Alpha-emitters and targeted alpha therapy in cancer treatment
Abstract
Alpha emitters are radionuclides with good pharmacological characteristics for the treatment of cancer because they decay by emitting high linear energy transfer particles. Recent advancements in isotope production and purification and the generation of novel techniques for optimum targeting have led to the development of targeted alpha therapy (TAT). The great cytotoxic potential of α-particle emissions combined with monoclonal antibodies, peptides, small compounds, or nanoparticles has led to investigations of TAT in the pre-clinical context and more recently, in oncology clinical trials. Numerous studies have shown that TAT is effective both in vitro and in vivo. The first α-emitter to obtain FDA approval for the treatment of prostate cancer with metastatic bone lesions was radium-223 dichloride. Many clinical trials are being conducted to evaluate the efficiency and safety of several radionuclides in cancer treatment, including radium-223, astatine-211, actinium-225, bismuth-213, lead-212, and thorium-227. This review provides an overview of the therapeutic use of these radionuclides and a summary of the studies that lay the groundwork for future clinical advancement.
Abbreviations
-
- 131I
-
- iodine-131
-
- 177Lu
-
- lutetium-177
-
- 211At
-
- astatine-211
-
- 212Pb
-
- lead-212
-
- 213Bi
-
- bismuth-213
-
- 225Ac
-
- actinium-225
-
- 226Ra
-
- radium-226
-
- 227Th
-
- thorium-227
-
- 229Th
-
- thorium-229
-
- 232Th
-
- thorium-232
-
- 233U
-
- uranium-233
-
- 90Y
-
- yttrium-90
-
- AML
-
- acute myeloid leukemia
-
- DOTA
-
- 1,4,7,10-tetraazcyclododecane-1,4,7,10-tetra acetic acid
-
- DOTATATE
-
- DOTA-DPhe1-Tyr3-octreotate
-
- DOTATOC
-
- somatostatin analog [DOTA0, Tyr3] octreopeptide
-
- DTPA
-
- diethylene triamine pentaacetic acid
-
- FAP
-
- fibroblast activating protein
-
- FAPI
-
- FAP inhibitors
-
- GBM
-
- glioblastoma multiforme
-
- h8C3
-
- humanized 8C3
-
- HER2
-
- human epidermal growth factor type 2
-
- PSMA-TTC
-
- PSMA-targeted thorium-227 conjugate
-
- RBE
-
- relative biological effectiveness
-
- RLT
-
- radioligand therapy
-
- sdAb
-
- single-domain antibody
-
- TAT
-
- targeted alpha therapy
-
- TCMC
-
- 1, 4, 7, 10-tetramethylcarboformyl-1, 4, 7, 10-tetrazazecyclododecane
1 INTRODUCTION
Nuclear medicine is used not only for imaging, which involves observing the distribution of radionuclides within an organism, but also for therapy, which involves the delivery of radiopharmaceuticals to specifically irradiate malignant cells. Radiopharmaceuticals typically consist of a radioactive moiety whose radiation enables the destruction of specific cells and a molecule to transport the radioactive moiety to the targeted organ or tissue. Various therapeutic strategies focused around the use of radiopharmaceuticals have been developed, including targeted radionuclide therapy, peptide receptor radionuclide therapy, radioimmunotherapy (RIT), etc.
In the past few years, β-emitters such as iodine-131 (131I), lutetium-177 (177Lu), and yttrium-90 (90Y) have been used as radionuclides for tumor treatment. With the increase in isotope production and purification technologies, α-emitters represent a promising direction in the treatment of tumors [1]. Compared to β-emitters, α-emitters have several advantages. The energy deposition per unit path length for α-emitters is much higher. Studies showed that α-emitters produce significantly more free radicals and fatal DNA double-strand breaks [2]. The limited range of α-particles (40–100 μm) allows for selective targeting of tumors without destroying healthy tissues. Additionally, α-emitters have the benefit of exhibiting cytotoxicity that is independent of the cell cycle or oxygen levels [3, 4], which make them useful for treating hypoxic cancers that are frequently resistant to radiation. The differences between α-emitter and β-emitter path lengths and ionization density are shown in Figure 1.
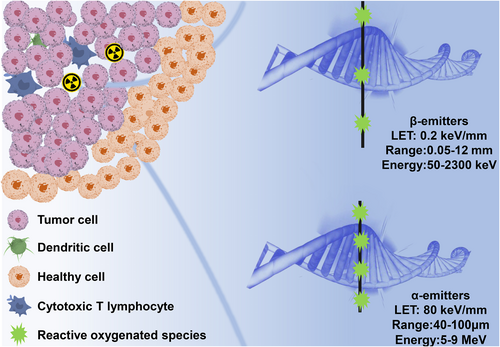
Path length and ionization density of α-emitters versus β-emitters.
In this review, we present a comprehensive overview of the most promising α-emitters, including radium-223 (223Ra), astatine-211 (211At), actinium-225 (225Ac), bismuth-213 (213Bi), lead-212 (212Pb), and thorium-227 (227Th). We discuss the production and the radiochemical design of these α-emitters and summarize the most recent clinical studies and preclinical research on α-emitters. The main objective of this review is to evaluate the potential utility of α-emitters for targeted alpha therapy (TAT).
