Value of Magnetic Resonance Spectroscopy for Examining Fetal Brain Development in Mid- to Late Pregnancy
Funding: This work was supported by China Society for Maternal and Child Health Research (Gant/Award Number: 2023CAMCHS003A17).
ABSTRACT
Background
Magnetic resonance spectroscopy (MRS) represents a significant advancement in the noninvasive assessment of brain metabolism. MRS can provide valuable metabolic information and facilitate more accurate diagnoses of intrauterine fetal brain development than was previously possible. To obtain information regarding normal intrauterine fetal brain metabolism and to establish gestational age-specific reference values for normal fetal brain metabolites for subsequent use in MRS, we conducted MRS scans of normal fetal brains during mid- to late-term pregnancies, along with related processing.
Methods
In this prospective study, MRS scans were conducted on 109 fetuses, with a total of 54 normal fetal brains enrolled on the basis of specific inclusion and exclusion criteria. We analyzed metabolic ratios, including the sum of N-acetylaspartate (NAA) and total N-acetylaspartate (tNAA), total choline (tCho), inositol (Ins), and total creatine (tCr), in relation to gestational age.
Results
Gestational age was significantly correlated with specific metabolic ratios (Ins/tCr: r = −0.75, p < 0.0001; tCho/tCr: r = −0.50, p < 0.0001), especially tNAA/tCho (tNAA/tCho: r = 0.54, p < 0.0001) and tNAA/Ins (r = 0.56, p < 0.0001), providing a baseline for fetal brain metabolic assessment. Linear regression analysis was used to calculate regression lines for fetal brain metabolite ratios. Slopes were tested at p of 0.05.
Conclusions
The current findings confirmed a significant correlation between fetal brain metabolites and gestational age, supporting the feasibility of establishing standard values for these metabolites in fetal brain assessment.
Abbreviations
-
- Asp
-
- L-aspartate
-
- Cho
-
- choline
-
- Cr
-
- creatine
-
- Cr/PCr/CK
-
- creatine/phosphocreatine/creatine kinase
-
- GA
-
- age of gestation
-
- Glu
-
- L-glutamate
-
- Ins
-
- myo-inositol
-
- MRS
-
- magnetic resonance spectroscopy
-
- NAA
-
- N-acetylaspartate
-
- NAAG
-
- N-acetylaspartylglutamate
-
- PRESS
-
- Point RESolved Spectroscopy sequence
-
- PtdCho
-
- phosphatidylcholine
-
- tCho
-
- total choline
-
- tCr
-
- total creatine
-
- TE
-
- echo time
-
- tNAA
-
- total NAA
-
- VOI
-
- volume of interest
1 Introduction
Magnetic resonance spectroscopy (MRS) represents a significant advancement in the noninvasive assessment of brain metabolism [1]. As maternal-fetal medicine has progressed, attention regarding neuroprotection has moved to the prenatal phase, during which the groundwork for enduring brain development and neurocognitive function is laid [2, 3]. The mid- to late gestation period is critical for fetal central nervous system development, during which most of the general structure of the fetal brain is formed. MRS can provide important metabolic information and facilitate more accurate diagnoses of intrauterine fetal brain development [4]. As a result, MRS is increasingly recognized as a valuable complement to morphological imaging techniques. Despite its growing significance, a substantial gap remains in our understanding of the metabolic processes and information linked to human brain development in utero [5-8]. This lack of knowledge highlights the need for further research into how metabolic changes during gestation can impact brain maturation and function, as well as the potential implications for understanding developmental disorders. Additionally, MRS could potentially be applied to elucidate the pathophysiological mechanisms of fetal brain injury, allowing for earlier intervention and improved prognostic predictions [9]. Therefore, it is essential to systematically assess fetal brain metabolism at each gestational week and establish standard values for fetal brain metabolites.
2 Materials and Methods
A total of 109 pregnant women who underwent fetal cranial magnetic resonance imaging (MRI) scans in the Department of Radiology, Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University were prospectively enrolled in this study. All pregnant women provided written informed consent. The inclusion criteria for the fetuses were: (1) singleton pregnancies, and (2) each fetus exhibited normal brain MRI findings and high-quality MRS scans, as confirmed by two radiologists. The exclusion criteria included: (1) maternal comorbidities such as gestational diabetes mellitus, hypertension, pre-eclampsia, and other conditions that could interfere with normal fetal growth and development; (2) maternal dysfunction of vital organs such as the liver and kidneys; (3) the use of sedatives during the study period; (4) poor quality of MRS scans. Ultimately, 54 healthy fetuses were included in this study, with gestational ages ranging from 22 to 39 weeks; specifically, 23 cases were classified as mid-gestation and 31 cases as late gestation. Fetal cranial imaging in axial, coronal, and sagittal planes was performed using the Siemens Healthcare MAGNETOM Vida 3.0 T scanner and a 32-channel body phased matrix coil manufactured in Germany. Guided by these conventional images, spectra were acquired using the single voxel technique in conjunction with a rotational echo Point RESolved Spectroscopy sequence featuring a short echo time (echo time = 35 ms). The volume of interest (VOI) was positioned in the region of the basal ganglia to obtain metabolic information regarding the normally developing brain. The VOI measured 3.375 cm3 (15 mm × 15 mm × 15 mm) at mid-pregnancy and expanded to 8 cm3 (20 mm × 20 mm × 20 mm) during late pregnancy (Figure 1). Linear regression analysis was used to calculate regression lines for fetal brain metabolite ratios. Slopes were tested at p of 0.05. The clinical trial was registered in Chinese Clinical Trial Registry with the registration number ChiCTR2500102512. The study was approved by the research ethics committee of Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University (Approval Number: 2024-108).
