The research progress of miRNA/lncRNA associated with spinal cord injury
Abstract
In recent years, with the rapid development of economy, the convenient transportation makes people travel more easily. However, the emergence of skyscrapers and various construction projects make high falling injury and traffic accident injury occur frequently, which are the most common types of spinal cord injury. Degeneration of nerve axons after spinal cord injury leads to paralysis of the distal limbs and death. So far, there have been no effective clinical therapy for the recovery of spinal cord function, but animal experiments have made encouraging progress. Recovery process of spinal cord injury involves axonal regeneration and the neural molecular genetic changes in the surrounding environment. Adult animals after spinal cord injury have different degrees of functional recovery in an appropriate growth environment with the benefit of some damaged neurons in the central nervous system. In this paper, we have discussed the changes of miRNA/LncRNA in the progress of regeneration and repair after spinal cord injury.
Introduction
Spinal cord injury (SCI) is a devastating condition that causes substantial morbidity and mortality for which no treatments are available (Antonic A, et al., 2013; Eckert MJ, et al., 2017). Complications, particularly pressure ulcers and pulmonary complications, occur frequently during the acute phase following traumatic spinal cord injury (van Weert KCM, et al., 2014; Ahuja CS, et al., 2017). Sprouting and axonal regrowth are key components of functional recovery but often counteracted by the inhibitory molecules (Plantman S, 2013). Once SCI occurs, re-establishing functional circuitry in the damaged central nervous system (CNS) faces multiple challenges including lost tissue volume, insufficient intrinsic growth capacity of adult neurons and the inhibitory environment in the damaged CNS. Dysfunction of the blood-spinal cord barrier (BSCB) which shares the same principal building blocks with the blood-brain barrier (BBB), plays a fundamental role in the etiology or progression of several pathological conditions of the spinal cord, such as SCI (Bartanusz V, et al., 2011).
Spinal cord injury therapeutics
Nowadays, clinic treatments for SCI are primarily confined to minizing secondary damage by reducing compression in trauma spots and stabilizing the spinal column (Cao HQ, et al., 2013). Although efforts and money have been spent in developing agents for acute and subacute SCI, no effective agents have been found up to now (Tator CH, et al., 2012; Assinck P, et al., 2017).
Acute traumatic central cord syndrome (ATCCS) is the most common type of incomplete SCI, characterized by predominant upper extremity weakness, less severe sensory and bladder dysfunction. Non-surgical treatment relies on external immobilization, maintenance of sufficient systolic blood pressure and early rehabilitation, which is mainly applied for patients suffering from mild ATCCS. In order to achieve spinal cord decompression, surgical management of ATCCS consists of posterior, anterior or combined approaches with or without stabilization (Molliqaj G, et al., 2014).
Cell transplantation
Recently, extensive preclinical literature has shown that stem-cell-based therapies may hold a promising future for SCI treatment. A meta-analysis study found that allogeneic stem cell treatment appears to improve both motor and sensory outcome, based on analyzing the impact of stem cell biology and experimental design. However, the efficacy is likely to be somewhat lower than reported (Antonic A, et al., 2013). Mesenchymal stem or stromal cells (MSCs) with anti-inflammatory and neuroprotective effects may reduce secondary damage of SCI after administration. A systematic review with quantitative syntheses was performed by Oliveri RS to assess the evidence of MSCs versus controls for locomotor recovery, which reported that MSCs would seem to demonstrate a substantial beneficial effect on locomotor recovery in a widely-used animal model of traumatic SCI (Oliveri RS, et al., 2014). Olfactory ensheathing cells (OECs) were injected into Fidyka’s spinal cord, who was left with an 8 mm gap in his spinal cord after being stabbed. Two years later, he could walk using a frame and also regain some bladder sensation and sexual function (Kmietowicz Z, 2014). Schwann cells (SCs) have been considered to be one of the most promising cell types for transplantation to treat SCI due to their unique growth-promoting properties. Wang XF showed that SCs-GFP can survive, proliferate, migrate along the central canal and regenerate myelinated axons when being grafted into a clinically-relevant contusive SCI in adult rats (Wang XF, et al., 2014). Bone marrow mesenchymal cells (BMSCs) are regarded as donor cells in cell transplantation therapies for SCI because their ability of favorable proliferation and multi-directional differentiation (Wang L, et al., 2014).