2 PRODUCTION OF α-EMITTERS
The nuclear properties and production of the clinically relevant α-emitters discussed in this review are summarized in Table 1. The radioactive decay chain is shown in Figure 2.
| Isotope | Half-life | Decays per atom | Production method | Max energy |
|---|---|---|---|---|
| 223Ra | 11.43 days | 4α,2β- | 227Ac generator | 5.87 |
| 211At | 7.21 h | 1α, 1EC | α-particles cyclotron | 5.87 |
| 225Ac | 9.92 days | 4α,2β- | 229Th generator | 5.83 |
| 213Bi | 45.61 min | 1α,2β- | 225Ac generator | 5.87 |
| 212Pb | 10.64 h | 1α,2β- | 224Ra generator | 6.09 |
| 227Th | 18.70 days | 5α,2β- | 227Ac generator | 6.04 |
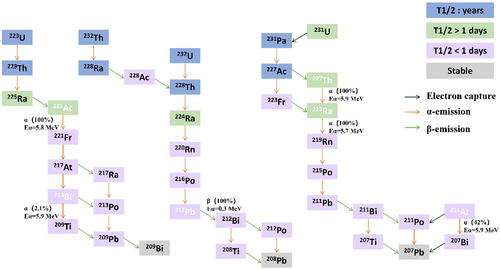
Radioactive decay chains of alpha emitters used in medicine.
2.1 Radium-223
Radium is an alkaline earth metal that originates from the decay of 235U or one of its daughter nuclides, such as 231Th (25.5 h), 231Pa (3.28 × 104 y), 227Ac (21.7 years), or 227Th (18.7 days) [5]. The production of 223Ra for clinical applications uses 227Ac/227Th generators in which the parent isotopes are on actinide chromatographic resins. A 223Ra-chloride solution is obtained after elution and purification on a cation exchange column. The disintegration sequence of 223Ra generates four high energy α particles, two β particles, and γ rays leading to total energy emitted of approximately 28 MeV, which includes 219Rn (3.96 s), 215Po (1.78 ms), 211Pb (36.1 min), 211Bi (2.14 min), 207Tl (4.77 min), or 211Po (0.52 s) and stable 207Pb. The cascade of α-particles produced (approximately 96% of the total energy) allows increasing the radiation dose received by the targeted tissues, while low energy γ-components (154 keV, 269 keV) are of interest for monitoring [6].
2.2 Astatine-211
The nuclear reaction 209Bi(α,2n) 211At is produced by bombarding solid 209Bi targets with α-beams at energies below 28.4 MeV [7]. When the energy is <28.4 MeV, 211At is created, which is isotopically pure. An undesired byproduct, 210At, is coproduced at energies >30 MeV. One stable 207Bi α-emission with a half-life of 7.21 h is produced by the radioactive decay of 211At, making it appropriate for use in medical procedures.
2.3 Actinium-225
With a half-life of approximately 10 days, 225Ac decays to 209Bi; the decay path generates a net four α-particles and two β-particles with maximal energies of 1.6 and 0.6 MeV. In preclinical research and clinical administration, 225Ac is primarily radiochemically isolated from thorium-229 (229Th) generated due to the decay of aged uranium-233 (233U). As an alternative, 225Ac can be produced by bombarding radium-226 (226Ra) in a medical cyclotron with <20 MeV beam energy, causing it to undergo 226Ra(p,2n) 225Ac reaction. 225Ac is also produced in substantial quantities by irradiating thorium-232 (232Th) with high-energy protons, where 226Ac and 227Ac are produced [8-10].
2.4 Bismuth-213
213Bi is a promising α-emitting radionuclide that has been successfully used in clinical trials for various blood and solid tumors [11]. It is a decay product of 225Ac with a relatively short half-life of 45.6 min and commonly used as an in vivo radioisotope generator in the form of a 225Ac/213Bi generator [12]. The 225Ac/213Bi generators use cation and anion exchange or extraction chromatography [13, 14]. The double generator using AG MP-50 cation exchange resin is the most mature, the most widely used, and the preferred system in clinical research.
2.5 Lead-212
Thorium nuclide 212Pb is part of the 232Th and 232U decay chains: 232Th (T1/2 = 1.4 × 1010 y) undergoes an α decay to produce 228Ra (T1/2 = 5.75 years); 228Ra undergoes a β− decay to produce daughter 228Ac (T1/2 = 6.15 h); 228Ac undergoes β− decay again with 228Th (T1/2 = 1.91 years), which then forms 224Ra (T1/2 = 3.6 days); and 212Pb can be separated from 228Th and 224Ra, the natural decay products of 232Th. Therefore, 228Th and 224Ra are parent radionuclides suitable for the 212Pb generator [12].
Friedman et al. deposited 228Th on a column containing sodium titanate and used the water flow to carry out 220Rn [15], and a D50 organic cation exchange resin column was used to elute 212Pb. Another approach is to use 224Ra, which has a shorter half-life than 228Th, as the parent nuclide. 224Ra is the preferred source of radionuclides because of its ability to reduce radiation hazards. Radium and thorium are separated by dissolving thorium in concentrated nitric acid to form the thorium nitrate anion; the solution is added to an anion exchange resin to elute 224Ra. Eluted 224Ra was loaded onto the cation exchange resin and used as a 212Pb/212Bi generator.
2.6 Thorium-227
227Thorium is the progenitor nuclide of 223Ra [16]. Its half-life is 18.7 days, and it primarily decays as α particle emission with five high-energy α particles and two β particles in the decay chain, which subsequently decays to stable 207Pb [17, 18]. 227Th is isolated from the 227Ac-based generator. Furthermore, high-purity 227Th is obtained through repeated extraction and separation processes of the short-lived decay product 223Ra.
