Catchment-Scale Eucalyptus Plantation Effects on Tropical Streams
ABSTRACT
Forestry plantations occur across the globe, and they are important in many tropical countries for timber supply. Plantations of non-native species, such as those of Eucalyptus species, may greatly affect the functioning of detritus-based ecosystems. However, despite eucalyptus plantations covering about 20 million hectares in tropical and subtropical regions (from a total of about 25 million hectares globally), there is scarce information about their effects on forest stream functioning. Our aim was to assess the effects of catchment-scale eucalyptus plantations (with native riparian buffers) on the functioning of tropical streams in the Cerrado biome. For that, three streams in catchments with eucalyptus plantations (eucalyptus streams) and three streams in catchments with native vegetation (native streams) were compared regarding water characteristics, litter inputs (vertical and lateral), decomposition of leaf litter (from a common native species, an eucalyptus species used in plantations, and a palatable exotic species) and litter-associated aquatic communities. We hypothesized that catchment-scale Eucalyptus plantations negatively affect stream characteristics (reduced water flow, heightened water acidity), litter inputs, aquatic communities, and litter decomposition in streams because dense, even-aged monocultures of eucalyptus trees have higher water consumption rates and produce recalcitrant litter. We also hypothesized that the effects of plantations would be stronger on shredders, as they are directly influenced by changes in litter input compared to other functional feeding groups. Finally, we hypothesized that plantation impacts on leaf litter decomposition would be stronger for palatable leaf litter, where invertebrate shredders play a major role, than for more recalcitrant and less palatable leaf litter. We found lower dissolved oxygen concentrations, lateral litter inputs, and litter decomposition in eucalyptus streams than in native streams. Conversely, fungal biomass on decomposing litter did not differ between eucalyptus streams and native streams. Eucalyptus plantations reduced overall invertebrate densities but did not affect shredder densities. Our study shows that catchment-scale eucalyptus plantations can change water characteristics and litter inputs to streams, thus slowing down litter decomposition in tropical streams, even when a native buffer is present. Increasing the width of the native buffer vegetation may contribute to increasing its protective role.
1 Introduction
Forestry activities for timber supply are important in many tropical countries, where plantation trees grow fast and avoid the unsustainable cuts of native forests (Onyekwelu et al. 2011). Eucalyptus is one of the tree genera most widely used in forestry, spanning approximately 20 million hectares in tropical and subtropical regions out of a total of around 25 million hectares globally (Elli et al. 2020). However, non-native species plantations may affect the functioning of detritus-based ecosystems (Larrañaga et al. 2021; Oester 2024).
Streams are particularly susceptible to changes in the catchment due to their position at the bottom of valleys, large aquatic-terrestrial interface, and strong dependence on litter inputs from the riparian vegetation (Tolkkinen et al. 2020). Therefore, large areas of eucalyptus plantations in the catchment can decrease runoff and aquifer levels (Lara et al. 2009) due to the high water demand of dense plantations of evergreen, young trees (Shi et al. 2012). Eucalyptus plantations also make the soils hydrophobic due to the accumulation of oils released from their leaves (Fernández et al. 2006). This increased soil hydrophobicity increases superficial water runoff when it rains, leading to peaks in stream discharge (Neary and Levia 2020). Soil hydrophobicity also reduces water infiltration, impairing the refilling of groundwater reservoirs and leading to reduced stream flow during dry periods (Shi et al. 2012). Changes in stream water availability can affect aquatic communities and the stream ecosystem functioning (Datry et al. 2011) and put water-related ecosystem services at risk (Bispo et al. 2023). Also, changes in litter inputs to streams (seasonality, diversity, quantity, and quality) may occur due to the replacement of native forests by eucalyptus plantations (Abelho and Graça 1996; Graça et al. 2002; Rezende et al. 2017). These changes in litter inputs may further affect aquatic communities and the functioning of forest streams due to their dependence on terrestrial-derived organic matter (Vannote et al. 1980; Wallace et al. 1997).
However, plantations are more likely to affect stream ecosystems when the exotic tree species used (e.g., Eucalyptus spp.) are functionally dissimilar from dominant native species than when they are functionally similar (Kominoski et al. 2013; Rabelo et al. 2024). In accordance, invertebrate density, biomass, and diversity, particularly of shredders, are generally lower in streams flowing through blue gum eucalyptus (E. globulus) plantations than through native mixed deciduous forests in the Iberian Peninsula (Larrañaga et al. 2009a, 2009b). Consequently, total leaf litter decomposition (i.e., carried out by both microbial decomposers and invertebrate shredders) is generally impaired in streams flowing through Eucalyptus plantations, while microbial-driven leaf litter decomposition is generally not affected (Ferreira et al. 2016, 2019). Distinct sensitivity of microbial-driven and total leaf litter decomposition to forest change likely results from high functional redundancy within microbial decomposer communities, while the loss of invertebrate shredders cannot be overcome (Ferreira et al. 2006). However, the effects of Eucalyptus plantations on leaf litter decomposition seem to be stronger for palatable than recalcitrant (e.g., eucalyptus) leaf litter (Ferreira et al. 2016), likely because the decomposition of palatable leaf litter has a higher contribution from shredders (Hieber and Gessner 2002). Eucalyptus trees in temperate regions produce leaves with high concentrations of secondary compounds (e.g., polyphenols and essential oils) compared to most native deciduous species, in addition to having lower nutritional quality and palatability (Canhoto and Graça 1995, 1996). Thus, Eucalyptus leaf litter is a poorer food resource than native leaf litter and leads to reduced growth and survival of invertebrate shredders and slow litter decomposition in eucalyptus streams (Canhoto and Graça 1995, 1996; Larrañaga et al. 2009a; Ferreira et al. 2015). Conversely, in tropical regions—including the Cerrado biome—eucalyptus (E. grandis) leaf litter has higher nutrient concentrations and lower concentrations of refractory compounds than leaf litter of many native species that are xerophytes (Gomes et al. 2016; Kiffer et al. 2018). Thus, Eucalyptus leaf litter may be colonized and decomposed faster by microbial decomposers and invertebrates compared with leaf litter of some native species (Gonçalves et al. 2012; Rezende et al. 2014; Gomes et al. 2016; Kiffer et al. 2018). Eucalyptus species, however, differ in leaf litter traits (Simões et al. 2021), which makes results dependent on the Eucalyptus species being considered (Gomes et al. 2016; Sena et al. 2024).