Transplantation effect
Functional improvement after implantation of biopolymer-based multimodal implants is modest as reported by Krishna et al. Cumulative mean improvements in BBB scores after biomaterial-based interventions are 5.93 and 4.44 for transection and hemisection models, respectively (Krishna V, et al., 2013).
Pathway inhibitors
Blocking small GTPase-RhoA signaling pathway is considered as a candidate translational strategy to improve functional outcome after SCI in humans. Watzlawick R conducted a systematic review and meta- analysis to study the effect of RhoA/ROCK inhibitors (C3-exoenzmye, fasudil, Y-27632, ibuprofen, siRhoA, and p21) on locomotor recovery of experimental models with spinal cord hemisection, contusion or transection. They found that RhoA/ROCK inhibition improved functional outcomes by 15% in experimental SCI taking into account publication bias (Watzlawick R, et al., 2014). Degradation of chondroitin sulfate proteoglycans using the chondroitinase ABC (ChABC) enzyme removes the regeneration barrier from the glial scar and increases plasticity in the CNS by removing perineuronal nets (Zhao RR, et al., 2013). FK506, an immunosuppressant widely used in clinic, exerts neuroprotective effects on rat models of SCI and cerebral ischemia (Saganova K, et al., 2012). Using monoclonal antibodies directed against 5-HT, NOS, Dyn A (1-17) and TNF-α in vivo result in marked neuroprotection and enhanced neuro-repair after trauma.
Furthermore, a suitable combination of monoclonal antibodies such as NOS and TNF-α could enhance neuroprotective ability against SCI after they are applied for 60-90 minutes (min) (Sharma A, et al., 2012). Reactive oxygen species (ROS) and oxidative stress play a significant role in the pathophysiology of SCI. Methods for alleviating oxidative stress such as using the glucocorticoid steroid methylprednisolone and the non-glucocorticoid 21-aminosteroid tirilazad have been shown to be feasibly antioxidant and can improve convalescence of SCI patients in clinical trials (Jia Z, et al., 2012). ROS and lipid peroxidation inhibitors reduce mechanical allodynia in a chronic neuropathic pain model of SCI in rats (Hassler SN, et al., 2014). NT-3 enhances myelination primarily by infiltrating Schwann cells, whereas over-expressing NT-3 on sonic hedgehog (SHH) substantially increased myelination by oligodendrocytes (Thomas AM, et al., 2014). Flavopiridol, a cell-cycle inhibitor, has been shown to improve the functional recovery of SCI models. Local delivery of flavopiridol in Poly lactic-co-glycolic acid (PLGA) nanoparticles (NPs) improves recovery of SCI models by inhibiting activation of astrocytes and synthesis of inflammatory factors (Ren H, et al., 2014). Mitochondrial dysfunction is becoming a pivotal target for neuroprotective strategies following SCI. N-acetylcysteine amide treatment significantly maintains acute mitochondrial bioenergetics and normalized GSH levels in SCI models, and prolongs delivery resulted in significant tissue sparing and improves recovery of hindlimb function (Patel SP, et al., 2014). Granulocyte colony-stimulating factor (G-CSF) promotes locomotor recovery and demonstrates neuroprotective effects in acute SCI model (Guo YJ, et al., 2014). Administration of high-dose high-potency steroidal drugs such as methylprednisolone and dexamethasone reduced the inflammation associated with primary injury and prevented the subsequent secondary injury (Kumar P, et al., 2014).