3 CHEMICAL LABELING
3.1 Radium-223
Radium belongs to group 2 of the periodic table and therefore has similar chemical properties to magnesium, calcium, and barium. With an electronic configuration of [Rn]7s2, the corresponding divalent cation Ra2+ is the only species formed. Because 223Ra2+ is the largest divalent cation in the periodic table (8-coordinate ionic radius, 148 p.m.), the identification of suitable chelators for 223Ra2+ remains challenging [19]. Studies on the complexation of radium cations by 1-,4-,7-,10-tetraazcyclododecane-1,4-,7-,10-tetra acetic acid (DOTA), amino carboxylic acids, cryptands, and calixarenes were unsuccessful. The coordination chemistry of radium remains rather limited. Thus, the lack of an efficient chelator has prevented the conjugation of 23Ra to biomolecules as with other alpha emitters. Furthermore, even if retention in structures such as nanoparticles is possible, 223Ra is mainly used in its chlorine salt form [223Ra]RaCl2 [20].
We reasoned that macropa would be an effective chelator for this promising therapeutic radiometal. Macropa quantitatively formed the [223Ra][Ra(macropa)] complex within 5 min at RT and pH 6. The concentration of ligand required to achieve 50% radiolabeling efficiency was 13 mM. Importantly, [223Ra][Ra(macropa)] remained ∼90% intact in human serum at 37°C after 12 days.
3.2 Astatine-211
While At has a metallic quality, no chelating ligand studied thus far has been able to create a 211At complex stable enough for TAT applications [21]. Nonactivated aryl-At bonding is used in several At labeling techniques, with electrophilic substitution processes using organometallic compounds, such as compound 2, N-succinimidyl 3-(tri-alkylstannyl)benzoate, as the most common technique.
Nonactivated arylboronic acid derivatives react rapidly with electrophilic At species. Recent research demonstrated that nucleophilic substitution processes using aryl boronic acids/esters can produce extremely effective 211At-labeling. Additionally, mAb conjugate labeling by nucleophilic substitution is possible with aryliodonium salts [22]. In preclinical and clinical research, the boron cage compound isothiocyanatophenyl-closo-decaborate(2-) (B10) was used as a reagent for 211At-labeling. The aromatic B10 moiety (75%–90% radiochemical yield, 1 min) contributes to excellent 211At tagging efficiency and in vivo stability. New boron cage labeling reagents are being investigated for more favorable pharmacokinetic properties and better tissue distribution to potentially stabilize 211At in a higher oxidation state.
3.3 Actinium-225
The free daughters of 225Ac such as 213Bi and 221Fr might spread out or be carried to different target organs, where they accumulate and induce radiotoxicity, particularly in the kidney. The kidney is a prime location for the accumulation of free radioactive bismuth because renal proximal tubular cells have proteins that resemble metallothionein and bind bismuth. Additionally, renal tubular cells may be able to collect 221Fr [23]. Chelators were used to improve the stability of 225Ac, and DOTA is the most widely used chelator. Previous studies reported that macrocyclic ligand macropa has better labeling characteristics for 225Ac radiometal compared with ligands [24]. However, 225Ac has yet to be incorporated into radiopharmaceuticals used in clinical settings. In vivo kinetic and thermodynamic instability prevents the use of linear bifunctional chelators such as ethylenediamine tetraacetic acid (EDTA) and diethylene triamine pentaacetic acid (DTPA) for tagging 225Ac-based radiopharmaceuticals.
3.4 Bismuth-213
The key consideration for 213Bi labeling is the appropriate rate of complex formation to accommodate its short half-life. The complexation kinetics of acyclic ligand DTPA is very fast at room temperature and has a high thermodynamic stability constant for many metal ions [25]. The stability constant of 10 for the complex of this ligand with Bi(III), the most common and stable oxidation state of Bi, is sufficient for the formation of a kinetically inert complex in vivo. However, despite the very high initial stability constant of the Bi(III)DTPA complex, the expected good in vivo stability was not observed with bifunctional DTPA derivatives conjugated with monoclonal antibodies. The division of the base of the cyclohexyl DTPA in the ligand of the DTPA derivative trans-cyclohexyl DTPA (CHX-A″-DTPA) into chelators increases rigidity, provides preorganization at metal ion binding sites, and increases kinetic inertness [25]. The complexation of CHX-A″-DTPA ligand with Bi(III) not only maintains relatively rapid complex formation kinetics but also provides very high in vivo stability [26]. The current 213Bi TAT studies mainly use CHX-A″-DTPA, which is the most suitable ligand for bismuth chelation [27].
3.5 Lead-212
212Pb has a strong affinity to nitrogen and oxygen, and the coordination properties of bifunctional chelators contribute to the formation of radioactive metal complexes that can be attached to carrier molecules. Ligands that stabilize the chelation of 212Pb and its decay to form 212Bi are more attractive because off-target free bismuth from the labeling complex tends to accumulate and lead to toxicity in kidneys [28].
A chelating agent of 1, 4, 7, 10-tetramethylcarboformyl-1, 4, 7, 10-tetrazazecyclododecane (TCMC) was developed; it was more stable to Pb(II) and more resistant to complex dissociation at lower pH in acidic cells compared with Pb(II)DOTA complexes. TCMC ligands also show other benefits, including more efficient conjugation with monoclonal antibodies and higher labeling yields [29-31]. These advantages support the use of TCMC as a ligand for the in vivo generator of 212Pb/212Bi.
