Analysis of the potential and mechanism of Ginkgo biloba in the treatment of Alzheimer's disease based on network pharmacology
Abstract
Objective
Study the principle and possible mechanism of Ginkgo biloba in the treatment of Alzheimer's disease (AD) which is based on network pharmacology.
Methods
The potential targets of active ingredients of Ginkgo biloba were collected by Traditional Chinese Medicine Integrated Database platform (TCMSP). TCMSP is a pharmacological system for drug discovery from Chinese herbal medicine. The disease targets of AD were searched and collected by the database of gene-disease associations (DisGeNET) and literature. The obtained targets were standardized by the UniProt database. STING network platform and Cytoscape were used to construct protein-protein interaction network (PPI) of the key targets. According to Materscape, we clarify the possible mechanism of action including Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene Ontology (GO) enrichment analysis.
Results
The compound-target network contains 27 active ingredients, 191 related targets, 18 key targets, including PLAU, HMOX1, TNF, INSR, MPO, MAOB, IGF2, IL1B, ESR1, BCL2, ACHE, BAX, GSK3B, PPARG, SLC2A4, NOS3, CASP3, VEGFA. GO enrichment analysis has got a total of 640 GO items, including 609 biological process (BP) items (95.1%), 16 molecular function (MF) items (2.5%) and 15 cellular component (CC) items (2.4%). After KEGG enrichment, 44 pathways were obtained.
Conclusion
Through the construction of “component-target-pathway”, GO biological function and KEGG pathway enrichment analysis were performed on core targets, and the possibility of Ginkgo biloba for the treatment of AD was explored from multiple targets and pathways, which provided a new approach for multi-target treatment.
Introduction
Alzheimer's disease (AD), whose key symptoms are memory dysfunction and progressive cognitive dysfunction, is the most common neurodegenerative disease. Amyloid-beta (Aβ) deposition and tau protein hyperphosphorylation are pathological characteristics of AD and also the main therapeutic targets (Jeong, et al., 2020). The prevalence of AD increases progressively with age. It is estimated that there will be approximately 50 million AD patients worldwide (Armstrong RA, 2019) with approximately one in three to four people over the age of 85 having AD. More female than male suffer from dementia (27 million vs 16.8 million) which is currently the fifth leading cause of death in the world. Risk factors for AD include high body mass index (BMI), high blood sugar, smoking, and high-sugar beverage intake (GBD 2016 Dementia Collaborator, 2019). However, there are still multiple opinions on the pathogenesis of AD, including the causes of amyloid hypothesis, tau protein transmission hypothesis, cholinergic hypothesis, inflammation hypothesis, mitochondrial cascade hypothesis, calcium homeostasis hypothesis, neurovascular hypothesis, metal ion hypothesis, and lymphatic system hypothesis. At present, four drugs approved by the U.S. Food and Drug Administration (FDA) for AD are applied in the treatment of clinical AD, the main purpose of which is to restore physiological acetylcholine (Ach) levels. Acetylcholine esterase (AChE) inhibitors are to inhibit the hydrolysis of acetylcholine, including Donepezil, Rivastigmin, and Galanthamine base, all of which belong to this category of drugs. Memantine, through non-competitive antagonism of N-Methyl-D-aspartic acid (NMDA) receptors, current flow (special calcium) is prevented and the excitotoxicity effect of glutamate is reduced. However, there is no clear and effective treatment for patients with advanced AD (Zagórska and Jaromin, 2020). Therefore, the AD multiple pathogenesis hypothesis and the imperfect single-target drug therapy require further exploration of multi-target comprehensive treatment.
Ginkgo is an ancient perennial tree of the genus Ginkgo, which survived after the Fourth Glacier. Ginkgo biloba, seed kernel and outer seed coat have medicinal value and have been used as medicine for more than 600 years, the earliest record of which could be traced in Sheng Nong's Herbal Classic. China is the birthplace of ginkgo which accounts for about 70% of the world's resources. At present, more than 160 compounds have been found in Ginkgo biloba leaves and the main effective ingredients are Ginkgo flavonoids, Ginkgolide, and Ginkgolic acid (Yang HP and Gao R, 2017; Yang Y, et al. 2016).