Leaf litter decomposition carried out by both microbes and invertebrates (i.e., total litter decomposition) is more sensitive to forest changes than microbe-driven decomposition when invertebrates are the main agents of decomposition, such as in temperate streams (Ferreira et al. 2016). In contrast, in regions where invertebrate shredders are scarce, and litter decomposition is primarily mediated by microbes, as in many tropical regions (e.g., Gonçalves et al. 2007), litter decomposition is likely less sensitive to forest changes due to functional redundancy of microbial communities (Gessner et al. 2007). However, forest changes can have impacts on microbial colonization and litter processing in some tropical streams (Gomes et al. 2016; Ferreira et al. 2019).
Our aim was to assess the effects of catchment-scale Eucalyptus plantations (with native riparian buffer) on tropical streams in the Cerrado biome. For that, streams in catchments covered with eucalyptus plantations and in catchments covered with native vegetation were compared regarding water characteristics, litter inputs (vertical and lateral), leaf litter decomposition (from a common native species, an eucalyptus species used in plantations, and a palatable exotic species) and litter-associated aquatic communities (microorganisms and invertebrates). We hypothesized that catchment-scale Eucalyptus plantations negatively affect water quality parameters (e.g., reduce water flow, increase water acidity), litter inputs, aquatic communities, and litter decomposition in streams because dense, even-aged monocultures of eucalyptus trees have higher water consumption rates and produce recalcitrant litter. We also hypothesized that the effects of plantations would be stronger on shredders, as they are directly influenced by changes in litter inputs compared to other functional feeding groups. Finally, we hypothesized that plantation impacts on leaf litter decomposition would be stronger for palatable leaf litter, where invertebrate shredders play a major role, than for more recalcitrant and less palatable leaf litter.
2 Methods
2.1 Study Area
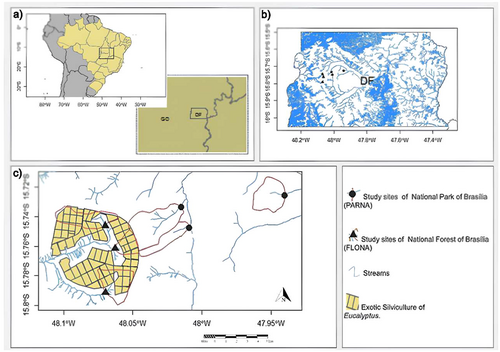
This study was carried out in six streams (1st–2nd order) in the Cerrado biome, Federal District, Brazil (Figure 1, Supporting Information S1: Table S1). Three streams (National Forest of Brasília—FLONA) are in sub-catchments dominated by exotic silviculture planted in the 1970s and abandoned in the 2000s; ~65% of the drainage area is covered by Eucalyptus grandis W. Hill ex Maiden, 23% by Eucalyptus saligna Sm., 11% by Eucalyptus urophylla S.T. Blake, and 1% by Eucalyptus alba Reinw. ex Blume (Brasil 1978; CODEPLAN 1991) (hereafter eucalyptus streams). Nevertheless, eucalyptus streams have a moderately well-preserved riparian zone (~87 m wide on each side of the stream) with native forest dominated by Tapirira guianensis Aubl., Lamanonia ternata Vell., Protium spruceanum Benth. The other three streams (Brasília National Park—PARNA) are in sub-catchments dominated by native vegetation; 100% of the drainage area is covered by strictu sensu Cerrado physiognomy with over 40 plant species in the riparian zone, which is dominated by Copaifera langsdorffii Desf. and Myrcia sp. (hereafter native streams). The sub-catchments were delimited using a Digital Elevation Model and digital hydrography bases on a scale of 1:10,000, available from the Cartographic System of the Federal District, Brazil (CODEPLAN 1991). The land use within catchments was determined using the free software QGIS 2.18.2 and Google Earth Pro, and the map was done using R version 4.4.2 (R Core Team 2024) and ArcGIS 10.3 (Figure 1, Supporting Information S1: Table S1). The climate is seasonal tropical (Aw, Köppen-Geiger climate classification) with a dry season (June–August) and a wet season (November–March), with an annual mean temperature of 21°C and annual precipitation of around 1477 mm (Tonin et al. 2021).
2.2 Stream Characterization
All streams were small (Table 1) and had the streambed dominated by boulders (56%–72%), with lower amounts of cobbles (9%–37%) and smaller substrates. The riparian zone was well preserved in all streams, with canopy cover of over 80%. The physical and chemical characteristics of stream water were determined three times over a 60-day period (August 4, 2015 to October 3, 2015). Stream water temperature, pH, conductivity, and dissolved oxygen concentration were measured using a portable multiparameter (JENWAY, Stone, Staffordshire, UK), and the current velocity with a flowmeter (FP10, Global Water, Randolph, Massachusetts, USA). On the same occasions, water samples (50 mL) were collected in plastic sterile bottles and stored cold (± 4°C). In the laboratory, stream water was filtered (Millipore filter GF/C, 0.45 μm, Billerica, MA, USA) and frozen (–20°C) for later determination of nitrate (NO3−), nitrite (NO2−), ammonia (NH4+) and phosphate (PO43−) concentrations by ion chromatography (930 Compact IC Flex, Metrohm, Herisau, Appenzell Outer, Switzerland).