3.6 Thorium-227
The therapeutic radionuclide 227Th is primarily used for labeling of numerous different vectors such as monoclonal antibodies and nanocarriers. Monoclonal antibodies with tumor-targeting properties can be efficiently labeled using 227Th via the covalent attachment of the ε-amino group of the lysine residue to the octadentate 3,2-HOPO chelator, resulting in a product with high yield, purity, and stability under physiological conditions [32].
A summary of the chelators of α-emitters is shown in Figure 3.
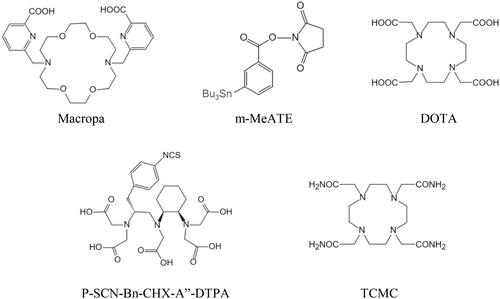
Chelators of α-emitters.
4 IONIZING RADIATION MECHANISM
Radiation exposure to cells leads to direct cellular effects (through energy transfer, such as DNA damage and cross-fire effects) and indirect cellular effects (reactive oxygen species generated by water radiolysis and radiation induced bystander effects, that is, signal transmission from radiation to adjacent cells, inducing apoptosis of cells not directly exposed to ionizing radiation or an immune response called an abscopal effect) [33, 34]. These effects can be quantified by determining the relative biological effectiveness (RBE). RBE is the ratio of two radiation doses, the dose of one type of radiation compared with a standard dose of radiation, usually from an external beam, needed to achieve a certain biological response. The RBE is reliant on several variables, including the particle type, absorbed dose, and tissue type, and provides a precise evaluation of the therapeutic potential for a given application.
5 PRECLINICAL STUDIES
Because of high cytotoxicity, α-particles need carriers for delivery to tumors. Peptides, monoclonal antibodies, nanoparticles, and other molecules can serve as carriers of α-particles. Small compounds frequently exhibit accelerated tumor accumulation and normal tissue clearance, which is consistent with the half-life of 211At. Because particles have a short route length and only cause minor cell damage, tiny molecules with higher penetration may be useful for tumor treatment.
The benefits of radiolabeled peptides include their accessible radiolabeling, relatively straightforward chemical synthesis, quick clearance from circulation, quick penetration, even tissue distribution, and reduced immunogenicity [35]. TAT based on antibodies is being explored in preclinical and clinical studies [36, 37]. Single-domain antibodies (nanobodies), with a molecular weight of roughly 15 kDa, are the variable domains of heavy chain antibodies from Camelidae. Nanobodies have several advantages compared with conventional monoclonal antibodies, including high affinity, enhanced stability, good water solubility, and powerful tumor penetration [38], and are suitable carriers for α-particles to target tumors. A summary of the preclinical studies on α-emitters is presented in Table 2.
| Targeted alpha therapy | Molecular target | Cancer type | References |
|---|---|---|---|
| 211At | - | Differentiated thyroid cancer | [39] |
| 211At-octreotide | SSTR2 | Lung cancer | [40] |
| 211At-trastuzumab | HER2 | Breast cancer | [41] |
| iso-211At-SAGMB-VHH_1028 | HER2 | Breast cancer | [42] |
| iso-[211At]AGMB-PODS-5F7 | HER2 | Breast cancer | [43] |
| 211At-ATE-MnO2-BSA | - | Breast cancer; colon cancer | [44] |
| 225Ac-FAPI-46 | FAP | Human pancreatic cancer | [45] |
| [225Ac]Ac-DOTA-2Rs15d | HER2 | HER2pos ovarian cancer | [46] |
| 225Ac-CD45 | CD45 | Acute myeloid leukemia | [47] |
| 213Bi-h8C3 | - | Melanoma | [48] |
| 213Bi-DTPA-2Rs15d | HER2 | Ovarian cancer | [49] |
| 213Bi-DOTATOC | SSTR2 | Pancreatic tumors | [50] |
| 213Bi-DOTATATE | SSTR2 | K562-SST2 cell line | [51] |
| 212Pb-sulfur colloid | - | Ovarian carcinoma | [52] |
| [212Pb]Fe(OH)2 | - | Ovarian carcinoma | [53] |
| 212Pb-NNV003 | CD37 | Leukemia | [54] |
| 212Pb-rituximab | CD20 | Lymphoma | [55] |
| 212Pb-L1-L5 | PSMA | Prostate cancer | [56] |
| 227Th-PSMA-TTC | PSMA | Prostate cancer | [57] |
| 227Th-MSLN-TTC | MSLN | Mesothelioma and ovarian cancer | [58] |
| 227Th-rituximab | CD20 | Lymphoma | [59] |
| 227Th-trastuzumab | HER2 | Breast cancer | [60] |
| 227Th-anti-CD22 antibody | CD22 | Non-Hodgkin lymphoma | [61] |
- Abbreviations: FAP, fibroblast activating protein; HER2, human epidermal growth factor type 2; SSTR2, somatostatin receptor subtype 2 .
5.1 Astatine-211
Patients with metastatic differentiated thyroid carcinoma are commonly treated with 131I. However, some tumors are resistant to this treatment. 211At is a radiohalogen with iodine-like chemical characteristics. In studies of in vitro treatments with thyroid cancer cells, [211At]NaAt significantly increased DNA double-strand damage compared with [131I]NaI, and a higher therapeutic efficacy was seen for [211At]NaAt in an animal model of preclinical thyroid cancer [39]. Therefore, the use of 211At in TAT to treat advanced differentiated thyroid carcinoma is quite promising.