The medicinal value of Ginkgo biloba was confirmed in Song Dynasty (960-1279), and was recorded in many kinds of herbal medicine books, such as Chinese Materia Medicain in Yuan Dynasty, Compendium of Materia Medicain in Ming Dynasty, and Mu Cao Feng Yuan in Qing Dynasty. Moreover, traditional Chinese medicine believes that Ginkgo biloba has effects of anti-oxidation, reducing blood viscosity, improving microcirculation, improving memory, increasing cerebral blood flow, protecting microvascular smooth muscle cells, reducing brain damage, improving neuroplasticity, improving neurodegenerative diseases, anti-radiation (Wang Y and Yang YF, 2001; Zhang PF, et al. 2017).
At present, there are a variety of ginkgo biloba prescriptions widely used in clinical practice, suggesting that it has an effect on improving neurodegeneration and cognitive impairment, while the mechanism remains unclear. We used network pharmacology methods to explore the drug-target-disease rationale and find new approaches for the treatment of AD in this research.
Materials and methods
Obtaining and screening the targets of Ginkgo biloba
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database (https://www.tcmspw.com/tcmsp.php) was used to search effective ingredients of Ginkgo biloba, and then filter under the following conditions: Oral Availability (OB) ≥ 30 %, Drug Likeness (DL) ≥ 0.18. The relevant target information was standardized through the UniProt platform (https://www.uniprot.org).
Collection and pretreatment of target proteins for AD
The related targets of AD were obtained through the DisGeNET database (https://www.disgenet.org), with AD as a key word. A total of 99 core targets were screened using the criterion of Score gad ≥ 0.3.
Construction and analysis of PPI network
The core targets of Ginkgo biloba from the TCMSP database and the core targets of AD from DisGeNET were imported into Venn to generate a Venn diagram, and the targets of the two interactions were obtained. In order to clarify the correlation between the common targets of Ginkgo biloba and AD, the common targets were imported into the STRING network platform (https://string-db.org/cgi/input.pl), and the protein type was set to “Homo sapiens” to construct PPI network then export related tsv data. Using the Cytoscape 3.6.1 software to process the target, calculating the degree value, and determining the core target of Ginkgo biloba treatment for AD according to the value.
KEGG pathway and GO bioinformatics analysis
Gene Ontology (GO) is an ontology widely used in the field of bioinformatics, including three aspects of biology: cellular component (CC), molecular function (MF), and biological process (BF). Kyoto Encyclopedia of Genes and Genomes (KEGG) is a database resource which was used to find the advanced functions and practicality of biological systems (such as cells, organisms, and ecosystems) from genomic and molecular level information. The common target of Ginkgo biloba and AD was imported into the metascape platform (http://metascape.org/gp/index.html#/main/step1), and set the species to “H.sapiens”. Biological analysis and KEGG pathway analysis of the target were carried out, and the mechanism and process of the target protein in BP, CC and MF were discussed.
Results
The target of Ginkgo biloba and AD
A total of 27 active ingredients and 191 corresponding targets were selected by the TCMSP platform. The basic information of the active ingredients of Ginkgo biloba is shown in Table 1. The target was standardized using the UniProt platform, and 191 target genes were obtained (Figure 1). Through the DisGeNET database, AD genes were obtained and a total of 99 target genes were screened out (Table 2).