| Water and stream characteristics | Eucalyptus streams | Native streams | F-value | Pr(>F) | Contrast analysis |
|---|---|---|---|---|---|
| Temperature (°C) | 21.1 ± 0.8 | 20.2 ± 2.1 | 3.1 | 0.098 | |
| Conductivity (µS cm–1) | 4.8 ± 1.2 | 4.5 ± 0.8 | 0.1 | 0.711 | |
| pH | 5.6 ± 1.0 | 6.1 ± 1.2 | 0.7 | 0.401 | |
| O2 (mg L–1) | 5.4 ± 0.8 | 7.1 ± 2.0 | 5.2 | 0.037 | EUC < NAT |
| N-NO3– (µg L–1) | 0.018 ± 0.01 | 0.018 ± 0.01 | 1.9 | 0.191 | |
| N-NH4+ (µg L–1) | 0.037 ± 0.09 | 0.008 ± 0.02 | 0.9 | 0.367 | |
| DIN (µg L–1) | 0.055 ± 0.09 | 0.027 ± 0.02 | 0.7 | 0.410 | |
| Width (m) | 1.00 ± 0.33 | 1.21 ± 0.15 | 1.0 | 0.326 | |
| Depth (cm) | 35 ± 8 | 21 ± 4 | 1.8 | 0.194 | |
| Water velocity (m s–1) | 0.54 ± 0.30 | 0.48 ± 0.4 | 0.2 | 0.627 |
2.3 Litter Inputs to Streams
Vertical and lateral coarse particulate organic matter (CPOM) inputs to streams were sampled three times over a 60-day period (August 4, 2015 to October 3, 2015). Sampling was cumulative over 15 days for the first two sampling dates and over 30 days for the third sampling date. The amounts of vertical and lateral CPOM inputs collected at each sampling date were summed across sampling dates to obtain the total cumulative amount produced since day 0 (August 4, 2015). For determination of the vertical input (directly from the canopy to the stream), nets (1 m2, 0.5 mm mesh size, n = 3) were suspended at 1.5 m above the stream surface, over a 30 m stream reach, 10 m apart from each other (Supporting Information S1: Figure S1). For determination of the lateral input (from the riparian bank to the stream), nets (0.25 m2, 0.5 mm mesh size, n = 4) were displayed at 10 m intervals, two in each stream bank (Supporting Information S1: Figure S1). The CPOM was removed from the nets, and the material was oven-dried at 60°C for 3–7 days (until a constant mass was reached). Afterward, the vegetative parts (leaves, branches, flowers, fruits, and miscellaneous) were sorted and weighed (accuracy 0.001 g). Results were expressed as g dry mass (DM) m–2.
2.4 Litter Decomposition Experiment
This experiment integrated an international effort to assess the effects of eucalyptus plantations on stream functioning (Ferreira et al. 2019). Senescent leaves of Inga laurina (Sw.) Willd. (June 2014, garden of the University of Brasilia, Distrito Federal), Eucalyptus grandis W. Hill ex. Maiden (June 2015, National Forest of Brasília—FLONA, Distrito Federal), and Alnus glutinosa (L.) Gaertn. (October 2014, Coimbra, Portugal) were collected with 1-m2 nets, air-dried, and stored in the dark. Three leaf litter samples (~3 g each) for each species were ground to a fine powder for subsequent determination of carbon (C) and nitrogen (N) concentrations using an elemental analyzer (LECO-CNS628, LECO Corporation, St. Joseph, USA) (Supporting Information S1: Table S2). Inga laurina is a native riparian species largely distributed throughout South America, which produces leaf litter with low quality to consumers due to its high C:N ratio and foliar toughness (Rezende et al. 2014; Supporting Information S1: Table S2). Eucalyptus grandis is one of the most common species used in forestry plantations in the study region (ICMBio 2016) and produces leaf litter that can be considered of intermediate quality between I. laurina and A. glutinosa, due to its intermediate C:N ratio and toughness, and high concentration of secondary compounds such as polyphenols that limit its palatability for consumers (Hepp et al. 2008; Supporting Information S1: Table S2). Alnus glutinosa is a common riparian species in Europe, which produces leaf litter that is highly palatable to aquatic decomposers due to its high softness and nutrient concentrations (low C:N ratio) and low concentrations of secondary compounds (Graça and Poquet 2014; Supporting Information S1: Table S2). It is exotic to Brazil, but it was used here as a surrogate for palatable leaf litter for comparative purposes. Air-dried leaf litter was weighed in batches of 2.5 ± 0.05 g (mean ± SD), moistened with distilled water, and enclosed in litter bags (12 × 15 cm). Fine-mesh litter bags (0.5 mm mesh) were used to restrict the access of macroinvertebrates, while coarse-mesh litter bags (10 mm mesh) allowed access of macroinvertebrates to the litter. Nine litter bags per mesh type and litter species were distributed by three blocks, spaced 10 m from each other, over a 30-m reach in each stream (9 litter bags × 2 mesh types × 3 litter species × 6 streams = 324 litter bags total; Supporting Information S1). After 15, 30, and 60 days, three litter bags were retrieved (one per block) and placed in a cool box for transportation to the laboratory. Three sampling dates spanning 60 days were used to increase the chances of detecting differences between treatments, which could be missed if a single date was used. A single sampling date increases the risk of sample loss, of missing important events (e.g., peaks in decomposer activity/colonization), and of not being sensitive enough (in case the selected sampling date is too late in the decomposition process).
In the laboratory, leaf litter was rinsed with distilled water over a 150-μm mesh to retain small litter fragments and invertebrates. From each sample, five leaves were randomly selected for the removal of two sets of five leaf discs (15 mm in diameter). Litter remains after the extraction of leaf discs were oven-dried (60°C) to constant mass and then weighed (accuracy 0.0001 g) to determine DM.
One set of leaf litter discs was used for the quantification of ergosterol concentration (used to indicate fungal biomass) (Gessner 2005). The discs were preserved in alkaline (KOH) methanol, and ergosterol was extracted in a hot bath (80°C, 30 min). The extract was purified with solid-phase extraction cartridges using a manifold equipped with a vacuum pump (Gessner 2005). Ergosterol was then eluted with isopropanol, and concentrations were determined by high-performance liquid chromatography (DIONEX—The UltiMate 3000 HPLC, Thermo Fisher Scientific, Waltham, Massachusetts, USA). Ergosterol concentration was expressed as μg g–1 leaf litter ash-free dry mass (AFDM), considering the mass of the second set of leaf litter discs (see next).