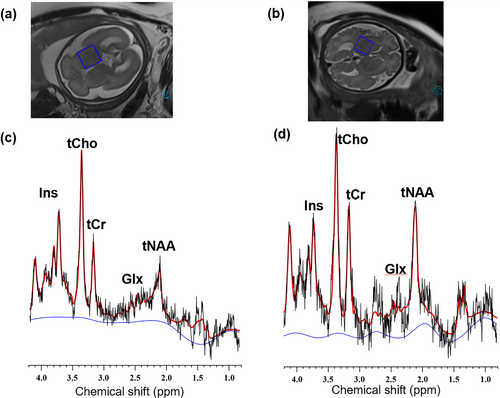
1H MRS of fetal brains at 26 and 36 weeks of gestation, respectively (blue squares represent MRS single voxel scans localized in the basal ganglia region.). (a and b) SSFSE T2WI images of the fetal at 26 and 36 weeks. (c and d) 1H MRS of the fetal brain at 26 and 36 weeks. SSFSE, single shot fast spin echo.
3 Results
As shown in Figure 2, the results revealed no significant correlation between total N-acetylaspartate (tNAA)/total creatine (tCr) and week of gestation (r = 0.08, p = 0.56), whereas tNAA/total choline (tCho) (r = 0.54, p < 0.0001) and tNAA/inositol (Ins) (r = 0.56, p < 0.0001) significantly increased with gestational week. Additionally, tCho/tCr (r = −0.50, p < 0.0001) and Ins/tCr (r = −0.75, p < 0.0001) decreased with increasing gestational weeks.
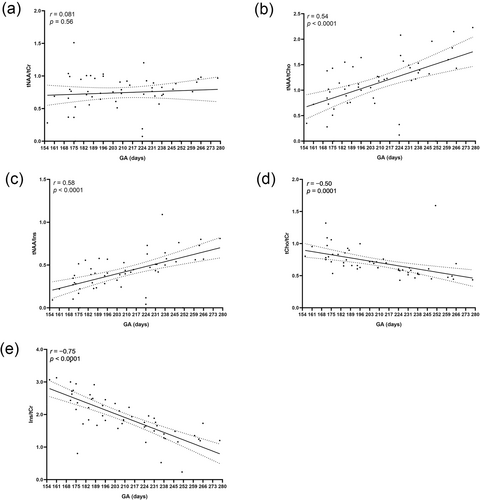
Correlation between the ratios of (a) tNAA/tCr (b) tNAA/tCho, (c) tNAA/Ins, (d) tCho/tCr, and (e) Ins/tCr in the fetal brain and gestational age. GA, gestational age; Ins, myo-inositol; tCho, total choline-containing compounds; tCr, sum of creatine and phosphocreatine; tNAA, sum of N-acetylaspartylglutamate and N-acetylaspartate.
4 Discussion
The most prominent signals in proton magnetic resonance spectra of a healthy brain originate from acetyl resonances at approximately 2.0 ppm [10-12]. NAA and its derivative, N-acetylaspartylglutamate (NAAG), have long been acknowledged as some of the most prevalent metabolites found within the central nervous system. NAA, which is an acetylated form of L-aspartate (Asp), plays a crucial role in various metabolic processes. NAAG is a dipeptide composed of both NAA and L-glutamate (Glu) and is also of considerable metabolic importance. Both of these substances are synthesized by neurons in the brain, underscoring their significance in neurobiological functions and potential implications in neurological research. These metabolites are found almost exclusively in neurons and their precursor cells, leading to their classification as neuronal markers. Increased NAA signaling is believed to indicate neurogenesis or maturation. During embryonic development, the fetal requirement for creatine (Cr) is partially met through the active transport of Cr from the mother to the fetus [13]. The creatine/phosphocreatine/creatine kinase (Cr/PCr/CK) system is crucial during brain development, as it provides sufficient adenosine triphosphate (ATP) to support neuronal growth and ensure axonal and dendritic development [14]. Consequently, Cr and its derivative, phosphocreatine (PCr), are closely associated with cellular energy metabolism in the brain and serve as markers reflecting cellular energy status. Although Cr is present in both neurons and glial cells, the ratio of the peak areas of these metabolites is thought to respond to the neuronal generation of neuroglial proliferation, thereby providing a quantitative measure of neuronal integrity [15-17]. NAA is detectable on the brain spectrum as early as 22 weeks of gestation and is believed to reflect axonal and synaptic development as demonstrated by Kato et al. [18]. In the present study, no significant correlation was found between the ratio of tNAA to tCr and gestational age. However, the ratios of tNAA to tCho and tNAA to Ins exhibited significant correlations [19]. This finding suggests that the tNAA to tCr ratio increases during pregnancy. Given that NAA is not detected in neuroglia and oligodendrocytes, the presence and increase of NAA in the fetal brain imply the development of axons and the process of myelination as gestational weeks progress [20]. Additionally, the rise in creatine (Cr) levels indicates adequate energy availability in neuroglia, which also reflects sufficient energy supply to neurons, correlating with an increase in neuron number and cellular energy metabolism in the fetal brain as gestational weeks advance [21]. This observation aligns with the findings of Evangelou et al., who reported an increase in fetal brain creatine levels between 18 and 40 weeks of gestation [22]. In contrast, we specifically placed the VOI in the basal ganglia region, utilizing dimensions of 15 mm × 15 mm × 15 mm for mid-trimester fetuses and 20 mm × 20 mm × 20 mm for late-trimester fetuses, to minimize errors associated with cerebrospinal fluid. By positioning the VOI in the basal ganglia, we facilitate the subsequent examination of the bilateral cerebral hemispheres within the same fetal brain, allowing us to investigate potential differences in the metabolic activity of the two hemispheres. Furthermore, postnatal studies have clearly demonstrated that brain creatine concentration increases during the first 3 months of life [23, 24].