Our group synthesized 211At-SPC-octreotide, which could target somatostatin receptor subtype 2. It exhibits rapid organ clearance 24 h after injection and increased uptake in the spleen, lung, stomach, and intestines in the early stages of the post-injection period. A radiation dose–dependent induction of apoptosis in cells by 211At-SPC-octreotide was observed. Therefore, 211At-labeled octreotide may be a potential cancer treatment [40].
In addition to peptides, monoclonal antibodies also serve as targets. Trastuzumab is a monoclonal antibody that targets human epidermal growth factor type 2 (HER2). By binding to HER2, it prevents human epidermal growth factors from adhering to HER2 and thereby limits the development of cancer cells. The capacity of 211At-trastuzumab to bind to breast cancer cells was found to be extremely selective. The biological effect of 211At-trastuzumab was twice as great as that of external radiation therapy [41], and 211At-trastuzumab inhibited the growth of HER2-positive tumor cells. Another study showed that 211At-trastuzumab extended median survival without posing a severe risk of harm in the treatment of liver metastases from primary gastric cancer [62].
Trastuzumab has shown some promising benefits, but the size of intact antibodies causes sluggish and uneven transport to tumors and prolonged residence time in healthy tissues [63].
Preclinical research showed that HER2-single-domain antibodies successfully transport to HER2-positive brain tumors and penetrate tumors much more quickly than whole antibodies [63, 64]. To investigate the effectiveness of the HER2 single-domain antibody, Feng et al. designed iso-211At-SAGMB-5F7 and iso-211At-SAGMB-VHH_1028. In most treated animals, full tumor regression was seen 200 days following therapy, and the treatments showed good therapeutic efficacy against HER2-positive breast cancer xenografts at a single dose [42]. However, radiolabeling prosthetic groups such as iso-[211At]SAGMB react with multiple lysine residues in the single-domain antibody (sdAb) fragment, resulting in a combination of radioconjugates with a range of properties. Because of the variability and irreproducibility inherent in random labeling, particularly at higher radioactivity levels required for therapeutic TAT, there are significant barriers to the efficient use of radioimmunoconjugates. Feng et al. thus described a method for radiohalogenating sdAbs at a particular location using a prosthetic compound that possesses both a residualized moiety and a thiol-reactive PODS activity. The in vitro stability was higher for iso-[211At]AGMB-PODS-5F7GGC than for iso-[211At]MEAGMB-5F7GGC, and tumor targeting was excellent [43].
In addition to directly killing tumor cells, the 211At-conjugates improved CD4+ T cell and macrophage recruitment [65]. More data is required to support the pro-inflammatory effects because CD4+ T cells also contain immunosuppressive T-regulatory cells. Our group constructed 211At-ATE-MnO2-BSA, which showed enhanced anti-cancer immune activity by increasing the proportion of dendritic cells. In combination with anti-PD-L1, distant tumor growth was inhibited from the abscopal effect [44].
5.2 Actinium-225
Many 225Ac labeled ligands are undergoing preclinical or early-stage clinical trials. Because of its long half-life, 225Ac is a suitable candidate for monoclonal labeling. Several targeted receptors overexpressed in cancer, such as HER2, fibroblast activating protein (FAP), and CD45, are in the development and preclinical stages [45-47]. HER2 is an important target for breast cancer. HER2-targeting mAb labeled with 225Ac α-particle emitters provides highly lethal and localized radiation to targeted cancer cells with minimal exposure to surrounding healthy tissues. FAP is highly expressed in cancer-associated fibroblasts in epithelial cancer stroma and its high expression is associated with poor prognosis. FAP inhibitors (FAPI) are used for theranostics in oncology. A study investigated the therapeutic effects of [225Ac]FAPI-04 in FAP-expressing human pancreatic cancer [66]. Another study suggested the possible application of FAPI radioligand therapy (RLT) in FAP-expressing pancreatic cancer [45]. Moreover, radio-labeled CD45 antibodies have shown clinical promise as a targeted modulator prior to bone marrow transplantation as a more effective alternative to chemotherapy and/or whole-body irradiation of a medullary conditioning regimen. In addition to monoclonal antibodies, peptides and small molecule compounds labeled with 225Ac are promising new radioconjugates for targeted α-particle therapy.
Quantitative imaging plays a significant role in improving therapeutic outcomes and guiding clinical trials. Without an intense emission from 225Ac decay, measuring 225Ac typically relies on detecting the 218 keV γ-ray from the 221Fr decay and the 440 keV γ-ray from the 213Bi decay; single-photon emission computed tomography (SPECT) is used to produce quantifiable dosage maps [67]. However, the required doses were two orders of magnitude over the mouse toxicity limit [68]. The requirement for adequate techniques to quantitatively assess 225Ac dosimetry in the clinic setting remains unfulfilled.
5.3 Bismuth-213
Many preclinical studies have been carried out on 213Bi in leukemia, non-Hodgkin/s lymphoma, malignant melanoma, brain tumors, neuroendocrine tumors, and other cancer types using multiple targeting strategies, antibodies, antibody fragments, peptides, liposomes, and other carriers.
In a highly aggressive B16F10 mouse melanoma model, 213Bi-labeled humanized 8C3 (h8C3) monoclonal antibody showed high uptake rates in tumors and virtually no uptake in natural melanotic tissues. The 213Bi-h8C3 treatment group exhibited a dose-dependent reduction in tumor volume without significant hematological or systemic side effects. Compared with 213Bi-h8C3, 177Lu-h8C3 had a worse effect on tumor growth at the same dose. No significant dose response was observed between 177Lu-h8C3 and 177Lu-H8C3 at a high dose (14.8 MBq) and low dose (7.4 MBq) [48].