The second set of leaf litter discs was used to estimate a conversion factor between litter DM and litter AFDM, for which it was oven-dried at 60°C to constant mass, weighed (accuracy 0.0001 g) to estimate DM of discs, ignited at 550°C for 4 h and the ashes weighed (accuracy 0.0001 g). Discs AFDM was calculated as discs DM-discs ash mass, and the fraction of discs AFDM was used to convert the litter DM remaining (also accounting for the extracted discs) into litter AFDM remaining. The percentage of litter AFDM remaining was calculated as litter AFDM remaining/litter initial AFDM × 100.
Leaf litter initial AFDM was estimated from an additional six sets of litter bags for each leaf species and mesh type, which were prepared as described above, transported to the field on day 0, submerged for approximately 10 min in one stream of each stream type, and returned to the laboratory. Leaf litter bags were processed as the experimental bags, and the initial AFDM was calculated as DM-ash mass. The ratio between initial AFDM and initial air-DM was used to estimate an air-DM to AFDM conversion factor, which was used to estimate the experimental samples’ initial AFDM, also accounting for handling losses.
Invertebrates (coarse-mesh bags only) were preserved with 70% ethanol and later identified until the family level according to specific bibliography (Cummins et al. 2005; Hamada and Ferreira-Keppler 2012; Hamada et al. 2014). Invertebrates were distributed by functional feeding groups (predators, collectors, collector-filterers, shredders, and scrapers), according to Merritt et al. (2017). Invertebrate density was expressed as no. ind. g–1 AFDM, and invertebrate taxa richness was expressed as no families sample–1.
2.5 Data Analysis
The litter decomposition rate was calculated as the slope of the regression between the fraction of litter AFDM remaining (ln-transformed) over time: ln (AFDMt/AFDM0) = – k × t, where AFDMt is the litter AFDM remaining at time t (in days), AFDM0 is the litter initial AFDM, and k is the decomposition rate, which is the linear form of the negative exponential model.
We used a one-way repeated-measures ANOVA (RM ANOVA) to compare stream water characteristics between eucalyptus and native streams (predictor variable; Crawley 2007). To analyze the influence of catchment-scale Eucalyptus plantations on the response variables (lateral and vertical inputs of total litter, leaf litter, fruits, flowers, branches, and miscellaneous; litter decomposition rate, and litter-associated invertebrates and fungal biomass) and identify specific differences between the levels of categorical factors (stream types [eucalyptus and native streams], litter species [I. laurina, E. grandis, and A. glutinosa], and mesh types [coarse and fine]; litter species only for litter decomposition and associated invertebrates and fungal biomass, and mesh types only for litter decomposition and fungal biomass), linear mixed models (LMM) were adjusted using the lme4 package in the R software (Bates et al. 2015). The models included the fixed factors of interest and a random effect to control the variability associated with data grouping. We used the AICctab function from the bbmle package (Bolker 2023) to select the best model. p-values of the models were obtained with the lmerTest package (Kuznetsova et al. 2017) using Satterthwaite's degrees of freedom method. Visual inspection of residual plots with the RVAideMemoire package (Hervé 2023) revealed no deviations from homoscedasticity or normality.
After adjusting the model, comparisons were made between the levels of the categorical factor of interest using the emmeans package (Lenth 2025), which calculates the adjusted or marginal means for the factor levels, controlling for the other terms in the model. Contrasts were assessed to identify specific differences between levels, using multiple comparison adjustments with Tukey's method to control the Type I error rate. All analyses were performed using software R version 4.4.2 (R Core Team 2024).
3 Results
3.1 Stream Water
Mean dissolved oxygen concentration was significantly lower in eucalyptus than in native streams (Table 1). There was no significant difference between stream types for the other water and stream characteristics (Table 1).
3.2 Litter Inputs to Streams
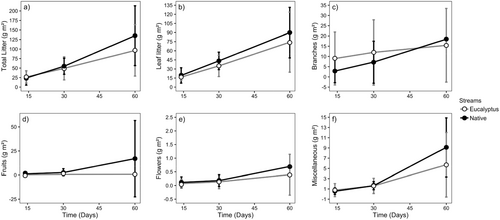
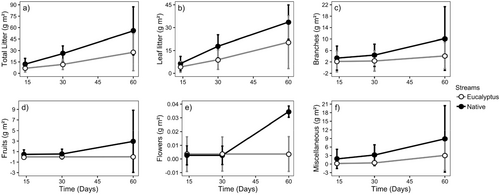
We found no significant difference in vertical inputs (total and for litter types) between stream types, but variation among streams was very high (Figure 2 and Table 2). The lateral inputs of total litter, leaf litter, fruits, branches, and miscellaneous were significantly lower in eucalyptus than in native streams, while the input of flowers did not differ significantly between stream types (Figure 3 and Table 2). The total lateral litter input was 50% lower in eucalyptus than in native streams, which reflects the lower leaf litter (39%), branches (59%), fruits (99%), and miscellaneous (65%) inputs to eucalyptus streams (Figure 3 and Table 2).
