Comparison of Collagen and Polylactic Acid Multifilament Yarns for Developing Textile Scaffolds for Tendon Tissue Engineering Applications
Funding: This work was supported by AATCC Foundation.
ABSTRACT
This study is the first to compare the mechanical properties and biocompatibility of multifilament yarns made from a natural biopolymer, collagen, and from a synthetic polymer, polylactic acid (PLA), with a view toward evaluating the suitability of these two yarns for fabricating tendon tissue engineering scaffolds. Genipin, the natural plant-based anti-inflammatory and antioxidant crosslinking agent, was used to stabilize the collagen structure. A range of different genipin concentrations, crosslinking temperatures, and crosslinking times were studied, and based on higher degrees of crosslinking, reduced swelling ratio, and greater tensile strength, the recommended conditions are crosslinking the wet-spun collagen yarns with 1.0% genipin at 37°C for 72 h. During a 6-week enzymatic degradation study, the PLA yarns were more resistant to degradation and retained greater tensile strength than the genipin-crosslinked collagen yarns under both dry (4.0 ± 0.2 N vs. 2.2 ± 0.4 N) and hydrated (3.7 ± 0.3 N vs. 0.4 ± 0.1 N) conditions. After coating the PLA yarns with collagen, their biocompatibility improved, and they were able to support tendon cell growth and proliferation. In conclusion, the selection of multifilament PLA yarns coated with collagen is recommended as a promising scaffold material for tendon tissue engineering applications.
1 Introduction
Rotator cuff tendon tears are one of the most common musculoskeletal disorders, affecting more than 50% of the population over 60 years old [1]. According to the American Academy of Orthopedic Surgeons, each year almost 2 million people seek treatment for rotator cuff tears, and 300,000 rotator cuff surgeries are performed, with a combined annual healthcare cost of $3 billion in the United States alone [2]. The re-tear rate remains as high as 30%–94%, and one of the major reasons for this high failure rate is the limited mechanical support provided by current biological grafts [3]. Thus, designing an implantable graft or scaffold with appropriate materials is crucial for providing sufficient mechanical support during the slow tendon tissue regeneration process.
In previous decades, textile technology has been widely used for the fabrication of grafts for medical applications using various yarns, due to the 3D structures with tunable properties at the micro- and macro-levels [4]. Critical factors that need to be considered are the yarn mechanical properties and degradation performance, which should match the demands for clinical tendon repair. Since collagen is the predominant structural component in tendons, accounting for 60%–85% of their dry weight, various approaches have been developed to produce continuous collagen fibers and yarns as raw materials [5-7]. These fibers are then fabricated into scaffolds for tendon and ligament repairs using techniques such as weaving, knitting, and electrospinning [8]. In 2020, wet-spun multifilament collagen yarns with a hierarchical structure were successfully manufactured by passing a collagen solution through a specially developed spin pack and long coagulation bath [6]. However, due to the self-assembled production process, these collagen yarns became swollen and bunched together when immersed in water or other solutions and showed limited mechanical strength [6]. So, stabilizing the fibrous collagen structure is necessary before these collagen yarn-based materials could be used effectively as tendon grafts.
Chemical crosslinking is a common technique used to stabilize the collagen structure, and typical agents used to crosslink collagen products include glutaraldehyde and 1-ethyl-3-(3- dimethylaminopropyl) carbodiimide hydrochloride (EDC) with N-hydroxysuccinimide (NHS) [9-11]. Genipin is another chemical crosslinker, which, though more expensive and less readily available than glutaraldehyde, is reported to be approximately 10,000 times less cytotoxic than glutaraldehyde and is able to promote significantly improved cell growth [12]. Genipin has also been reported to enhance biostability compared to EDC/NHS when used for crosslinking an extracellular matrix (ECM) hydrogel [13]. However, the optimal processing conditions for using genipin to crosslink wet-spun multifilament collagen yarns have not previously been studied.
When selecting a material for a tendon repair graft, maintaining sufficient strength and mechanical stability during the healing process is essential. Since complete healing and tendon tissue regeneration require 6 to 12 months following human rotator cuff or Achilles tendon repair, the materials should have good resistance to degradation over this time period [14]. A previous study found type I collagen yarns lost about 80% of their mass and mechanical integrity over only 8 weeks [15]. Polylactic acid (PLA) has been approved by the U.S. Food and Drug Administration (FDA) as a suitable candidate for tendon repair, because it usually takes between 6 months and 2 years for PLA to break down hydrolytically into a non-toxic degradation byproduct, such as lactic acid, which can be readily metabolized by the human body [16]. However, due to its initial hydrophobic surface, PLA promotes limited cell proliferation compared to other synthetic polymers, such as polycaprolactone (PCL) and polylactic-co-glycolic acid (PLGA) [17]. As a result, PLA grafts have been coated with collagen to improve their suitability for tendon repair and tissue regeneration [4].
Although several different materials have been shown to be suitable for tendon repair grafts, no previous study has directly compared crosslinked collagen yarns with collagen-coated PLA yarns. Our primary objective was to determine which of these yarns is better suited for fabricating tissue-engineered grafts for tendon repair, based on their tensile properties, resistance to degradation, and biocompatibility with primary tendon cells.