The 213Bi-labeled HER2-targeted sdAb 213Bi-DTPA-2Rs15d sdAb demonstrated excellent in vivo stability and rapid tumor-specific uptake in HER2-positive tumor models with peritoneal metastasis. Efficacy evaluation showed significantly improved median survival and lower normal tissue toxicity in mice compared with 0.9% NaCl group [49]. Thus, 213Bi-DTPA-2Rs15d sdAb is a promising new radioconjugate for the treatment of HER2-positive metastatic cancers.
Peptides with low molecular weight and strong diffusivity enable rapid targeting, making them a promising combination with 213Bi with a short half-life. A 213Bi-labeled somatostatin analog [DOTA0, Tyr3] octreopeptide (DOTATOC) was evaluated for antitumor efficacy in a rat pancreatic cancer model and showed good therapeutic efficacy in a linear relationship with an absorbed dose [50]. In in vitro studies, 213Bi-labeled DOTA-DPhe1-Tyr3-octreotate (DOTATATE) showed better tumor killing effects than 177Lu-DOTATATE. Tumor cell viability was only 10% at a 3 Gy dose of 213Bi-DOTATATE, while the cytotoxicity of 177Lu-DOTATATE was much lower than that of 213Bi-DOTATATE at the same absorbed dose. 177Lu-DOTATATE requires six times the dose of 213Bi-DOTATATE to achieve a 10% cell survival rate [51].
5.4 Lead-212
Preclinical studies have reported the antitumor effects of untargeted 212Pb without a targeting vector. Intraperitoneal injection of 212Pb-labeled sulfur colloids in an ovarian cancer mouse model showed effective tumor killing with ascites reduction and longer survival. However, when the dosage of 212Pb reached 70 mCi, severe gastrointestinal toxicity occurred, resulting in mouse death [52]. Based on the fact that the intraperitoneal residence time of ferric hydroxide is longer than that of ferric sulfide or ferric hydroxide, [212Pb]Fe(OH)2 was further prepared as a colloidal carrier for 212Pb [53]. In animal studies, dose-dependent survival was observed after intraperitoneal infusion of [212Pb]Fe(OH)2. 212Pb significantly increased the radiosensitivity and chromosome aberration of tumor cells. [212Pb]Fe(OH)2 up to 2.6 mCi was used in dogs without any significant adverse effects or toxicity.
Targeted therapy studies were carried out for the labeling of 212Pb with monoclonal antibodies, peptides, and other targeting molecules. Ruble et al. demonstrated the effectiveness of 212Pb-labeled monoclonal antibody 103A targeting Rauscher leukemia virus in the treatment of leukemia. All leukemia model mice treated with 212Pb-DOTA-103A were cured histologically. However, at a dose of only 20 mCi, there was severe myelotoxicity, and all animals died of leukopenia and secondary infection. TAT was also conducted for non-Hodgkin's lymphoma with the 212Pb-labeled rituximab and anti-CD37 antibody NNV003 [54, 55]. Median survival was significantly prolonged after intravenous administration of 212Pb-rituximab or 212Pb-NNV003, demonstrating the efficacy of α nuclide in the treatment of hematologic tumors.
The combination of 212Pb with small molecules such as PSMA inhibitors is also being investigated. PSMA ligands L1-L5 were synthesized for chelating 203Pb/212Pb. 212Pb-L2 showed high tumor-specific uptake in PSMA-positive tumor-bearing mice and achieved a good antitumor effect. When combined with the 203Pb labeled PSMA ligand, it enables real-time adjustment of treatment [56]. 203Pb, which has the same element as the therapeutic 212Pb, has a half-life of 52 h and emits 80.1% γ rays at 279 keV, so it is capable of SPECT imaging. Therefore, 203Pb is highly expected to be used as a radionuclide tracer matching 212Pb for accurate in vivo biodistribution imaging and targeted determination of 212Pb radionuclide molecules, thus achieving integration of diagnosis and treatment.
5.5 Thorium-227
The therapeutic radionuclide 227Th is primarily used for labeling targeting vectors to enhance treatment efficacy. Targeted thorium-227 conjugate (TTC) therapy is a promising potent tool for TAT. Several studies have shown potential clinical applications of 227Th.
The 3,2-HOPO chelator and PSMA-targeting antibody are covalently linked to form the alpha particle emitter PSMA-targeted thorium-227 conjugate (PSMA-TTC). PSMA-TTC is preferentially internalized into cells with positive PSMA and demonstrated uptake by and efficacy against PSMA-positive tumors [57].
Mesothelin is a GPI-anchored membrane glycoprotein that is overexpressed in mesothelioma, ovarian, pancreatic, lung, and breast cancers, while showing limited expression in healthy tissues. A mesothelin-targeted 227Th conjugate (MSLN-TTC) has strong antitumor efficacy against disseminated lung cancer, resulting in a significant survival benefit [58].
Initial studies showed 227Th conjugated to antibodies such as trastuzumab and anti-CD20 monoclonal antibodies had significant antitumor effects in breast, ovarian, and lymphoma models [59, 60]. Later reports demonstrated 227Th-labeled CD22-targeted antibodies were safe and tolerated in patients with CD22-positive relapsed/refractory B-cell non-Hodgkin's lymphoma (R/R-NHL), promising clinical translation [61].
