05) Aa | 3.12 (0.11) Bb | 7.82 (0.02) Aa |
| 14 days | 2.89 (0.01) Bb | 7.35 (0.07) Aa | 3.09 (0.11) Bb | 7.59 (0.27) Aab |
| 21 days | 2.65 (0.20) Bb | 7.27 (0.35) Aa | 2.84 (0.32) Bb | 7.13 (0.15) Aabc |
| 40 days | 2.62 (0.66) Bb | 7.11 (0.51) Aa | 3.16 (0.10) Bb | 7.07 (0.14) Aabc |
| 60 days | 2.43 (0.50) Bb | 6.96 (0.71) Aa | 2.96 (0.32) Bb | 6.63 (0.38) Abc |
| 90 days | 2.49 (0.11) Cb | 6.55 (0.75) Aa | 3.74 (0.33) Bb | 6.25 (0.79) Ac |
- Note: G1: 300 μm membrane; G2: 300 μm membrane associated with HA/TCP; G3: 600 μm membrane; G4: 600 μm membrane associated with HA/TCP. Distinct letters (uppercase horizontally and lowercase vertically) indicate statistically significant differences (p ≤ 0.05). p(group) < 0.0001; p(time) < 0.0001; p(interaction) < 0.0001.
3.5 Histological and Histomorphometric Analyses
All animals recovered successfully following the surgical procedures. No animals died or experienced any complications that could affect the results during the healing periods at 7, 30, and 60 days.
Representative histological images in critical defects at different time points are shown in Figures 2 and 3. Lymphocytic inflammatory infiltrate was widely distributed only at 7 days of evaluation, particularly in G1 and G3 (Figures 2A,C and 3). At this time point, slight new bone formation was present in the defect area, mostly at the edges.
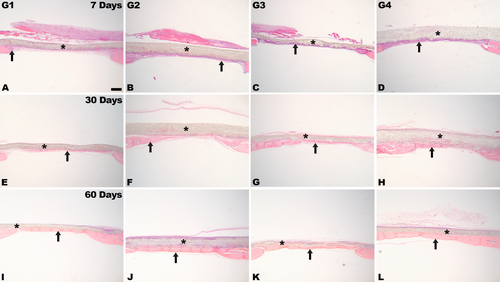
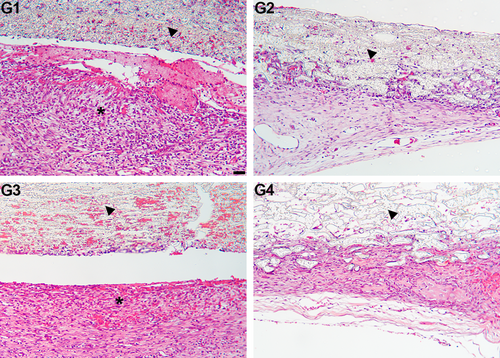
Table 2 illustrates the intensity of inflammatory infiltrates in the defect region. The inflammation score was significantly higher in the pristine polymeric membranes, regardless of thickness (G1 and G3), compared to the membranes incorporating HA/TCP (G2 and G4) (p < 0.05). No significant differences were observed between G1 and G3 or between G2 and G4 (p > 0.05).
| Groups | Median (minimum and maximum values) |
|---|---|
| G1 | 3.0 (2.0–3.0) a |
| G2 | 0.0 (0.0–1.0) b |
| G3 | 3.0 (2.0–3.0) a |
| G4 | 0.0 (0.0–1.0) b |
- Note: G1: 300 μm membrane; G2: 300 μm membrane associated with HA/TCP; G3: 600 μm membrane; G4: 600 μm membrane associated with HA/TCP. Distinct letters indicate statistically significant differences (p < 0.05). Absent (0); Mild (1), up to 25%; Moderate (2), from 25% to 50%; Intense (3), above 50%.
At 30 days, new bone formation was observed in the areas near the edges of the defect, and in the center of the defect. Newly formed bone was evident in the center of the defect for G2, G3, and G4 (Figure 2F,H). In G3 and G4, there was partial membrane degradation, with connective tissue cells and focal areas of newly formed bone interspersed (Figure 2G,H). At 60 days, more advanced membrane degradation was observed, with bone formation in close contact with membrane remnants from both the edges and the defect center (Figure 2I–L). Complete defect closure was observed occasionally, particularly in G4, along with the presence of more lamellar bone (Figure 2L), as evidenced by the red areas in the histological sections, in relation to the other group (Figure 4).