2 Experimental Section
2.1 Fabrication of Multifilament Wet-Spun Collagen Yarns
The multifilament collagen yarns were produced using a wet-spinning technique as described previously [6] but with an advanced spin pack design assembled from individual 3D printed stackable polymethyl methacrylate plates (Figure 1). Ten glass capillaries with an inner diameter of 0.5 mm and a length of 20 mm were used as spinnerets. The spinnerets had space surrounding them for the coagulation liquid. A tube with an inner diameter of 3.5 mm and a length of 1200 mm was connected to the spin pack for long-lasting coagulation of the extruded collagen filaments. At the bottom exit of the tube, the coagulated filaments were transported through 2 baths, each 400 mm long and about 350 mL in volume. Finally, the filaments passed through an 800 mm long drying section, and the dried filaments were continuously wound up on a winder.
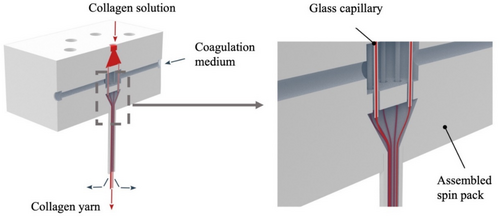
The spinning solution was prepared by stirring 4% collagen pellets that contained 50% water (KBM Collagen and Biomaterials for Medical Products GmbH, Wald-Michelbach, Hesse, Germany) with 0.1% acetic acid and 95.9% water at 22°C for 24 h. The solution was then poured into a 120-mL syringe and degassed by keeping the syringe upright in a refrigerator at 10°C for at least 48 h. The pH of the spinning solution was 3.3. The coagulation solution was prepared by stirring 10% polyethylene glycol, 0.4% NaH2PO4, 1.2% Na2HPO4, 0.6% triethylsilane, 0.8% NaCl, and 87% water for 1 h at room temperature [19]. Two 120-mL syringes were filled with the coagulation solution and stored overnight at 10°C. The pH of the coagulation solution was 7.7. Wet spun collagen yarns were comprised of 10 single filaments, each with a linear density of 35 tex and a diameter of 55 μm.
2.2 Crosslinking Condition Optimization
Wet-spun collagen yarns were divided into eight groups: a control group consisting of a non-crosslinked collagen yarn and seven crosslinked groups based on different genipin concentrations, crosslinking temperatures, and crosslinking times (Table 1).
| Group | Crosslinking conditions | ||
|---|---|---|---|
| Genipin (%) | Temp (°C) | Time (h) | |
| Control | 0.0 | 20 | 12 |
| 1 | 0.5 | 20 | 12 |
| 2 | 0.5 | 37 | 12 |
| 3 | 0.1 | 37 | 12 |
| 4 | 1.0 | 37 | 12 |
| 5 | 0.5 | 37 | 2 |
| 6 | 0.5 | 37 | 24 |
| 7 | 0.5 | 37 | 72 |
The yarns were crosslinked by immersion in 10 mL of 100% (v/v) ethanol with 0.1%, 0.5%, or 1.0% (w/v) genipin (FUJIFILM Wako Chemicals USA Corporation, Richmond, VA) at either 20°C or 37°C for time periods of 2, 12, 24, or 72 h. The crosslinked collagen yarns were then washed with deionized (DI) water three times to remove any remaining reagents. Both crosslinked and non-crosslinked yarns were vacuum dried at room temperature for 24 h and stored at 4°C until used experimentally. The optimum crosslinking conditions for the wet-spun collagen yarns were assessed based on which provided a higher degree of crosslinking, reduced swelling ratio, and greater tensile properties.
2.2.1 Degree of Crosslinking
2.2.2 Swelling Ratio
2.2.3 Ultimate Tensile Load
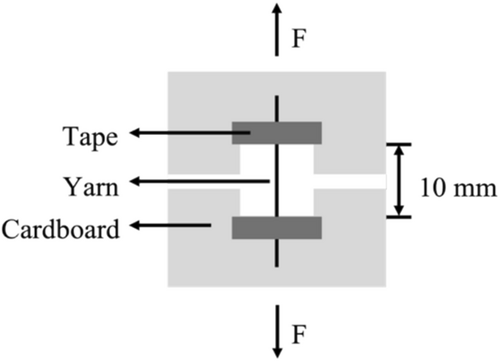
The tensile properties of the crosslinked and non-crosslinked collagen yarns under both dry and hydrated conditions were measured on an MTS Criterion 43 tensile tester with a 50-N load cell following the protocol outlined in our previous study [15]. The dry samples were conditioned in a desiccator for 24 h before testing, and the hydrated samples were then immersed in 1× phosphate buffered saline (PBS, Cytiva HyClone) at room temperature for 2 h before testing. To achieve the 10-mm gauge length, a 40-mm long specimen was mounted on a 30 mm × 30 mm cardboard frame with a 10 mm × 10 mm window. The cardboard frame was mounted between the flat clamps of the tensile tester, and both sides of the frame were cut to isolate the yarn specimen before testing (Figure 2). The yarns were tested to failure under uniaxial tension using a crosshead speed of 10 mm/min. The ultimate tensile force was measured and recorded at the failure point. The chosen 10-mm gauge length ensures accurate measurement of the yarn's mechanical properties over a small, representative section, while the 10 mm/min testing speed reflects loading conditions typically observed in tendon and ligament repair applications, where moderate loading rates are crucial for mimicking physiological stress. Additionally, this speed ensures failure occurs within 20–40 s, as required by ASTM D2256—Standard Test Method for Tensile Properties of Yarns by the Single-Strand Method [20].