Hypothermia
The efficacy of systemic and regional hypothermia for treatment of traumatic SCI was determined by a series of review and meta-analyses which showed that systemic hypothermia improved behavioral outcomes by 24.5% and a similar magnitude of improvement was seen across a number of high quality studies. Meanwhile, overall behavioral improvement using regional hypothermia was 26.2% (Batchelor PE, et al., 2013). Therefore, regional hypothermia appears to be a promising potential method for treating acute SCI.
Extracorporeal shock wave therapy (ESWT)
Low-energy ESWT significantly increased the expression of VEGF and Flt-1 in the spinal cord without any detrimental effect, reduced neuronal loss in the damaged neural tissue and improved locomotor function of SCI (Yamaya S, 2014).
Elongated-needle penetration (ENP)
ENP stimulation could attenuate injury and cell apoptosis in the spinal injury rabbits due to its effects of up-regulating p-Akt and p-ERK1/2 and down-regulating Cyt C and Caspase-3 expression in the spinal cord and reducing serum TNF-alpha content (Chen RL, et al., 2014).
Activity-based therapy (ABT)
ABT has the potential to promote neurologic recovery and enhance walking ability in patients with chronic motor-incomplete SCI. However, time, effort and resources required to undertake ABT for these patients (Jones ML, et al., 2014). Activity-based therapies are routinely integrated in SCI rehabilitation programs because of its effects of reduction of hyperreflexia and spasticity. Exercise could contribute to reflex recovery and restoration of endogenous inhibition through a return to chloride homeostasis after SCI (Cote MP, et al., 2014).
Combination treatment
The combined treatment of transplantation (TP) of neural stem cells (NSCs) and treadmill training (TMT) resulted in tissue sparing, increased myelination and restoration of serotonergic fiber innervation to the lumbar spinal cord a larger extent than that induced by either TP or TMT alone (Hwang DH, et al., 2014). The combination of PTEN deletion and salmon fibrin injected into the lesion can significantly improve voluntary motor function after SCI by enabling regenerative growth of CST axons (Lewandowski G, et al., 2014). Combined with physical therapy, autologous adherent bone marrow cell therapy appears to be a safe and promising therapy for patients with chronic SCI (El-kheir WA, et al., 2014).
Others
Improvements of balance and gait have significant implications for functional recovery for people with incomplete spinal cord injury (iSCI). Sensory tongue stimulation combined with task-specific training may be a feasible method for improving balance and gait in people with iSCI (Chisholm AE, et al., 2014). In contrast to biologic therapies, emerging neural interface technologies e.g. brain machine interface (BMI) and limb reanimation through electrical stimulators provide a foundation of the development of bypass system to improve functional outcome after traumatic SCI (Lobel DA, et al., 2014).
Changes of mitochondria after SCI
Local mitochondrial respiratory function was significantly affected after spinal cord injury. Once mitochondrial membrane permeability increased, swelling, edema and the H+ pump obstacles are happening. Ca2+ induced by trauma flow into the neurons, which activates the phospholipase mediated hydrolysis and releases large amounts of free fatty acid to damage mitochondrial membrane (Liu D, et al., 2001). ASC I produces some mitochondrial respiratory inhibitors or uncouplers, which inhibit a variety of mitochondrial charge transfer to directly influence energy metabolism, such as active nitrite nitrogen and oxygen free radicals (Bolanos JP, et al., 2010). After SCI, there are excessive reactive oxygen species produced including the ultra-oxygen anion free radical (O2-) and hydrogen peroxide (H2O2). These reactive oxygen species attack mitochondria membrane through the lipid peroxidation and then destroy the membrane integrity. In the process of lipid oxidation, toxic byproducts are produced such as acrolein and hydroxyl nonene aldehyde , which play an important role in mitochondrial damage (Luo J, et al., 2005). The excessive produced NO and O2- after SCI cause mitochondrial dysfunction up-regulate the nuclear PARP-1 and translocate mitochondria Bax, trigger the AIF and caspase-dependent apoptosis signaling pathways (Wu KL, et al., 2009; Rong YL, et al., 2019). Mitochondria fusion genes (Mfn1 and Mfn2) and division related gene (Drp1 and Fis1) increased in 3 hours (h) and 6 h after spinal cord injury, but decreased in 12 h and 24 h later. These findings provided important instructions for understanding mitochondrial function and kinetic energy after SCI (Grohm J, et al., 2010).