| Syringetin | Diosmetin |
|---|---|
| Luteolin-4′-glucoside | Flavoxanthin |
| Isogoycyrol | sesamin |
| Ginkgolide M | Mandenol |
| ginkgolide J | bis[(2S)-2-ethylhexyl] benzene-1,2-dicarboxylate |
| ginkgolide C | (+)-catechin |
| ginkgolide B | Stigmasterol |
| Bilobalide | kaempferol |
| Laricitrin | beta-sitosterol |
| Linolenic acid ethyl ester | isorhamnetin |
| Genkwanin | quercetin |
| campest-5-en-3beta-ol | (-)-catechin |
| Chryseriol | luteolin |
| Ethyl oleate (NF) |
| ABCA7 | EPHA1 | INPP5D | SLC2A4 | LEP |
|---|---|---|---|---|
| ACE | CST3 | SOD2 | CHRNB2 | INSR |
| TREM2 | BDNF | MS4A4A | SNAR-I | HMOX1 |
| BCHE | TNF | PLCG2 | CASP3 | INS |
| CLU | CR1 | PRNP | MIR3622B | IGF1R |
| MTHFR | PCDH11X | RELN | CALM1 | IGF2 |
| BIN1 | NOS3 | DHCR24 | SLC30A4 | HFE |
| MAPT | CYP46A1 | TF | MIR766 | MPO |
| PSEN1 | TFAM | CHRNA7 | MIR4467 | AMFR |
| CD33 | CD2AP | HLA-DRB5 | VEGFA | SLC30A6 |
| PICALM | IGF1 | IQCK | TPI1 | ENO1 |
| APOE | NECTIN2 | NCSTN | PGRMC1 | MIR708 |
| IDE | PLAU | ARC | TPP1 | F2 |
| APP | APOC1 | BAX | ADAMTS1 | WWOX |
| TOMM40 | ADAM10 | CRH | DPYSL2 | ABI3 |
| BACE1 | ESR1 | MIR505 | EIF2S1 | ATP5F1A |
| PPARG | GSK3B | BCL2 | MAOB | MIR375 |
| PSEN2 | ACHE | CASS4 | MIR296 | NPY |
| A2M | IL1B | PYY | MIR146A | GAPDHS |
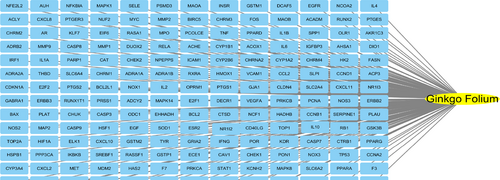
191 target gene of Ginkgo Folium.
Construction of Venn graph
The genes of AD and Ginkgo biloba were introduced into Venn to obtain an intersection (Figure 2). The blue circle on the left represents the target gene of AD, the red circle on the right represents the target gene of Ginkgo biloba, and the overlapping part is the common target of the two. The overlapping target genes contain 18 such as plasminogen activator, urokinase (PLAU), heme oxygenase 1 (HMOX1), tumor necrosis factor (TNF), insulin receptor (INSR), myeloperoxidase (MPO), monoamine oxidase B (MAOB), insulin like growth factor 2 (IGF2), interleukin 1 beta (IL1B), estrogen receptor 1 (ESR1), B-cell CLL/lymphoma 2 (BCL2), acetylcholinesterase (AChE), BCL2-associated X (BAX), glycogen synthase kinase 3 beta (GSK3B), peroxisome proliferator activated receptor gamma (PPARG), solute carrier family 2 member 4 (SLC2A4), nitric oxide synthase 3 (NOS3), caspase 3 (CASP3) and vascular endothelial growth factor A (VEGFA).
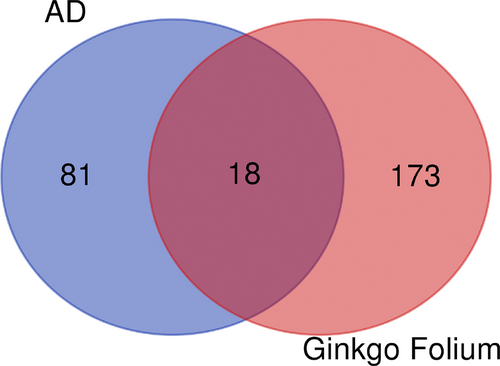
Ginkgo folium and AD target gene Venn figure.