The choline peak occurs at 3.2 ppm and comprises choline phosphate, choline glycerol 3-phosphate, and choline, which is an intermediate metabolite of the neurotransmitter acetylcholine and membrane phospholipids. Phosphatidylcholine (PtdCho) plays a crucial role as a primary constituent of cellular membranes and is vital for the growth and division of cells, especially in the developing brain [25]. Derived from PtdCho, sphingolipids are important for the myelination of axons in the central and peripheral nervous systems [26]. Consequently, it is widely accepted that total choline compounds serve as markers of cell membrane synthesis and cell division, with both neuronal and glial cells participating in choline uptake [27]. Fetuses and newborns have a particularly high demand for choline, which is vital for normal development [28]. This division and growth occur rapidly during the 6th month of gestation and continue until approximately 3–5 years of age [29, 30]. The choline peak represents the highest peak in the MRS sequence of the fetal brain, reflecting elevated substrate levels as myelination progresses. Fetal brain choline peak levels exceed those of neonates and adults, with a gradual decline in the months following birth because of the formation of myelin and a reversal of the relationship with the NAA peak. Researchers have observed a twofold increase in ceruloplasmin in the cerebral cortex and a threefold increase in ceruloplasmin in the white matter from the 10th week of gestation to 2 years of age [31]. Myelin formation in human brain tissue primarily occurs during the late embryonic period and in the years following birth, aligning with the trend of decreasing choline content in the MRS of the fetal brain. In the current study, total choline levels gradually decreased with advancing gestational weeks, reflecting ongoing fetal brain development as myelin forms in late pregnancy. This finding is consistent with previous studies by Evangelou et al. and Kok et al. [22, 32].
Ins is recognized as a neuroglial marker, and the current findings indicate that its peak levels decrease with advancing gestational weeks (23–39 weeks of gestation), consistent with the findings of Girard et al. [33].
The current study involved several limitations. First, this study focused solely on the correlation between fetal cerebral metabolites and gestational age during the middle and late stages of pregnancy, leaving the changes in fetal cerebral metabolites during the early stages inadequately understood. Second, we did not include fetuses with abnormal brain development in our study, which limits our ability to compare brain metabolites between normal and abnormal fetuses. Finally, the small number of late-gestation fetuses (for instance, only one case each of 38- and 39-week gestation) may have introduced bias.
5 Conclusions
MRS is not commonly employed in fetal testing or clinical decision-making. However, it can provide valuable insights into brain metabolism, particularly when subtle lesions are observed on conventional MRI. In light of the diagnostic potential of 1H-MRS within pediatric neurology, this innovative approach could also assist in identifying pathological features in the developing fetal brain [34].
Author Contributions
Dejuan Shan: data curation (equal), writing – original draft (lead). Yi Zhang: conceptualization (equal). Maobo Wang: data curation (supporting), validation (supporting). Yanyan Liu: data curation (supporting). Yudong Wang: data curation (supporting). Lianxiang Xiao: conceptualization (equal), writing – review and editing (lead).
Acknowledgments
The authors have nothing to report.
Ethics Statement
The clinical trial was registered in Chinese Clinical Trial Registry with the registration number ChiCTR2500102512. The study was approved by the research ethics committee of Shandong Provincial Maternal and Child Health Care Hospital Affiliated to Qingdao University (Approval Number: 2024-108).
Consent
All pregnant women provided written informed consent.
Conflicts of Interest
This article belongs to a special issue (SI)-Fetal imaging, maternal and children imaging. As the SI's Guest Editor, Professor Lianxiang Xiao is excluded from all the editorial decision related to the publication of this article. The remaining authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to ethical restrictions. Imaging will not be shared due to privacy reasons.