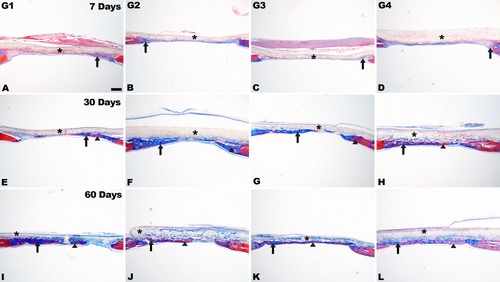
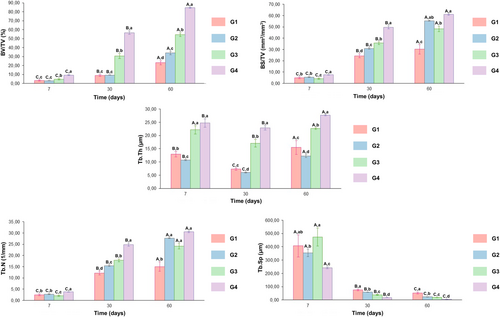
Stereological analysis revealed a significant increase in BV/TV and BS/TV at G4 when compared to G2 and G1 (p < 0.05) in all time points evaluated (Figure 5). Furthermore, the Tb.Th at 7 days, exhibited a significantly higher value in G3 and G4 when compared to G2 and G1 (p < 0.05). At 30 and 60 days, G4 displayed a higher value in comparison to the other groups. For Tb.N, the higher values were observed for G4 when compared to the other groups, especially at 7 and 30 days (p < 0.05). Regarding the Tb.Sp parameter, G4 exhibited lower values compared to the other groups (p < 0.05).
4 Discussion
The current study, for the first time, assessed the efficacy of electrospinning a membrane composed of a biodegradable polymeric blend of 70% PLGA and 30% PTMC, and the biological parameters involved in inducing bone neoformation with this alternative alloplastic material. Alloplastic materials have emerged as an alternative to xenogeneic or allogeneic biomaterials to perform GBR procedures that can minimize the drawbacks and limitations presented by other biomaterials, while also promoting predictable bone neoformation [7, 23]. Our data suggest that the PLGA/PTMC blend combined with HA/TCP provides an attractive alternative to current alloplastic biomaterials for bone healing.
PLGA is a well-known biodegradable polymer widely used in other medical applications such as absorbable sutures and meshes [24]. PTMC has the advantage of containing no acidic degradation products and is a biocompatible, biodegradable polymer with good flexibility and surface erosion properties [25]. The combination of these polymers (PLGA + PTMC) was proposed to obtain a membrane with predictable biodegradation and tissue integration, as well as mechanical stability and stiffness to ensure proper regeneration [17]. PTMC appears to have a slower degradation rate than PLGA [26]. Furthermore, electrospun PLGA membranes have some hydrophobicity, which could hinder cell adhesion [27]. The results of the present study revealed that different membrane thicknesses accounted for modifications in tear resistance, with greatest tear resistance observed for the 600 μm thickness, regardless of HA/TCP incorporation. These findings could be useful in clinical procedures that require the use of devices such as pins and screws, which increase the tension on the membranes [28].
When biodegradable polymers are implanted in an aqueous environment, such as a biological system, they hydrate, undergo hydrolysis, and lose mass integrity, which can cause an inflammatory reaction. The degree of hydrolysis is significantly affected by the nature of the polymer, pH, temperature, degree of polymer crystallization, membrane volume, and thickness [29]. Therefore, considering that tissue occlusiveness, space maintenance ability, and new bone formation are important and dependent on membrane thickness [30, 31], the present study evaluated two different thicknesses. Thicker membranes may be related to an increase in the robustness of the scaffold in larger defects, which may affect their mechanical properties and ability to maintain space during implantation [32]. Previous studies have demonstrated that the placement of a thicker membrane reduces soft tissue ingrowth and facilitates more effective bone formation [6, 30]. In addition, the permeability of the membranes promotes adequate fluid and gas exchange, which is also related to their thickness [33]. Importantly, our study demonstrated that the use of a thicker membrane was associated with significantly greater bone formation, reinforcing the critical role of membrane thickness in optimizing regenerative outcomes.