2.3 Degradation Evaluation
The resistance to enzymatic degradation of the genipin-crosslinked collagen (Gen-Col) yarns, the non-crosslinked collagen (Col) yarns, and the polylactic acid (PLA) yarns (146 denier, 72 filament, Xinxiang Sunshine Textile Company Ltd., Henan, Chian) was evaluated by immersing the yarns in 1× PBS (pH = 7.4) containing 100 μg/mL collagenase type I [21] and incubating at 37°C for up to six weeks. The Gen-Col yarns were crosslinked using 1.0% genipin at 37°C for 72 h, based on the previous optimization experiment. The degradation solution was renewed every two weeks. The yarn mass and tensile properties were measured under both dry and hydrated conditions at 0, 2, 4, and 6 weeks of degradation.
2.3.1 Mass
2.3.2 Ultimate Tensile Load
Using the tensile test method outlined above, a 40-mm length of each yarn was used to measure the maximum tensile performance after 0, 2, 4, and 6 weeks of degradation. At each time point, the ultimate load was determined for each group under both dry and hydrated conditions (n = 4 per condition).
2.4 In Vitro Biocompatibility
2.4.1 Tendon Cell Harvest
Primary tendon cells were harvested from the Achilles tendons of Sprague Dawley rats aged 2 to 4 weeks old. After rinsing in sterile 1× PBS containing 10% penicillin–streptomycin, the tendons were cut into pieces measuring approximately 1 mm × 1 mm using surgical scissors. The tendon pieces were placed in serum-free Dulbecco's Modified Eagle Medium (DMEM) containing 2 mg/mL type 1 collagenase and incubated in a 37°C water bath overnight. To isolate the tendon cells, the solution was filtered through a 70-μm mesh cell strainer and centrifuged at 2000 rpm for 3 min. The supernatant liquid was discarded. The tendon cells were resuspended in growth medium composed of 89% DMEM, 10% fetal bovine serum (Corning Inc., Corning, NY) and 1% penicillin–streptomycin. The cells were plated in T75 flasks at a density of 2.1 × 106 cells and cultured under standard conditions of 37°C and 5% CO2. The culture medium was changed every other day. When the cells in each flask reached confluence, they were trypsinized and sub-cultured in a new T75 flask under the same culture conditions to promote proliferation.
2.4.2 Yarn Preparation and Cell Seeding
Biocompatibility was evaluated for four yarn groups: non-crosslinked collagen yarns, Gen-Col yarns, PLA yarns, and collagen-coated PLA (Col-PLA) yarns. The Gen-Col yarns were prepared using the previously described protocol by immersing the collagen yarns in a 1.0% genipin solution at 37°C for 72 h. The Col-PLA yarns were prepared by coating PLA yarns with 500 μg/mL of type I collagen (RatCol, Advanced BioMatrix, Carlsbad, CA) at 4°C for 2 h.
The yarns were cut into 30-cm lengths, and the specimens were sterilized in an Anprolene AN74i ethylene oxide sterilizer (Andersen Products Inc., Haw River, NC) for a period of 12 h, followed by at least 2 h of aeration. The yarn samples were placed into 24-well culture plates, with 3 yarn specimens per group for each assay. Tendon cells were seeded at a density of 2 million cells per well by adding 20 μL of a cell suspension at a concentration of 100 million cells/mL. The samples were incubated for 30 min at 37°C and 5% CO2 to allow for cell attachment. The samples were then moved to a new 24-well culture plate, and 1 mL culture medium was added to each well. The culture medium was changed every other day throughout the 7-day cell culture experiment.
2.4.3 Cell Viability
Cell viability was monitored by a Live/Dead assay (Invitrogen R37601, Thermo Fisher Scientific), which was performed on Days 1, 3, 5, and 7 (n = 3 per group). The cell culture medium was replaced by a diluted Live/Dead reagent in a 1:1 ratio with 1× PBS, and the cells were incubated in the dark at 37°C and 5% CO2 for 15 min. The cells were then imaged at a magnification of 4× using the green fluorescent protein (GFP) channel and the Texas Red channel (EVOS FL Auto 2 Cell Imaging System, AMAFD2000, Thermo Fisher Scientific). After imaging, the samples were washed three times with sterile 1× PBS, and 1 mL of fresh growth medium was added to each well before returning the samples to the incubator for subsequent Live/Dead assays.