6 CLINICAL STUDIES
6.1 Radium-223
Because its chemical properties are similar to those of calcium, Ra2+ naturally targets this mineral component and is incorporated into the bone matrix via substitution for calcium ions. Thus, radium could be incorporated into hydroxyapatite. Consequently, 223Ra irradiation may be a promising option for the treatment of skeletal metastases. Studies in mice have shown that <1% of 223Ra decay products migrate away from the bone, thereby limiting the toxic effects and maximizing the exposure of bone metastases to alpha particle radiation [69].
223RaCl2 was approved for clinical use by the FDA and the European Medicines Agency in 2013 and has been widely used in men with symptomatic mCRPC; a major milestone in the ALSYMPCA trial showed a survival benefit with 223Ra uptake and favorable safety profile. With no subsequent redistribution, peak skeletal uptake occurs within 1 h of injection. Blood clearance is rapid after intravenous administration and skeletal uptake is estimated to be 40%–60% of administered activity. Additionally, non-negligible levels of 223RaCl2 are found in the spleen, stomach, and intestines and are rapidly excreted in the gastrointestinal tract [70]. In clinical reports, 223RaCl2 produced a pain response in approximately 71% of patients and had significant positive effects on bone alkaline phosphatase and prostate-specific antigen levels and overall survival. These studies show no evidence of long-term toxicity during the 2 years of follow-up; the most common adverse events were transient diarrhea, fatigue, nausea, and vomiting [71].
Treatment with 223RaCl2 may potentially have abscopal effects. Kwee et al. reported observations in two patients with castrate-resistant prostate cancer that supported a 223RaCl2 abscopal effect and good response. Plasma interleukin 6 levels increased, indicating that immunological activation may have played a role in the response [72].
6.2 Astatine-211
Seven clinical investigations using 211At are ongoing. As reported in ClinicalTrials.gov, there are five early-phase clinical trials at the Fred Hutchinson Cancer Center using 211At-based radionuclide therapy to enhance outcome following hematopoietic cell transplantation. Investigations are now being conducted on the 211At-BC8-B10 and 211At-OKT10-B10 authorized constructions.
The NCT03128034 study is testing increasing doses of 211At-BC8-B10 for high-risk acute myeloid leukemia (AML) and acute lymphocytic leukemia. The patient population, transplantation technique, and conditioning regimen are different from those of the NCT03670966 phase I/II trial on 211At-BC8-B10 followed by donor stem cell transplantation for the treatment of patients with relapsed or refractory high-risk acute leukemia.
Two clinical trials are being conducted in Japan. At Fukushima Medical University, one trial is exploring 211At-MABG for patients with malignant pheochromocytoma. The study will follow the 3 + 3 study design and may increase to doses of 1.3 and 2.6 MBq kg−1 depending on toxicity. Another trial at Osaka University Hospital is investigating [211At]NaAt for differentiated thyroid carcinoma. This dose-escalation phase I study employing a single intravenous dose of TAH-1005 includes patients with differentiated thyroid carcinoma who have not responded to conventional therapy. The starting dose is 1.25 and 10 MBq kg−1 is the maximum dose.
6.3 Actinium-225
Some 225Ac-labeled ligand/antibody portions have also reached routine clinical practice for the treatment of neuroendocrine tumors, prostate cancer, and acute myelocytic leukemia. Somatostatin receptors are highly expressed in most neuroendocrine tumors. In 2011, the first human clinical study of a 225Ac-labeled small molecule for the treatment of patients with neuroendocrine tumors was conducted. Advanced and metastatic neuroendocrine tumors were treated with 225Ac-DOTA-TOC, and the results showed that most patients were stable and their symptoms were relieved, without side effects such as hematoxicity and liver and kidney function injury. Recently, a retrospective analysis of 39 patients who received 225Ac-DOTA-TOC was conducted to determine the safe level of 225Ac-DOTA-TOC. The analysis found that approximately 20 MBq per cycle (treatment every 4 months) and cumulative doses of 60–80 MBq of 225Ac-DOTATOC were reasonable for patients with advanced stage malignancies. Chronic renal toxicity was observed at these doses but pre-existing renal risk factors were important cofactors. In a long-term study of patients with metastatic gastro-pancreatic neuroendocrine tumors receiving both 225Ac-DOTATATE-TAT and capecitabine, 225Ac-DOTATATE TAT showed satisfactory outcomes and improved overall survival. In patients previously refractory to 177Lu-DOTATATE, there were brief and acceptable adverse reactions [73, 74].
Several small-molecule PSMA ligands that can be conjugated to radioisotopes, such as 18F, 68Ga, 177Lu, and 225Ac, have been developed. The value of 68Ga-PSMA (FDA approved in 2020) and [18F]DCFPyL for the treatment of prostate cancer patients has been widely recognized. PSMA-based radioactive tracers, such as 177Lutetium-PSMA (177Lu-PSMA) and 225Ac-PSMA (225Ac-PSMA), have shown unique diagnostic and therapeutic value [75]. 225Ac-PSMA, which emits a very narrow range of radiation equivalent to a few cell diameters, is more effective for treatment than 177Lu-PSMA, which emits β rays. A recent meta-analysis by Lee et al. showed that prostate specific antigen decreased in approximately 84% of patients and prostate specific antigen decreased more than 50% in approximately 61% of patients after receiving 225Ac-PSMA RLT, which is far better than the 46% and 57% for 177Lu-PSMA RLT [76]. Clinical data on PSMA TAT was available only for 225Ac-PSMA-617. TAT for prostate cancer using 225Ac-PSMA-617 showed a significant therapeutic effect and has the potential to overcome resistance to beta-transmitter therapy. However, TAT of 225Ac PSMA-617 can cause side effects including xerostomia and hematotoxicity. To reduce toxic side effects, new approaches are needed. PSMA-I&T was introduced in 2014 as a theranostic PSMA-targeting small molecule. Mathias and colleagues reported the first clinical experience of patients receiving 225Ac-PSMA-I&T; the results showed a promising antitumor effect in advanced metastatic castration-resistant prostate cancer, and grade 3/4 hematological side effect were reduced, indicating that this may be an additional therapeutic option in end-stage mCRPC patients [77].