PPI interaction network
The PPI network of Ginkgo biloba core gene for AD treatment was established by string database, including 18 points and 72 edges (Figure 3). The relevant data of core targets were imported into Cytoscape, and further filter the degree value and the Betweenness Centrality value. Using the screening results, the PPI network was constructed, and a total of 11 central targets were screened (Figure 4). The size of the target and the thickness of the line can clearly show the tightness of the important target and the line. From the visual analysis of PPI network, VEGFA, TNF, CASP3, PPARG, IL1B, NOS3, HMOX1, ESR1, MPO, AChE, play a pivotal role in Ginkgo biloba intervention in AD, link other core targets together, and play a synergistic effect.
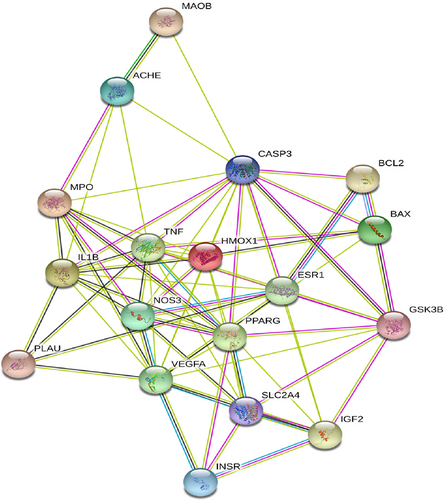
PPI network diagram of Ginkgo biloba and AD.
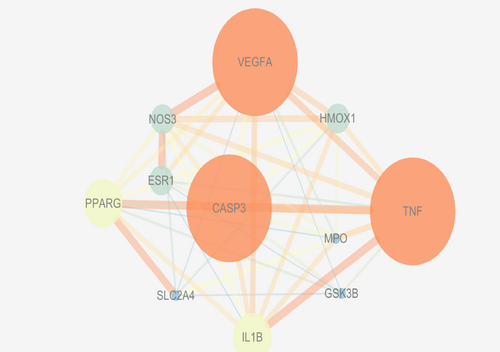
Ginkgo biloba and AD PPI network after screening.
KEGG and GO enrichment analysis
Perform analysis of GO function (Figure 5) and KEGG pathway (Figure 7) analysis using Metascape database.GO analysis includes three parts: biological process (BP), cellular component (CC) and molecular function (MF), enriching AD related genes, and setting a threshold P <0.05. A total of 640 GO items were collected, including 609 BP items, accounting for 95.1%, 16 MF items, accounting for 2.5%, and 15 CC items, accounting for 2.4% (Figure 6). Among the BP, developmental process involved in reproduction, reproductive structure development, reproductive system development, response to toxic substance, extrinsic apoptotic signaling pathway, positive regulation of cell migration, positive regulation of cell motility, positive regulation of cellular component movement, apoptotic signaling pathway, and positive regulation of locomotion are in the front position (Figure 5). Among the CC, membrane raft, membrane microdomain, membrane region, caveola, plasma membrane raft, external side of plasma membrane, side of membrane, mitochondrial outer membrane, organelle outer membrane, outer membrane are in the front position (Figure 5). Among the MF, protein homodimerization activity, protein domain specific binding, protease binding, cytokine receptor binding, receptor ligand activity, receptor regulator activity, cofactor binding, oxidoreductase activity, transcription factor binding, cytokine activity are in the front position (Figure 5).
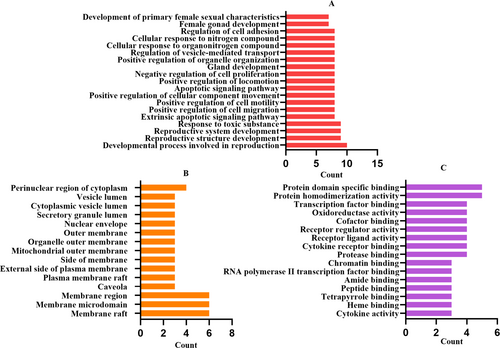
GO enrichment analysis. (A) Biological process. (B) Cellular component. (C) Molecular function.