| Factor | Sum Sq. | DF | DenDF | F-value | Pr(>F) | Contrast analysis |
|---|---|---|---|---|---|---|
| Vertical input | ||||||
| A. Total litter model: Total litter ~ Stream + Time + (1|Time) | ||||||
| Stream | 1251 | 1 | 53 | 0.66 | 0.416 | |
| Time | 828 | 2 | 53 | 0.22 | 0.801 | |
| B. Leaf litter model: Leaf litter ~ Stream + Time + (Time|Stream) | ||||||
| Stream | 23 | 1 | 53 | 0.03 | 0.859 | |
| Time | 44,460 | 2 | 53 | 33.48 | < 0.001 | |
| C. Fruits model: Fruits ~ Stream × Time + (1|Time) | ||||||
| Stream | 582 | 1 | 51 | 2.11 | 0.152 | |
| Time | 234 | 2 | 51 | 0.42 | 0.498 | |
| Stream × Time | 688 | 2 | 51 | 1.24 | 0.295 | |
| D. Flowers model: Flowers ~ Time + (1| Stream) | ||||||
| Time | 2 | 2 | 54 | 2.94 | 0.061 | |
| E. Branches model: Branches ~ Stream + Time + (Time|Stream) | ||||||
| Stream | 6 | 1 | 53 | 0.03 | 0.871 | |
| Time | 1355 | 2 | 53 | 3.37 | 0.041 | |
| F. Miscellaneous model: Miscellaneous ~ Stream + Time + (1|Time) | ||||||
| Stream | 9 | 1 | 51 | 0.75 | 0.388 | |
| Time | 1 | 2 | 51 | 0.05 | 0.948 | |
| Stream × Time | 26 | 2 | 51 | 1.04 | 0.358 | |
| Lateral input | ||||||
| G. Total litter model: Total litter ~ Stream × Time + (1|Time) | ||||||
| Stream | 4455 | 1 | 66 | 15.69 | < 0.001 | EUC < NAT |
| Time | 7 | 2 | 66 | 0.01 | 0.987 | |
| Stream × Time | 1544 | 2 | 66 | 2.65 | 0.077 | |
| H. Leaf litter model: Leaf litter ~ Stream × Time + (1|Time) | ||||||
| Stream | 1172 | 1 | 66 | 12.90 | < 0.001 | EUC < NAT |
| Time | 23 | 2 | 66 | 0.13 | 0.877 | |
| Stream × Time | 362 | 2 | 66 | 1.99 | 0.143 | |
| I. Fruits model: Fruits ~ Stream × Time + (1|Time) | ||||||
| Stream | 31 | 1 | 66 | 5.26 | 0.024 | EUC < NAT |
| Time | 8 | 2 | 66 | 0.69 | 0.662 | |
| Stream × Time | 23 | 2 | 66 | 1.98 | 0.145 | |
| J. Flowers model: Flowers ~ Stream + (1|Time) | ||||||
| Stream | 0.002 | 1 | 70 | 0.80 | 0.373 | |
| L. Branches model: Branches ~ Stream × Time + (1|Time) | ||||||
| Stream | 174 | 1 | 66 | 5.23 | 0.025 | EUC < NAT |
| Time | 9 | 2 | 66 | 0.13 | 0.872 | |
| Stream × Time | 82 | 2 | 66 | 1.23 | 0.288 | |
| M. Miscellaneous model: Miscellaneous ~ Stream × Time + (1|Time) | ||||||
| Stream | 208 | 1 | 66 | 6.75 | 0.011 | EUC < NAT |
| Time | 1 | 2 | 66 | 0.02 | 0.973 | |
| Stream × Time | 56 | 2 | 66 | 0.91 | 0.406 | |
3.3 Leaf Litter Decomposition
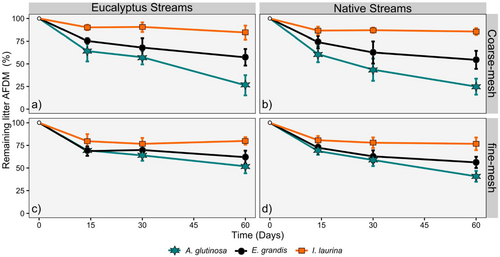
After 60 days incubation, leaf litter AFDM remaining varied between 16% and 90% in eucalyptus streams and between 15% and 97% in native streams across leaf litter species, and between 61% and 98% for I. laurina, 39% and 90% for E. grandis and 16% and 76% for A. glutinosa across streams (Figure 4). Consequently, leaf litter decomposition rates (k, d–1) were significantly lower (23%) in eucalyptus than in native streams (mean across streams ± SE: 0.010 ± 0.001 and 0.013 ± 0.001, respectively) and significantly higher for A. glutinosa than for E. grandis than for I. laurina, but no significant interaction was found between stream type and litter species (Tables 3 and 4). Mesh type did not significantly affect leaf litter decomposition, but the interaction between litter species and mesh type was significant, with higher litter decomposition rates for A. glutinosa in coarse mesh litter bags (Tables 3 and 4).
| Stream type | Litter species | Mesh type | k | SE | R2 |
|---|---|---|---|---|---|
| Eucalyptus | Inga laurina | Coarse | 0.0031 | 0.0007 | 0.77 |
| Fine | 0.0053 | 0.0011 | 0.67 | ||
| Eucalyptus grandis | Coarse | 0.0106 | 0.0014 | 0.89 | |
| Fine | 0.0096 | 0.0013 | 0.82 | ||
| Alnus glutinosa | Coarse | 0.0229 | 0.0017 | 0.92 | |
| Fine | 0.0125 | 0.0011 | 0.90 | ||
| Native | Inga laurina | Coarse | 0.0033 | 0.0005 | 0.75 |
| Fine | 0.0057 | 0.0012 | 0.76 | ||
| Eucalyptus grandis | Coarse | 0.0136 | 0.0011 | 0.86 | |
| Fine | 0.0114 | 0.0016 | 0.89 | ||
| Alnus glutinosa | Coarse | 0.0255 | 0.0017 | 0.92 | |
| Fine | 0.0161 | 0.0013 | 0.95 |
| Factor | Sum Sq. | DF | DenDF | F-value | Pr(>F) | Contrast analysis |
|---|---|---|---|---|---|---|
| Litter remaining mass model: Litter remaining mass ~ Stream × Litter species × Mesh + (1|Time) | ||||||
| Stream | 885 | 1 | 310 | 12.04 | < 0.001 | EUC < NAT |
| Litter species | 50,546 | 2 | 310 | 344.05 | < 0.001 | I. laurina < E. grandis < A. glutinosa |
| Mesh | 124 | 1 | 310 | 1.68 | 0.195 | |
| Stream × Litter species | 319 | 2 | 310 | 2.17 | 0.115 | |
| Stream × Mesh | 8 | 1 | 310 | 0.1 | 0.741 | |
| Litter species × Mesh | 6358 | 2 | 310 | 43.28 | < 0.001 | I. lau CM < I. lau FM < E. gra FM < E. gra CM < A. glu FM < A. glu CM |
| Stream × Litter species × Mesh | 16 | 2 | 310 | 0.11 | 0.895 | |
3.4 Fungal Biomass
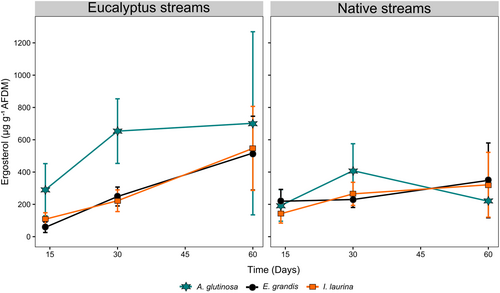
Fungal biomass did not significantly differ between stream types (Figure 5 and Table 5), but it was significantly higher (1.9×) in A. glutinosa than in E. grandis and I. laurina (Figure 5 and Table 5). No significant interaction was found between factors (Table 5).