2.4.4 Cell Proliferation
Cell proliferation was assessed using an alamarBlue assay (Invitrogen DAL1100, ThermoFisher Scientific) (n = 3 per group) over a period of 7 days. On Days 1, 3, 5, and 7, the cell culture medium was replaced by growth medium with 10% alamarBlue reagent, and the cells were incubated in the dark at 37°C and 5% CO2 for 4 h. The fluorescence of each sample was measured on a Tecan GENios microplate reader (Tecan Trading AG, Switzerland) using an excitation wavelength of 550 nm and an emission wavelength of 590 nm. Three replicates were performed on each yarn. Growth medium with 10% alamarBlue was used as the negative control, and autoclaved growth medium with 10% alamarBlue was used as the positive control. The samples were then washed three times with 1× PBS, and 1 mL of fresh growth medium was added to each well before returning the samples to the incubator for subsequent alamarBlue assays.
2.5 Statistical Analysis
Statistical analysis was performed using Prism 9 software (GraphPad Software Inc., San Diego, CA) with a significance level of 0.05. Data are reported as mean ± standard deviation. For the crosslinking evaluation, the degree of crosslinking and swelling ratio were compared across genipin concentrations (0.1%, 0.5%, 1.0%) and crosslinking times (2, 12, 24, 72 h) using one-way analysis of variance (ANOVA) with Tukey's adjustments for multiple comparisons and across crosslinking temperature (20°C, 37°C) using unpaired t-tests. The ultimate tensile load was compared across the hydrated condition (dry, hydrated) and each of the other conditions (genipin concentration, crosslinking time, and crosslinking temperature) using a two-way ANOVAs with Tukey's adjustments for multiple comparisons.
For the degradation study, yarn mass and ultimate tensile load were compared across yarn type (Col, Gen-Col, PLA) and degradation time (0, 2, 4, 6 weeks) using a two-way ANOVA with Tukey's adjustments for multiple comparisons. For the tendon cell proliferation evaluation, tendon cell number was compared across yarn type (Col, Gen-Col, PLA, Col-PLA) and culture time (1, 3, 5, 7 days) using a two-way ANOVA with Tukey's adjustments for multiple comparisons.
3 Results
3.1 Crosslinking Condition Optimization
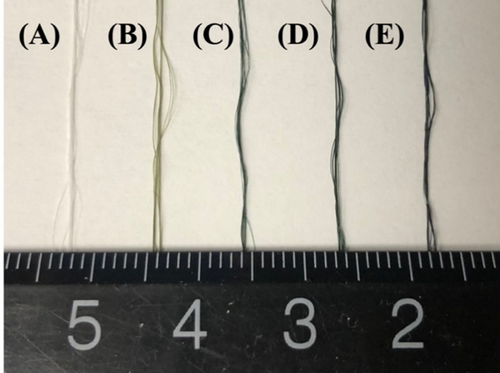
The original color of the non-crosslinked collagen yarns was white. After being crosslinked with genipin, the collagen yarns turned blue, with deeper shades of blue for longer crosslinking times (Figure 4).
3.1.1 Degree of Crosslinking
The degree of crosslinking was significantly greater with higher genipin concentration, higher crosslinking temperature, and longer crosslinking time (Table 2). When analyzing crosslinking temperature, while keeping genipin concentration (0.5%) and crosslinking time (12 h) constant, a 455% greater degree of crosslinking was achieved when the yarns were crosslinked at 37°C compared to 20°C (p < 0.0001). When analyzing genipin concentration, while keeping crosslinking temperature (37°C) and time (12 h) constant, the degree of crosslinking with 1.0% genipin was 153% higher compared with 0.1% genipin (p < 0.0001) and 45% higher compared with 0.5% genipin (p < 0.0001). When analyzing the crosslinking time, while maintaining genipin concentration (0.5%) and crosslinking temperature (37°C), the degree of crosslinking for collagen yarns crosslinked for 72 h was 516% higher compared to 2 h (p < 0.0001), 87% higher compared to 12 h (p < 0.0001), and 51% higher compared to 24 h (p < 0.0001).