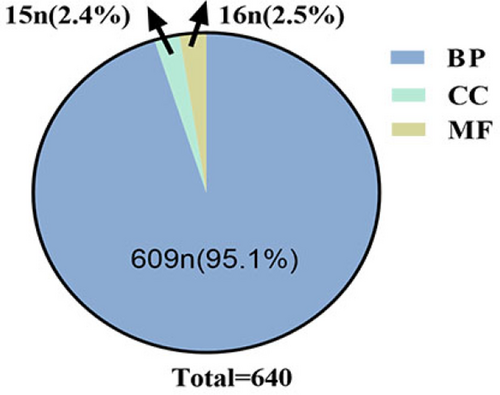
The total amount and percentage of biological process (BP), molecular function (MF) and cellular component (CC).
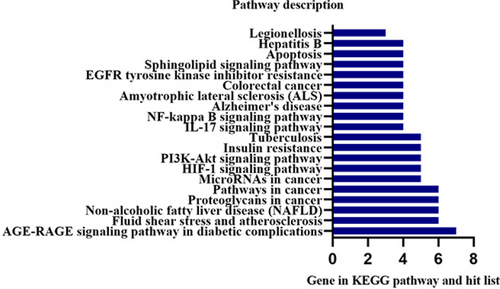
KEGG pathway.
A total of 44 pathways were obtained after KEGG enrichment. Advanced glycation end (AGE)-Receptor of Advanced Glycation End products (RAGE) signaling pathway in diabetic complications, fluid shear stress and atherosclerosis, non-alcoholic fatty liver disease (NAFLD), proteoglycans in cancer, pathways in cancer, microRNAs in cancer, hypoxia-inducible factor-1 (HIF-1) signaling pathway, phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway, insulin resistance, tuberculosis, are in the front position (Figure 7).
Discussion
AD with a variety of pathophysiological mechanisms is a chronic degenerative disease. Neural plaques and neurofibrillary tangles (NTF) are the pathological features of AD, which is related to the accumulation of amyloid-beta peptide (Aβ) in brain tissue and the cytoskeletal changes caused by the hyperphosphorylation of microtubule-associated tau protein in neurons (De-Paula, et al., 2012). Neural plaques are cleaved from amyloid precursor protein (APP) by β-amyloid and amyloid secretase. At present, the primary treatment of AD still revolves around the relief of symptoms, with cutting-edge therapy of the inhibition of acetylcholinesterase (AChEI), such as Donepezil, Galantamine, etc., which mainly improve cognitive symptoms and weaken the disease. AChEI approved for treatment in the mild and moderate stages of AD (MMSE 26-10), and Memantine (N-Methyl-D-aspartic acid receptor inhibitor), which can effectively bind to AChE, was used in the middle and late stages of AD (Kovács, 2009). However, these drugs can only treat the symptoms of AD. There is no evidence that they can abrogate or reverse the progression of the disease. In addition, it is impossible to predict whether it will be effective for an individual. In the world, there is an AD patient approximately every 3 seconds. By 2019, there were more than 10 million AD patients in China, which is the country with the largest number of patients in the world. It is estimated that China will have 28 million patients in 2050 (Armstrong RA, 2019). According to the theory of traditional Chinese medicine and clinical practice, it is found that Ginkgo biloba has advantages in improving memory and alleviating neurodegenerative diseases, which is consistent with the pathogenesis of AD.