The changes of microRNA and lncRNA
MicroRNA
MicroRNAs (miRNAs) expression levels are changing in the SCI of contusion rodent models. Most SCI lead to permanent paralysis in mammals. By contrast, the remarkable regenerative abilities of salamanders enable full functional recovery even from complete spinal cord transections. Therefore, a group of miRNAs that are conserved between the Mexican axolotl salamander (Ambystoma mexicanum) and mammals were identified. Results found that miR-125b was essential for functional recovery. Increasing miR-125b levels in the rat through mimic treatments following spinal cord transection downregulated Sema4D and other glial-scar-related genes and enhanced the animal's functional recovery (Quiroz JFD, et al., 2014). Through the bioinformatics strategy to identify gene expression profile of SCI in rat, down-regulated genes of 3 days post-injury and the up-regulated genes of 2 weeks post-injury were significantly enriched as the target genes of microRNAs (miR-129 and miR-124) (Jin LJ, et al., 2014). In the compression model of SCI, expression levels of different miRNAs are altered in the acute phase of injury (Ziu MT, et al., 2014). Knockdown of miR-21 by antagomir-21 in the contusion spinal cord injury rats attenuated recovery in locomotive function of hindlimbs, increased lesion size, decreased tissue sparing and increased the expression of pro-apoptosis genes such as FasL and PTEN. These indicate the anti-apoptotic effect of microRNA-21 (Hu JZ, et al., 2013). Besides, another research showed that overexpression of miR-21 in astrocytes attenuated the hypertrophic response to SCI and inhibition of miR-21 augmented the hypertrophic phenotype which resulted in an increased axon density within the lesion site. These results suggest that miR-21 regulates astrocytic response following SCI (Bhalala OG, et al., 2012). Overexpression of miR-124 can promote the differentiation of neuron stem cells (NSCs ) and play an important role in the repair of SCI. Transfection of miR-124 in NSCs dramatically increased the percentage of NeuN-positive cells, reduced the percentage of GFAP-positive cells and achieved a better functional recovery (Xu WW, et al., 2013). Knocking down miR-486 induced the expression of NeuroD6, which effectively ameliorated the spinal cord injury and improved recover of motor function. These findings demonstrated a new role for NeuroD6 in neuroprotection (Jee MK, et al., 2012).
Blocking miR-20a in SCI animals promote neural cell survival and eventual neurogenesis with rescued expression of the key target gene neurogenin 1 (Ngn1). While inhibition of miR20a expression effectively reduced definitive motor neuron survival and neurogenesis, resulting in functional deficit in SCI models. These indicate that silencing of miR20a is crucial for Ngn1-mediated neuroprotection in injured spinal cord (Jee MK, et al., 2012). Microarray comparisons of the injury sites of contusion showed that 32 miRNAs including miR-124, miR-129 and miR-1 were significantly down-regulated, whereas SNORD2, atranslation-initiation factor, was induced (Strickland ER, et al., 2011). In the non-mammal SCI study, miR-133b is an important determinant factor in spinal cord regeneration of adult zebrafish through reduction in RhoA protein levels by direct interaction with its mRNA (Yu YM, et al., 2011).