| Group | Crosslinking conditions | Crosslinking degree (%) | Swelling ratio (%) | Ultimate load (N) | |||
|---|---|---|---|---|---|---|---|
| Genipin (%) | Temp (°C) | Time (h) | Dry | Hydrated | |||
| Control | 0.0 | 20 | 12 | — | 1241.0 ± 206.5 | 3.7 ± 0.3 | 0.1 ± 0.0 |
| 1 | 0.5 | 20 | 12 | 2.2 ± 0.9 | 293.8 ± 6.3 | 4.5 ± 0.5 | 0.8 ± 0.1 |
| 2 | 0.5 | 37 | 12 | 12.2 ± 0.6 | 127.3 ± 9.1 | 4.5 ± 0.3 | 1.2 ± 0.1 |
| 3 | 0.1 | 37 | 12 | 7.0 ± 0.4 | 133.7 ± 12.8 | 4.2 ± 0.4 | 1.1 ± 0.1 |
| 4 | 1.0 | 37 | 12 | 17.7 ± 3.2 | 79.0 ± 5.1 | 4.7 ± 0.3 | 1.5 ± 0.1 |
| 5 | 0.5 | 37 | 2 | 3.7 ± 0.8 | 152.6 ± 2.8 | 4.7 ± 0.4 | 0.8 ± 0.1 |
| 6 | 0.5 | 37 | 24 | 15.1 ± 0.5 | 123.2 ± 6.4 | 4.9 ± 0.4 | 1.2 ± 0.2 |
| 7 | 0.5 | 37 | 72 | 22.8 ± 0.5 | 90.5 ± 4.1 | 4.8 ± 0.3 | 1.8 ± 0.1 |
3.1.2 Swelling Ratio
The swelling ratio of the wet-spun collagen yarns was significantly lower with higher crosslinking temperature, higher genipin concentration, and longer crosslinking time (Table 2). The swelling ratio was significantly lower when the collagen yarns were crosslinked with genipin compared to the control non-crosslinked collagen yarns. The collagen yarns crosslinked with 0.5% genipin at 20°C for 12 h had a 76% lower swelling ratio compared to the control yarns (p = 0.015). When analyzing crosslinking temperature, the swelling ratio was 57% lower for yarns crosslinked at 37°C compared to 20°C (p < 0.0001). When analyzing genipin concentration for the yarns crosslinked at 37°C for 12 h, the swelling ratio was 41% lower for 1% versus 0.1% genipin concentration (p = 0.0010) and 38% lower for 1% versus 0.5% genipin concentration (p = 0.0020). When analyzing crosslinking time for the yarns crosslinked with a 0.5% genipin concentration at 37°C, for yarns crosslinked for 72 h, the swelling ratio was 41% lower compared with 2 h (p < 0.0001), 29% lower compared with 12 h (p = 0.0004), and 27% lower compared with 2 h (p = 0.0008).
Based on these results, the wet-spun collagen yarns crosslinked with 1.0% genipin at 37°C for 72 h will provide the optimal results in terms of a higher degree of crosslinking, a reduced swelling ratio, and a stronger tensile strength. As a result, yarns exposed to these conditions were used in the subsequent degradation study.
3.1.3 Ultimate Tensile Load
The ultimate tensile load was lower for the collagen yarns tested under hydrated conditions compared with yarns tested under dry conditions (p < 0.0001) for both crosslinked and non-crosslinked samples regardless of the crosslinking conditions (Table 2). The ultimate tensile load of the wet-spun collagen yarns under hydrated conditions was significantly stronger for longer crosslinking times but was not affected by crosslinking temperature or genipin concentration. The ultimate tensile load of the collagen yarns was enhanced with genipin crosslinking; compared with the non-crosslinked collagen yarns, collagen yarns crosslinked with 0.5% genipin at 20°C for 12 h had a 22% higher ultimate tensile load compared with the control sample under dry conditions (p = 0.0026) and a 7-fold higher tensile load compared with the control sample under hydrated conditions (p = 0.0062). When analyzing crosslinking temperature, ultimate tensile load was not significantly different for yarns crosslinked at 20°C and 37°C. Likewise, when analyzing genipin concentration for yarns crosslinked at 37°C for 12 h, ultimate tensile load was not significantly different for 1.0%, 0.5%, and 0.1% genipin concentration samples under either dry or hydrated conditions. When analyzing crosslinking time for yarns crosslinked with 0.5% genipin at 37°C, ultimate tensile load under hydrated conditions was 125% greater for yarns crosslinked for 72 h compared to yarns crosslinked for 2 h (p < 0.0001), 50% greater for 24 h versus 2 h (p = 0.016), and 50% greater for 12 h versus 2 h (p = 0.038).
3.2 Degradation Evaluation
3.2.1 Mass
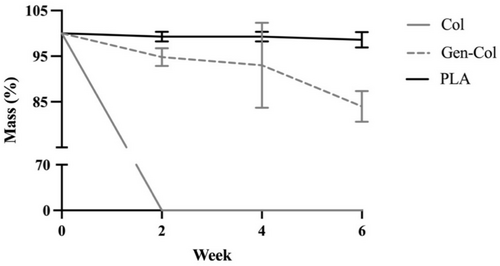
Enzymatic degradation with collagenase over six weeks had the most significant impact on non-crosslinked collagen yarns, with less impact on the genipin-crosslinked collagen yarns and minimal impact on the polylactic acid yarns (Table 3, Figure 5). Since no intact non-crosslinked collagen yarns were found in the degradation solution after 2 weeks, we assumed they were completely degraded within that period, and a 0% retained mass was recorded for this sample for Weeks 2, 4, and 6. Genipin-crosslinked collagen yarns experienced much less degradation, with 94.8% retained mass after 2 weeks that was not significantly different from Week 0, and then slightly reduced mass to 93.0% at Week 4 (p = 0.0077) and 84.0% at Week 6 (p < 0.0001). PLA yarns experienced no significant changes in retained mass between Week 0 and Week 6, with retained mass values of 99.3% at Week 2 and Week 4 and 98.6% at Week 6.