Through the TCMSP database, a total of 27 effective active ingredients of Ginkgo biloba including kaempferol, ginkgolide, syringaresin, luteolin, bilobalide, and quercetin were screened for target information. Through UniProt query, 191 gene abbreviations including VEGFA, MPO, MAOB, AChE, IL6, NOS3, and TNF were obtained. NOS is a nitric oxide synthase, an isoenzyme, which exists in endothelial cells, macrophages, phagocytes and nerve cells. At present, oxidative stress induced by nitric oxide is suggested to be one of the pathogenesis of AD. Furthermore, the abnormal expression of NOS gene is also believed to induce the degeneration of brain neurons and glia. MPO is a myeloperoxidized protein, which is rich in neutrophils. It can catalyze and oxidize chloride ions to generate hypochlorous acid, kill microorganisms in phagocytes, and produce and regulate inflammation in the body. Studies have shown that there is a significant increase in MPO in the peripheral blood of AD patients, which is considered to be related to the progression of AD disease. Besides, MPO plaques and neurofibrillary tangles were found in the frontal cortex and hippocampus of AD patients (Glass, et al. 2010; Wu CY, et al. 2020).
Through DisGeNET database, with AD as the key word, 99 core targets including ATP binding cassette subfamily A member 7 (ABCA7), Angiotensin Converting Enzyme (ACE), triggering receptor expressed on myeloid cells 2 (TREM2), butyrylcholinesterase (BCHE), clusterin (CLU), methylenetetrahydrofolate reductase (MTHFR), bridging integrator 1 (BIN1), microtubule associated protein tau (MAPT), presenilin 1 (PSEN1), CD33 molecule (CD33), phosphatidylinositol binding clathrin assembly protein (PICALM), apolipoprotein E (APOE), insulin degrading enzyme (IDE), APP were screened out. Currently the pathogenesis of AD has not been fully elucidated, and is considered to be related to the cholinergic hypothesis (Bateman, et al., 2012), amyloid hypothesis (Hardy and Higgins, 1992), tau protein transmission hypothesis (Frost, et al., 2009), mitochondrial cascade hypothesis, and Ca2+ homeostasis hypothesis (Kim, et al., 2000), inflammatory hypothesis (Liu, et al., 2019), neurovascular hypothesis, metal ion hypothesis (Abelein, et al., 2015), lymphatic hypothesis.
The acetylcholinergic hypothesis is mainly due to choline deficiency, with AChE and butyrylcholinesterase (BuChE) as the main targets. AChE can degrade acetylcholine between cholinergic synapses, terminate the excitatory effect of neurotransmitters on the postsynaptic membrane, and ensure the correct transmission of nerve signals in the organism. At present, the most commonly used drugs are acetylcholinesterase inhibitors, such as donepezil, galanthamine and so on. However, there are also many reports that the accumulation of anticholinergic drugs is related to poor cognitive ability and functional outcome (Salahudeen, et al., 2015). The characteristic histology of AD is neurofibrillary tangle, which is mainly elicited by phosphorylated tau protein. These lesions can lead to activation of microglia and astrocytes, leading to synaptic loss and neuronal death. GSK3 β is a serine/threonine kinase, which widely exists in mammalian eukaryotic cells. GSK3β can regulate cell apoptosis by affecting signal protein structural proteins and transcription factors. Currently, GSK3 β is selected as a therapeutic target in the research of neurodegenerative diseases, neuropsychiatric diseases, cancer and other major diseases. GSK3 β regulates apoptosis by affecting glucose concentration in blood, mitochondrial permeability and cytochrome c release. It is also an important target for tau protein phosphorylation, which is also the source of tau hypothesis (Toral-Rios, et al., 2020).