| Factor | Sum Sq. | DF | DenDF | F-value | Pr(>F) | Contrast analysis | |
|---|---|---|---|---|---|---|---|
| Fungal biomass model: Fungal biomass ~ Stream × Litter species × Mesh + (1|Time) | |||||||
| Stream | 981,537 | 1 | 271 | 1.77 | 0.183 | ||
| Litter species | 3,487,916 | 2 | 271 | 3.16 | 0.043 | I. laurina = E. grandis < A. glutinosa | |
| Mesh | 237,251 | 1 | 271 | 0.43 | 0.512 | ||
| Stream × Litter species | 1,126,725 | 2 | 271 | 1.02 | 0.361 | ||
| Stream × Mesh | 112,252 | 1 | 271 | 0.20 | 0.652 | ||
| Litter species × Mesh | 597,108 | 2 | 271 | 0.54 | 0.582 | ||
| Stream × Litter species × Mesh | 190,166 | 2 | 271 | 0.17 | 0.841 | ||
3.5 Litter Associated Invertebrates
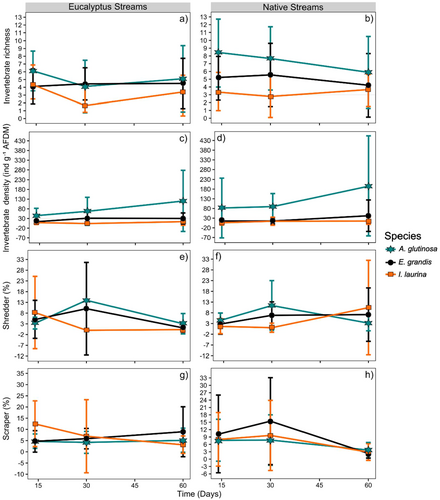
Invertebrate density was not significantly affected by stream type, but it significantly differed between leaf litter species (Table 6). Invertebrate density on leaf litter was 4× higher for A. glutinosa than for E. grandis and 9.2× higher than for I. laurina (Figure 6). Invertebrate taxa richness also did not significantly differ between stream types (Figure 6 and Table 6), but it significantly differed between litter species, being 1.2× higher for A. glutinosa than for E. grandis and 2× higher than for I. laurina (Figure 6 and Table 6). No significant interaction was found between stream type and litter species (Table 6). Shredder and scraper relative density were not significantly affected by stream type, litter species, and their interaction (Figure 6 and Table 6).
| Factor | Sum Sq. | DF | DenDF | F-value | Pr(>F) | Contrast analysis | |
|---|---|---|---|---|---|---|---|
| A. Invertebrate density model: Invertebrate density ~ Stream × Litter species × Time + (1|Time) | |||||||
| Stream | 7993 | 1 | 144 | 1.10 | 0.299 | ||
| Litter species | 261,796 | 2 | 144 | 17.78 | < 0.001 | I. laurina < E. grandis < A. glutinosa | |
| Time | 7663 | 2 | 144 | 0.50 | 0.595 | ||
| Stream × Litter species | 14,388 | 2 | 144 | 0.91 | 0.379 | ||
| Stream × Time | 2516 | 2 | 144 | 0.14 | 0.843 | ||
| Litter species × Time | 54,534 | 4 | 144 | 1.81 | 0.122 | ||
| Stream × Litter species × Time | 3197 | 4 | 144 | 0.13 | 0.979 | ||
| B. Invertebrate taxa richness model: Invertebrate richness ~ Stream × Litter species × Time + (1|Time) | |||||||
| Stream | 28.3 | 1 | 144 | 2.98 | 0.089 | ||
| Litter species | 252.1 | 2 | 144 | 12.98 | < 0.001 | I. laurina < E. grandis < A. glutinosa | |
| Time | 14.8 | 2 | 144 | 0.79 | 0.471 | ||
| Stream × Litter species | 30.6 | 2 | 144 | 1.57 | 0.214 | ||
| Stream × Time | 27.4 | 2 | 144 | 1.37 | 0.251 | ||
| Litter species × Time | 30.8 | 4 | 144 | 0.72 | 0.537 | ||
| Stream × Litter species × Time | 12.8 | 4 | 144 | 0.39 | 0.859 | ||
| C. Shredder relative density model: Shredder relative density ~ Stream × Litter species × Time + (1|Time) | |||||||
| Stream | 3 | 1 | 144 | 0.02 | 0.874 | ||
| Litter species | 249 | 2 | 144 | 0.81 | 0.443 | ||
| Time | 292 | 2 | 144 | 0.95 | 0.385 | ||
| Stream × Litter species | 30 | 2 | 144 | 0.09 | 0.905 | ||
| Stream × Time | 515 | 2 | 144 | 1.68 | 0.188 | ||
| Litter species × Time | 1169 | 4 | 144 | 1.91 | 0.11 | ||
| Stream × Litter species × Time | 395 | 4 | 144 | 0.64 | 0.628 | ||
| D. Scraper relative density model: Scraper relative density ~ Stream × Litter species × Time + (1|Time) | |||||||
| Stream | 49 | 1 | 144 | 0.56 | 0.469 | ||
| Litter species | 161 | 2 | 144 | 0.85 | 0.425 | ||
| Time | 179 | 2 | 144 | 0.94 | 0.391 | ||
| Stream × Litter species | 80 | 2 | 144 | 0.43 | 0.650 | ||
| Stream × Time | 476 | 2 | 144 | 2.53 | 0.082 | ||
| Litter species × Time | 240 | 4 | 144 | 0.64 | 0.634 | ||
| Stream × Litter species × Time | 357 | 4 | 144 | 0.95 | 0.436 | ||
4 Discussion
Lateral litter inputs and leaf litter decomposition significantly differed between eucalyptus and native streams, despite eucalyptus streams having native riparian buffers. According to our hypotheses, litter decomposed slower in tropical streams within catchments dominated by Eucalyptus plantations compared to streams in catchments covered with native vegetation in the Cerrado biome. This result agrees with that of a meta-analysis that found 22% lower litter decomposition rates in eucalyptus streams than in native streams in Mediterranean climates (Ferreira et al. 2016). Previous studies on the effects of Eucalyptus plantations on litter decomposition suggested that maintaining native riparian forests could mitigate plantation impacts by limiting Eucalyptus litter inputs to eucalyptus streams and ensuring that they would still receive native litter inputs (Ferreira et al. 2016). This was not the case here, which could suggest that the riparian buffers in eucalyptus streams were not effective enough to mitigate plantation effects.