| Group | Week | |||
|---|---|---|---|---|
| 0 | 2 | 4 | 6 | |
| Col | 100.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Gen-Col | 100.0 ± 0.0 | 94.8 ± 1.9 | 93.0 ± 9.3 | 84.0 ± 3.4 |
| PLA | 100.0 ± 0.0 | 99.3 ± 1.1 | 99.3 ± 1.1 | 98.6 ± 1.7 |
3.2.2 Ultimate Tensile Load
3.2.2.1 Dry Conditions
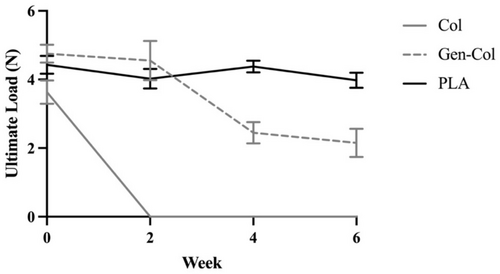
During the 6 weeks of enzymatic degradation, the ultimate tensile load for the samples measured under dry conditions decreased for both non-crosslinked collagen and genipin-crosslinked collagen yarns but remained constant for PLA yarns, which maintained over 90% of the initial ultimate load at Week 6 (Table 4, Figure 6). Since none of the Col yarns were intact when examined at Week 2, the ultimate tensile load was recorded as 0 N for all samples in this group at Weeks 2, 4, and 6. At Week 2, the ultimate tensile load was similar between Gen-Col and PLA yarns (p > 0.05) and then decreased significantly in Gen-Col yarns, with a 46% lower ultimate tensile load at Week 4 compared to Week 2 (p < 0.0001) and values that were 1.8-fold lower compared to PLA yarns at both Week 4 (p < 0.0001) and Week 6 (p < 0.0001).
| Group | Week | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | ||
| Dry | Col | 3.6 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Gen-Col | 4.8 ± 0.3 | 4.6 ± 0.6 | 2.5 ± 0.3 | 2.2 ± 0.4 | |
| PLA | 4.4 ± 0.3 | 4.0 ± 0.3 | 4.4 ± 0.2 | 4.0 ± 0.2 | |
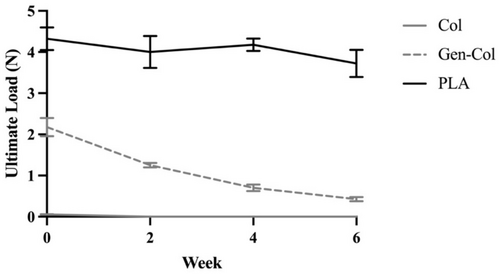
| Hydrated | Col | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Gen-Col | 2.2 ± 0.2 | 1.3 ± 0.1 | 0.7 ± 0.1 | 0.4 ± 0.1 | |
| PLA | 4.3 ± 0.3 | 4.0 ± 0.4 | 4.2 ± 0.2 | 3.7 ± 0.3 | |
3.2.2.2 Hydrated Conditions
Similar results were observed for ultimate tensile loads measured under hydrated conditions throughout the 6-week enzymatic degradation study. Non-crosslinked collagen yarns had no measurable tensile strength, and genipin-crosslinked collagen yarns lost substantial amounts of tensile strength, but PLA yarns were able to retain tensile strength (Table 4, Figure 7). For Col yarns, the initial ultimate tensile load was only 0.1 N, and since none of these yarns were intact at Week 2, the ultimate load was recorded as 0 N for all samples at Weeks 2, 4, and 6. Gen-Col yarns retained only 18% of the initial ultimate tensile load at Week 6 of enzymatic degradation (p < 0.0001). PLA yarns retained 86% of the initial ultimate tensile load at Week 6 (p = 0.0032), suggesting these yarns exhibit good mechanical stability during the critical early phases of tendon repair when mechanical support is essential. A major difference from the results measured under dry conditions was that the initial ultimate tensile load was about 2-fold lower for Gen-Col yarns compared to PLA yarns (p < 0.0001). In summary, enzymatic degradation had the greatest impact on Gen-Col yarns, with over 9-fold lower ultimate tensile load at Week 6 compared to PLA yarns (p < 0.0001).
3.3 In Vitro Biocompatibility
3.3.1 Cell Viability
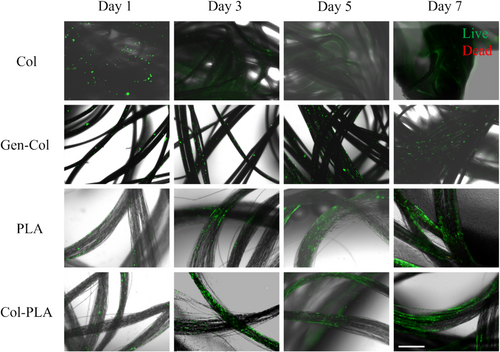
Tendon cell viability differed for the different yarns during the 7 days of static cell culture (Figure 8). On Day 1, more live cells, stained green, were observed to be attached to the non-crosslinked collagen yarns than to the other yarns. The extent of live cell staining increased over 7 days for genipin-crosslinked collagen, PLA, and collagen-coated PLA yarns. In contrast, Col yarns showed a downward trend in cell viability over the 7-day period, possibly due to the limited surface area available for nutrients, oxygen, and CO2 exchange as the yarns clumped together. On Day 7, the live cell staining was greater on PLA and Col-PLA yarns compared to Col yarns, indicating a greater density of viable tendon cells was attached.