MAO-B levels were found to increase in the frontal cortex and hippocampus of AD, which causing reactive oxygen activity (ROS) and hydrogen peroxide to increase, leading to oxidative damage and neurodegeneration. Based on the amyloid hypothesis of AD, the overproduction of Aβ is the result of the destruction of the steady-state process, which regulates the proteolytic cleavage of amyloid precursor protein (APP). The core of the amyloid plaque in the brain of AD patients is composed of fibrils formed by Aβ, which plays a key role in the pathogenesis of AD. It is suggested that MAO-B can regulate the level of a β in the nerve, which may be mediated by γ - secretase, which is also the reason why MAO-B is considered as the target of AD (Schedin-Weiss, et al., 2017). The “component-target-pathway” network prompts the degree of targets such as VEGFA, TNF, CASP3, IL1B, PPARG, NOS3, ESR1, HMOX1, SLC2A4, MPO, GSK3β, IGF2, BAX, ACHE, INSR, PLAU, BCL2, MAOB targets are ranked front position. Combining the above content, it is found that Ginkgo biloba can achieve the purpose of treating AD through core targets such as ACHE, MAOB, GSK3β, NOS3, and MPO. The key targets were analyzed by GO function analysis. The biological functions of 18 main targets were mainly reflected in protein binding, receptor binding, receptor regulation, redox and other processes. Enrichment analysis of KEGG pathway showed that the main biological processes involved in core targets include HIF-1 signaling pathway, NF-kappa B signaling pathway, PI3K-Akt signaling pathway, Apoptosis pathway, etc. The ATP efficiency of the thread body in AD decreases, leading to the increase of ROS and oxidation levels, and the lack of Ca+ buffering capacity, which promotes the release of apoptotic factors and induces apoptosis (Gadhave, et al., 2020). HIF-1 signaling pathway can increase the response of cells to hypoxia, hypoglycemia and oxidative stress by increasing glucose uptake, glycolysis and pyruvate to lactic acid transformation, so as to ensure the supply of intracellular ATP and reduce cell apoptosis, so as to achieve the effect of treating AD (Yu and Blair, 2019).The neuropathological feature of AD is the formation of senile plaques (SP) composed of small peptide Aβ and intracellular neurofibrillary tangles (NFT) (De Strooper, 2010). NF-κB pathway plays an important role in neuronal damage and synaptic plasticity in the central nervous system (Zhang, et al., 2017). In addition, the up-regulation of NF-κB activity induced by Aβ not only promotes the expression of pro-inflammatory factors, but also increases the production of Aβ, thereby participating in the development of AD (Chami, et al., 2012). Therefore, this is one of the reasons why the NF-κB pathway has become a new direction for AD treatment. PI3K-Akt signaling pathway is a downstream signaling pathway of brain-derived neurotrophic factor (BDNF)/tyrosine kinase receptor B (TrkB), which is widely present in cells. Glycogen synthase kinase-3 (GSK-3) is an important downstream target of PI3K-Akt signaling pathway. GSK-3 activation can lead to abnormal phosphorylation of tau protein and accumulation of neurofibrillary tangles, which ultimately leads to learning and memory impairment. These pathways are all related to the cause of AD. Ginkgo biloba affects oxidative and inflammatory responses through multiple core targets including GSK3β, TNF, and IL-1B through multiple pathways, and act on AD with multiple targets to compensate for the lack of single-target drug therapy.
In summary, the network system of Ginkgo biloba-component-target-AD was constructed by using the method of network pharmacology, public database and related software. KEGG pathway and GO biological function analysis of core targets were carried out, and the multi-target mechanism of Ginkgo biloba in the treatment of AD was explored, which provided new ideas for clinical drug research.
Ethical statement
Not applicable.
Conflict of interest
No conflicts of interest were declared.
Acknowledgements
The authors extend gratitude to Professor Ting-Hua Wang (Institute of Neurobiological Disease, West China Hospital, Sichuan University).
Funding
This study was supported by the Science and Technology Fund of Guizhou Provincial Health Commission No. gzwjkj2020-1-014.
Transparency statement
All the authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors' contribution
Xue-Yan Huang, Chang-Yin Yu and Liu-Lin Xiong contributed the central idea, analysed most of the data, made figures & tables and wrote the initial draft of the paper. Ting-Ting Li, Lin Zhou and Tao Liu contributed to refining the ideas, carrying out additional analyses and finalizing this paper.