Differences in leaf litter decomposition between stream types were consistent across leaf litter species and mesh types, suggesting that microbial decomposer activity was also impaired in eucalyptus streams. This could have resulted from the lower dissolved oxygen concentration in eucalyptus streams. Invertebrate metrics and fungal biomass associated with leaf litter were not influenced by catchment-scale eucalyptus plantations but were influenced by litter species, showing the preponderance of physical and chemical litter characteristics over environmental conditions in controlling leaf litter biological colonization in the study streams (Sena et al. 2020, 2021).
4.1 Impacts of Eucalyptus Plantations on Water Characteristics
Dissolved oxygen concentration was the only measured water characteristic that differed between eucalyptus and native streams. This result indicates that catchment-scale eucalyptus plantations can cause less environmental alterations than other anthropogenic impacts in tropical streams, such as agriculture (Taniwaki et al. 2016; Gutiérrez et al. 2024) or urbanization (Martins et al. 2015).
The low dissolved oxygen concentrations in eucalyptus streams may be related to an increase in the biological oxygen demand (O'Driscoll et al. 2016), resulting from slightly higher water temperature and dissolved nutrient (DIN) concentration. However, this potentially faster microbial activity in eucalyptus streams did not translate into faster leaf litter decomposition. Maybe higher biological oxygen demand was driven by stimulated bacteria activity, which generally has a low impact on the decomposition of CPOM when compared with fungi (Gessner et al. 2007, 2010; Baldrian et al. 2012).
4.2 Impacts of Eucalyptus Plantations on Litter Inputs
We found lower lateral inputs of CPOM (total litter and by litter type) in eucalyptus streams than in native streams. These differences can be attributed to differences in floristic composition and retention structures in the soil between plantations and native forests. It has been shown that Eucalyptus plantations can impact the flow of matter and energy from terrestrial to aquatic ecosystems in Mediterranean climates when plantations are present in or very close to the riparian vegetation (Abelho and Graça 1996; Pozo et al. 1997; Laćan et al. 2010), due to differences in the phenology, quantity, and quality of litter production in comparison with native forests. In the Cerrado biome, differences in floristic composition between plantations and native forests may, however, be more evident at other times of the year since vertical litter inputs (total litter and by litter type) did not significantly differ between stream types. Nevertheless, these differences in floristic composition may be retained in the litter accumulated on the soil, which later enters the streams. Also, the accumulation of eucalyptus woody material on the plantation floor (especially because the plantations have been abandoned) may contribute to retain more litter, thus reducing its movement to the stream during rain events. Differences in lateral litter inputs to streams also point to the importance of surface water runoff in mediating the effects of eucalyptus plantations (which are > 80 m from the stream) on organic matter flow between terrestrial and stream ecosystems (Leite et al. 2016; Tonin et al. 2017).
4.3 Impacts of Eucalyptus Plantations on Litter Decomposition and Associated Aquatic Communities
Leaf litter decomposition was slower in eucalyptus streams than in native streams. Effects of forest changes on litter decomposition are observed mainly if exotic plantations reach the riparian area and cause changes in litter inputs to streams (Ferreira et al. 2016). Although our streams had a native riparian buffer, we found lower lateral (by 25%) litter inputs to streams surrounded by catchment-scale Eucalyptus plantations. This lower organic matter supply in eucalyptus streams may result in lower benthic invertebrate abundance and microbial inoculum and explain the lower leaf litter decomposition in these streams.
Also, Eucalyptus leaf litter in eucalyptus streams, with the presence of antibiotic compounds (such as polyphenols and tannins), may contribute to lower fungal species diversity in these streams (Gomes et al. 2016), potentially reducing the decomposition rate of leaf litter compared to native streams (e.g., Jabiol and Chauvet 2012).
The effects of the catchment-scale Eucalyptus plantations on leaf litter decomposition were not influenced by the mesh type, inhibiting decomposing on both coarse- and fine-mesh bags to a similar degree. Invertebrate shredders generally are key players in the decomposition of allochthonous organic matter in streams (Hieber and Gessner 2002). However, shredders were rare (< 10%) in our study. Invertebrate shredders are often scarce in tropical regions, where leaf litter decomposition is primarily mediated by microbes (Gonçalves et al. 2007). Invertebrate scrapers can also play a role in leaf litter decomposition when they scrape the periphyton on litter surfaces (Wantzen and Wagner 2006), but they were also rare (< 10%) in our study, suggesting that scrapers also had little influence on leaf litter decomposition. Thus, although microbial communities are generally less sensitive to forest changes due to their functional redundancy (Ferreira et al. 2016), forest changes appear to affect the microbial colonization and leaf litter processing in tropical streams, as observed in other studies (Gomes et al. 2016; Ferreira et al. 2019). The lower dissolved oxygen concentration in eucalyptus streams may have inhibited fungal activity and slowed down leaf litter decomposition (Gessner et al. 2007; Medeiros et al. 2009; Gomes et al. 2018).