3.3.2 Cell Proliferation
While tenocytes proliferated on all four yarn groups over the 7 days of culture, the growth rate varied depending on the material and surface treatment (Table 5, Figure 9). At Day 1, more tendon cells were attached to the collagen yarns than to the PLA yarns (p = 0.0016). In particular, 60% more cells were attached to the collagen-coated (Col-PLA) yarns compared to PLA yarns. Between Day 1 and Day 3, tendon cells proliferated rapidly on all four yarn groups (p < 0.0001), but particularly on the Gen-Col, PLA, and Col-PLA yarns. They continued to proliferate through Day 7, when the tendon cell number for Col-PLA yarns was about 2.4-fold greater than for Col yarns (p < 0.0001) and was comparable to both Gen-Col and PLA yarns.
| Group | Culture day | |||
|---|---|---|---|---|
| 1 | 3 | 5 | 7 | |
| Col | 71,200 ± 12,200 | 95,300 ± 20,000 | 153,100 ± 1600 | 149,100 ± 50,900 |
| Gen-Col | 56,100 ± 13,100 | 124,400 ± 22,800 | 281,700 ± 49,700 | 316,500 ± 40,300 |
| PLA | 35,300 ± 10,800 | 169,400 ± 38,700 | 244,200 ± 37,300 | 332,200 ± 24,700 |
| Col-PLA | 56,300 ± 6500 | 149,300 ± 6900 | 220,000 ± 67,600 | 350,400 ± 37,000 |

4 Discussion
An ideal material for a tendon repair graft is expected to have excellent mechanical properties, maintenance of mechanical strength during post-implantation time, and biocompatibility that will not lead to a negative inflammatory response or immune reaction. In this study, we stabilized the structure of wet-spun collagen yarns by genipin crosslinking and found the tensile properties of collagen yarns were enhanced after being crosslinked, especially under the optimized condition of 1.0% genipin at 37°C for 72 h. At the same time, the tensile properties and resistance to enzymatic degradation for Gen-Col yarns were not as strong as for yarns made from the synthetic polymer PLA, which is a raw material often used for fabricating rotator cuff grafts. We also coated collagen on PLA yarns to improve their biological performance. By seeding tenocytes on both collagen yarns and collagen-coated PLA yarns, we demonstrated that collagen-coated PLA yarns had a comparable biological performance to collagen yarns in terms of cell adhesion and proliferation. This study has provided a reference baseline for selecting the materials and surface coatings for tendon repair scaffolds to be used for tendon tissue engineering.
Stabilizing the collagen structure is necessary for wet-spun collagen yarns to achieve their maximum tensile strength and minimize the degree of swelling [22]. In our optimization study, we found that the best properties for multifilament collagen yarns were achieved with a 1.0% genipin concentration, crosslinking at 37°C for 72 h. These conditions resulted in a 1.3-fold increase in ultimate tensile load of 4.8 ± 0.3 N under dry conditions and a 22-fold increase of 2.2 ± 0.1 N under hydrated conditions compared to non-crosslinked collagen yarns. These tensile strengths were similar to those reported in a previous study, which found ultimate loads of 3.9 N under dry conditions and 1.5 N under hydrated conditions for wet-spun collagen yarns crosslinked with glutaraldehyde [6]. Our results agree with previous studies in which electrospun chitosan nanofibers and freeze-dried collagen scaffolds were crosslinked with genipin. They reported that when the degree of crosslinking increased from 10% to 30% and the crosslinking temperature increased from 20°C to 37°C, the swelling ratio decreased from 20% to 17.5%. In addition, by increasing the genipin concentration from 0.1% to 1.0%, the tensile strength of the chitosan nanofibers was enhanced, and the swelling ratio was decreased [21, 23].