Considering the parameters used for electrospinning, ultrastructural analyses revealed that the membranes exhibited a network structure composed of micro- and nano-sized fibers and intermediate pores spread across the surface. These findings are critical for in vivo regeneration, considering that the appropriate pore size allows vascular ingrowth and regulates cell adhesion and bone formation within the defect [34].
Not only were different thicknesses investigated, but also the effect of HA/TCP nanoparticles incorporated into the membranes during electrospinning. The addition of inorganic materials such as HA and TCP to biomaterials is often explored for their osteogenic potential [13, 14]. Although the clinical efficacy of these materials is widely recognized, questions persist regarding possible inflammatory reactions and residual residues [35].
The present study demonstrated that the association with HA/TCP resulted in an increase in surface area and in total pore volume, compared with their respective thickness without particle incorporation, regardless of the membrane thickness. The size and quantity of pores have a direct impact on both permeability and mechanical properties [36]. Surfaces with higher porosity presented more cell adhesion sites, hence higher cell viability and differentiation [34].
In fact, the results demonstrated that the incorporation of HA/TCP promoted an increase in bone formation at the initial time point, with a more lamellar and mineralized tissue of newly formed bone. These findings underscore the beneficial effect of ceramic addition on membrane bioactivity and its ability to accelerate bone regeneration at the defect sites [37]. This positive effect was further supported by the stereological analysis that revealed a significant increase in bone volume (BV/TV) accompanied by an increase in trabecular number (Tb.N), thickness (Tb.Th) and bone surface density (BS/TV) in 600 μm thickness membranes associated with HA/TCP, reflecting a complex bone microstructure, with a smaller size of the marrow spaces (Tb.Sp).
It is important to highlight that the presence of HA/TCP ceramics promoted a significantly higher pH in the solution during dissolution in PBS than the non-association of the ceramic. PLGA degrades via hydrolysis into glycolic acid and lactic acid, leading to the formation of an acidic extracellular microenvironment [38]. Although the acidic degradation of PLGA reduces local pH, resulting in an autocatalytic environment, HA/TCP increases the pH of the environment and neutralizes the effects of PLGA hydrolysis products [39]. This pH increase can be attributed to the release of calcium and phosphate ions from the HA/TCP ceramics during dissolution, which leads to the formation of slightly basic compounds such as calcium hydroxide and phosphate salts in the medium [37]. These findings, associated with the physical properties of the membrane, could contribute to the positive effect on bone neoformation, particularly of the thicker membrane associated with ceramics. It is well documented in the literature that an acidic pH environment induces autophagy in osteoblasts, negatively modulating bone matrix formation [40]. Furthermore, it is known that low pH values may influence the emergence of macrophages, which are directly related to inflammatory processes [41], thus hindering tissue healing.
Further investigations are essential to gain a more in-depth understanding of the biological performance of these membranes, particularly in randomized clinical trials with a long-term follow-up, and in regard to different thicknesses and clinical needs. These parameters directly relate to the size of the defect area, which is influenced by the membrane's flexibility and its conformation to the region. In addition, they are influenced by the necessity for a barrier effect, which may affect the permeability and degradation rate of the membrane. These studies will contribute to fully understanding the potential of this innovative biomaterial, potentially leading to significant improvements in bone regeneration outcomes and advancing the current state of guided bone regeneration technologies.
5 Conclusions
Based on the methodology used in this animal study, and its limitations, the findings evidenced that the polymeric blend of a 70% PLGA and 30% PTMC, particularly when combined with HA/TCP, produces a promising material for use as a membrane in clinical GBR procedures, and represents a potential alloplastic alternative to the currently available products. Additionally, the superior performance of the 600 μm thick membrane highlights its potential in bone regeneration, providing enhanced mechanical strength and augmented osteogenic capacity, which are essential for effective tissue integration and bone healing.
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Acknowledgments
The authors would like to thank Dr. Fernanda Barchesi Zanelatto for her technical assistance with the animals, and also acknowledge Nancy Cristina Martorana for promptly volunteering to review this manuscript regarding its English language content, and Dr. Rafael Bovi Ambrosano for helping with the statistical analysis of the data. The Article Processing Charge for the publication of this research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) (ROR identifier: 00x0ma614).
Ethics Statement
The study was approved by the Research Ethics Committee for Animal Experimentation from Faculdade São Leopoldo Mandic (Protocol #2020/01).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