The effects of Eucalyptus plantations on leaf litter decomposition were also not influenced by the litter species identity. The lack of interaction between stream type and litter species reinforces the idea that plantation effects on leaf litter decomposition are not mediated by changes in invertebrate shredder metrics. The fact that we used a native litter species and two exotic species was probably of no consequence because home-field advantage (HFA) seems unlikely to have played a role. On the one hand, E. grandis leaf litter decomposed slower in eucalyptus than in native streams, contrary to what is postulated by the HFA hypothesis that litter decomposition is faster in its “home” environment than “away” due to the adaptation of decomposer communities to the local resource (Yeung et al. 2019; Fenoy et al. 2024). On the other hand, A. glutinosa leaf litter, new to both stream types, was the fastest decomposing leaf litter in eucalyptus and in native streams (even faster than the native species I. laurina), showing that the local decomposers had no major difficulty in processing this high-quality resource. In fact, HFA has been found especially when a recalcitrant novel leaf litter is introduced into an ecosystem that lacks such recalcitrant litter and where decomposers may lack the necessary enzymatic set to properly decompose it (Yeung et al. 2019; Fenoy et al. 2024). Thus, in this study, decomposers reacted to leaf litter characteristics and not to its origin.
Litter decomposition rates were higher for A. glutinosa, followed by E. grandis and I. laurina, which is explained by differences in litter characteristics among species. Recalcitrant compounds (lower concentrations in A. glutinosa, followed by E. grandis and I. laurina) commonly have negative effects on consumers (Boyero et al. 2017). These compounds (e.g., lignin and cellulose) can impede litter colonization, microbial degradation, and invertebrate fragmentation (Gonçalves et al. 2012; Kiffer et al. 2018). Also, A. glutinosa litter has high nutrient concentrations (lower C:N ratio), followed by E. grandis and I. laurina litter, making it a more attractive litter in the nutrient-poor Cerrado streams (Gonçalves et al. 2007; Callisto et al. 2015), where decomposer communities may be highly dependent on litter to satisfy their nutritional needs (Rezende et al. 2010; Sales et al. 2015). Our findings confirm that the innate physical and chemical characteristics of leaf litter significantly impact litter decomposition, regardless of litter origin (native or exotic) and stream type (Rezende et al. 2014; Sena et al. 2024).
Our study shows that the impact of catchment-scale Eucalyptus plantations on water quality is low compared to other anthropogenic disturbances such as agriculture (Taniwaki et al. 2016; Gutiérrez et al. 2024) or urbanization (Martins et al. 2015). However, catchment-scale Eucalyptus plantations change litter inputs to streams and litter decomposition in tropical streams despite the presence of a native riparian buffer. This suggests that the riparian buffer was not completely effective in protecting the streams from the impacts of Eucalyptus plantations. One possibility is that buffers were not wide enough, as previous studies have shown that buffer width is a crucial factor influencing buffer efficacy in protecting streams from human activities (Gilliam 2006; Sweeney and Newbold 2014) and that larger buffers promote faster leaf litter decomposition (Gutiérrez et al. 2024). Riparian buffer length may be another variable controlling buffer efficacy in protecting streams from human activities. Riparian buffers that are too local may not be as effective as buffers that are established over longer distances along the stream, especially in protecting streams from a catchment-scale land-use change (Gilliam 2006; Sweeney and Newbold 2014). Further studies should consider buffer characteristics (e.g., composition, width, length) when evaluating the effects of catchment-scale human activities on stream ecosystems.
Author Contributions
V.F. and J.F.G.J. conceived the ideas and designed the methodology. A.P. and G.S. collected the data. G.S. and R.D.S.R. analyzed the data. A.P. and G.S. led the writing of the manuscript with substantial inputs from R.D.S.R., V.F., A.M.T., R.S.R., and J.F.G.J. All authors contributed critically to the drafts and gave final approval for publication.
Acknowledgments
This study was supported by PROCAD-NF/CAPES (No. 173/2010), CAPES/Edital PNADB/2009 (No. 1098/2010), MCTI/CNPq No. 14/2013 - Universal/Universal 14/2013 (No. 471767/2013-1), CNPQ/ICMBIO/FAPs (No. 421288/2017-5 and 405290/2018-7), CNPq/Bolsas PQ (No. 302957/2014-6 and 310641/2017-9) MCTI/PELD/CNPq (No. 558233/2009-0), MCTI/CNPq/CT-AGRO/CT-SAÚDE/CT-HIDRO (No. 37/2013), MCT/CNPq/FNDCT/FAPs/MEC/CAPES/PRO-CENTRO-OESTE (No. 031/2010), EMBRAPA/Edital called 01/2011, FAP-DF/Edital 03/2015 (No. 193.000.870/2015), FAP-DF/Edital 05/2016 - Demanda Induzida - Água (No. 0193.000716/2016), FAPEMIG (No. APQ-00274-12), Fundação Universidade de Brasília (DPP No. 121366/2011) and the Portuguese Foundation for Science and Technology (FCT; projects UIDP/04292/2020 and UIDB/04292/2020 granted to MARE and project LA/P/0069/2020 granted to the Associate Laboratory ARNET). VF is grateful to FCT for financial support (SFRH/BPD/76482/2011, IF/00129/2014 and CEECIND/02484/2018). GS is grateful to the Brazilian National Council for Scientific and Technological Development (CNPq) through a postdoctoral fellowship (152642/2024-2). RSR is grateful to CNPq for project 403945/2021-6 and research productivity grant 302044/2022-1, and JFGJr is grateful to CNPq for the resources for the TWRA structuring project (Proc. 400439/2022-0) and the PDE grant (Proc. 200356/2022-4).
Conflicts of Interest
The authors declare no conflicts of interest.