The ability to resist enzymatic degradation also plays a pivotal role in the success of scaffold materials used for tendon repair, since a mismatch between the rates of yarn absorption and wound healing will cause the failure of the repair. By stabilizing the structure with genipin crosslinking, collagen yarns maintained over 90% of their initial mass after 2 weeks, displaying a superior resistance to degradation compared to non-crosslinked collagen yarns, which were completely degraded within 2 weeks. In addition, the genipin-crosslinked collagen yarns in this study exhibited a better degradation resistance compared to two previous studies, one in which glutaraldehyde-crosslinked, electrochemically aligned collagen yarns were exposed to 0.01 M PBS solution for the same 6-week time period [24], and another in which genipin-crosslinked collagen scaffolds were exposed to 100 μg/mL collagenase type I solution for 12 h [21]. Even after 6 weeks of degradation, our optimized genipin-crosslinked collagen yarns maintained 84% of their initial mass and, more importantly, maintained 46% of their initial tensile strength under dry conditions, further demonstrating that genipin crosslinking stabilizes the collagen structure. However, even with optimized crosslinking conditions, the tensile strength of the genipin-crosslinked collagen yarns after 6 weeks of degradation was 45%–89% lower compared to the PLA yarns, which retained at least 86% of their initial tensile strength under both dry and hydrated test conditions. Differences in the degradation rate are likely related to different mechanisms of degradation for these two materials. When collagen is exposed to proteases, such as type 1 collagenase in this study, the protein structure is broken down, while PLA degradation results from hydrolysis of the ester bonds [25]. Although the human body produces enzymes like lipase that are capable of catalyzing ester bond cleavage along the polymer chain, lipase is released by the pancreas, mouth, and stomach and would not be delivered to the tendon or ligament repair site [26, 27]. Therefore, when considering these two degradation profiles, PLA yarns are likely more suitable than genipin-crosslinked collagen yarns for manufacturing textile-based scaffolds for tendon tissue engineering.
Beyond the issue of degradation, and because collagen is the main component in native tendon ECM, a major consideration for using collagen or collagen-coated multifilament yarns in the fabrication of tendon repair scaffolds is their biological performance. Several previous studies have used genipin-crosslinked collagen scaffolds to culture a variety of cell types, such as mouse fibroblasts and human mesenchymal stem cells. For all of these, the collagen surface showed low cytotoxicity and was amenable to supporting cell adhesion and proliferation [21, 28-30]. In the current study, primary tendon cells attached well to both collagen and PLA multifilament yarns on Day 1 and showed good viability and proliferation through 7 days of culture, also confirming that PLA is non-cytotoxic and well-tolerated by cells. On Day 1, collagen-coated PLA yarns exhibited better cell adhesion compared to the PLA yarn control sample, most likely due to lower surface hydrophobicity and the promotion of cell binding through peptide domains in the collagen coating [31]. The number of tendon cells on Col-PLA yarns was greater than on Col yarns, possibly due to the slower, more controlled degradation of Col-PLA yarns, which provided a more stable environment for cell growth over time, along with enhanced biological performance from the collagen coating. These findings are consistent with a recently published study in which NIH 3 T3 fibroblasts cultured on collagen fibers had higher bioactivity compared to those cultured on PLA fibers [32].
The current study had several limitations. First, the 22.8% ± 0.5% degree of crosslinking of our multifilament collagen yarns was relatively low compared to the ~60% reported in a previous study using the same crosslinking conditions at 37°C for 72 h [33]. This difference is possibly due to the tight multifilament structure of our collagen yarns, which may have impeded the genipin-crosslinking process between interior yarns. Nevertheless, the crosslinked multifilament collagen yarns in the present study had a higher ultimate tensile strength of 4.8 ± 0.3 N compared to 3.8 N for the previously reported crosslinked collagen yarns, which were crosslinked with 2.0% genipin [31]. Second, a 6-week period of enzymatic degradation may not be sufficient to fully test the in vivo performance for rotator cuff tendon repair, because rotator cuff tendon injuries usually take 6 to 12 months to heal. However, assessing the 6-week degradation performance of potential graft materials is critical, because after patients complete the required six weeks of postoperative immobilization, the graft would then be expected to provide sufficient mechanical support as they begin rehabilitative shoulder movements [34]. Third, the current study examined only the biocompatibility of collagen-coated PLA yarns. While we expect the mechanical performance and resistance to enzymatic degradation of these yarns to be similar to those of PLA yarns, these metrics should be assessed directly. Lastly, this study assessed the interaction of primary tendon cells with the yarns only in terms of cell viability and proliferation. Additional in vitro and in vivo studies are needed to fully evaluate the biocompatibility of the yarns, including a hemocompatibility test.
5 Conclusions
This study was the first to crosslink wet-spun multifilament collagen yarns with genipin and to identify how genipin concentration, crosslinking temperature, and crosslinking time enhanced the ultimate tensile strength of the collagen yarns. A greater genipin concentration, higher crosslinking temperature, and prolonged crosslinking time resulted in a greater degree of crosslinking, with improved ultimate tensile strength and reduced swelling ratio under both dry and hydrated conditions. Among the crosslinking conditions tested in this study, crosslinking with 1.0% genipin at 37°C for 72 h was found to be the optimal combination for wet-spun collagen yarns and was subsequently used for the experiments comparing the performance of collagen and PLA multifilament yarns. Compared to genipin-crosslinked collagen yarns, PLA yarns showed superior resistance to enzymatic degradation. They were better able to retain their original mass and exhibited higher ultimate tensile strength after exposure to collagenase type I for 6 weeks, which is a key clinical timepoint for the onset of rehabilitative movements. When coated with collagen, PLA yarns showed excellent biocompatibility and promoted better cell attachment for primary tendon cells. In summary, PLA yarns coated with collagen are a promising material for fabricating scaffolds for rotator cuff tendon repair.
Acknowledgments
This study was financially supported by the Student Research Support Grant, funded by the American Association of Textile Chemists and Colorists (AATCC).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