Quantitative polymerase chain reaction analysis revealed high expression of miR-223 at 12 h after SCI. Meanwhile, double staining of in situ hybridization and immunohistochemistry showed that the signals of miR-223 colocalized with Gr-1 positive neutrophils. These data indicate that miR-223 may regulate neutrophils in the early phase after SCI (Izumi B, et al., 2011). Increased expression of miR Let-7a and miR-16 were showed after SCI. While, exercise leaded to elevated levels of miR-21 and decreased levels of miR-15b. These changes in microRNAs expression are correlated with changes in expression of their target genes i.e. pro-apoptotic (decreased PTEN, PDCD4, and RAS mRNA) and anti-apoptotic (increased Bcl-2 mRNA) target genes, which indicate cycling exercise affects the expression of apoptosis-associated microRNAs after SCI in rats (Liu G, et al., 2010).
LncRNA
So far, few articles referring to the lncRNA and SCI are searched in the database of PubMed or others. Recent studies of the lncRNA were concentrated in the nerve cells differentiation and neurodegenerative disease, which are needed investigating in SCI via more experiments.
Changes of lncRNA and miRNA after SCI in the mitochondria
Mitochondrial DNA (mtDNA) only have 37 genes encoding 13 kinds of mitochondrial oxidative phosphorylation (oxidative phosphorylation, OXPHOS) complex subunits, 22 kinds of tRNA and 2 kinds of rRNA (Ryan MT, et al., 2007). While, other metabolic enzymes and protein expression is regulated by the nuclear gene. Mir-181-c, nuclear encoding gene, can be transferred to the mitochondria, and then affect mitochondrial energy metabolism COX1 through regulation of mitochondrial protein (Das S, et al., 2012). Other studies found that mirRNA-15a and mirRNA-16-1 could cause apoptosis by regulating the function of mitochondria (Calin GA, et al., 2005).
By Northern blot and qPCR detection, a study confirmed that the mitochondrial RNA enzyme protein 1 (MRPP1) was important for three lncRNA transcription process which come from the mitochondria (Rackham O, et al., 2011).
Conclusion
In conclusion, the lncRNA researches are less in the studies of mitochondria, and studies of miRNA are not special. Additionaly, researches of miRNA and lncRNA in the mitochondria have less been touched in the area of SCI. In order to further explore the regeneration and repair after SCI, we need to pay more attention to the function of mitochondria, which may be benefit of finding therapy candidates for SCI ( ).
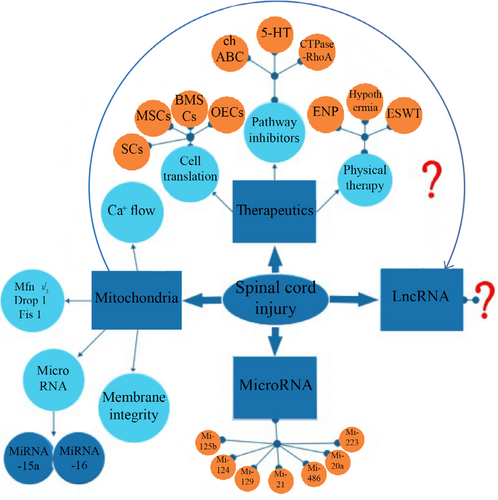
The research progress of miRNA/lncRNA associated with spinal cord injury. chABC, chondroitinase ABC. 5-HT, 5-hydroxy trptamine. SCs, schwann cells. MSCs, mesenchymal stem or stromal cells. BMSCs, bone marrow mesenchymal cells. OECs, olfactory ensheathing cells. ENP, Elongated-needle penetration. ESWT, extracorporeal shock wave therapy. Mfn, mitochondria fusion genes. Drp, division related gene.
Ethical statement
Not applicable.
Acknowledgements
Not applicable.
Conflict of interest
There is no conflict of interest in this article.
Funding
None.
Transparency statement
The authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors' contributio
Hao Yuan and Ting-Hua Wang contributed the central idea, collecting literatures, and wrote the initial draft of the paper; Yue Hu and Ling Jiang contributed to refining the ideas, carrying out additional literatures’ searching; Ting-Hua Wang finalized and approved the this paper.