In addition to prostate cancer and neuroendocrine tumors, hematological diseases can also be treated by 225Ac radiopharmaceuticals. Lintuzumab targeting HL60 leukemia shows strong specificity [78]. The first-in-human, phase I dose-escalation trial of 18 patients with relapsed or refractory AML showed that targeted therapy with 225Ac-lintuzumab is feasible, has an acceptable safety profile, and exhibits activity against advanced AML. Clinical trials for RIT for glioblastoma are also underway. While therapeutic efficacy of 225Ac radiopharmaceuticals was observed, further studies should focus on the improvement of the biological and chemical properties of 25Ac radiopharmaceuticals, patient selection, and fractionation regimes.
6.4 Bismuth-213
213Bi is the first α particle emitter to enter clinical studies. The labeled lintuzumab, HuM195, was tested in a phase I clinical trial in 18 patients with relapsed or refractory AML. Studies showed that 213Bi-HuM195 was rapidly ingested in the bone marrow, liver, and kidney, where leukemia cells were aggregated, and 78% of patients exhibited a reduced proportion of myeloblasts. 213Bi-HuM195 was well tolerated, with no evidence of obvious extramedullary toxicity [79]. The effectiveness and safety of 213Bi-HuM195 against leukemia were demonstrated in this study, which was the first validation for targeted α-emitting radionuclide immunotherapy in humans.
Peptide receptor alpha therapy with 213Bi combined with low molecular weight, rapidly diffusing peptides has shown promise for glioma treatment. Substance P labeled with 213Bi targeting G protein-coupled receptor neurokinin Type 1 receptor (NK-1R) was used for the treatment of recurrent glioblastoma multiforme (GBM). 20 GBM patients treated with 213Bi-DOTA-SP showed significant improvements in progression-free survival and overall survival compared with patients treated with other therapies such as surgery or chemotherapy [80].
213Bi represents a new treatment option for the targeted treatment of prostate cancer with PSMA-617. Prostate-specific antigen levels decreased nearly four-fold (from 237 to 43 μg/L) [81]. However, the dosimetric estimation of 213Bi from 68Ga-PSMA-617 imaging showed that 213Bi-PSMA-617 was less effective than 225Ac-PSMA-617 [82].
6.5 Lead-212
The first clinical trials of the 212Pb/212Bi in vivo generator were conducted from 2011 to 2015. Peritoneal treatment was administered to 18 individuals with HER2-positive tumors (ovarian and colon cancers). In the first human study, conducted by Meredith et al., the biological distribution, pharmacokinetics, and safety of 212Pb-TCMC-trastuzumab were evaluated. Six doses of 0.2–0.74 mCi/m2 (7.4–27.4 MBq/m2) were tested using a single intraperitoneal injection of 212Pb-TCMC-trastuzumab (7.4 MBq/m2), followed by a dose escalation study. Minimal extraperitoneal radiopharmaceutical redistribution and no significant myelosuppression were observed; the maximum dosage did not induce major hematologic, renal, or hepatic damage, suggesting the dose might be increased in conjunction with other medications. However, none of the patients showed a partial response, and all patients had disease progression within <8 months [83].
Another study, based on successful preclinical studies of 212Pb-DOTAMTATE for somatostatin receptor–positive metastatic neuroendocrine tumors [84], is a phase I clinical trial initiated in 2018. The optimal dose was found, and the objective radiological response was as high as 83%, much higher than the 13% of 177Lu-DOTATATE. The clinical study for dosage escalation of 212Pb-DOTAMTATE in humans in 2022 demonstrated good treatment tolerability, no drug-related serious adverse events, and encouraging therapeutic effects in 10 subjects with neuroendocrine tumors [85].
6.6 Thorium-227
Four clinical development initiatives based on TTC are in progress. 227Th conjugates targeting PSMA are currently being evaluated in patients with metastatic castration-resistant prostate cancer. The study is determining the safety and tolerability profile of PSMA-TTC alone or in combination with darolutamide, the maximum tolerated dose, the recommended dose for further clinical development, and how the drug is distributed and cleared in vivo.
Additionally, 227Th labeled antibody-chelator conjugate is being evaluated for the treatment of patients with advanced recurrent epithelioid mesothelioma or serous ovarian cancer in the NCT03507452 trial. The patients received a single intravenous dose on day 1 of each cycle for a total of 6 weeks (42 days), starting at 1.5 MBq and increasing by increments of 1.0 or 1.5 MBq.
A 227Th-labeled antibody-chelator conjugate that targets HER2 on cancer cells is being studied in an open-label study (NCT04147819). The study included patients with HER2-overexpressing breast cancer, HER2 low expressing breast cancer, and other HER2-expressing cancers. Each patient will receive the drug once every 6 weeks for up to 6 cycles and will be monitored for safety and efficacy for up to 3 years.
In the phase I study by Lindén et al., 21 patients with CD22-positive relapsed/refractory R/R-NHL were treated with 227Th-labeled CD22-targeting antibodies. While the drug was safe and tolerable with an objective response rate of 25% (5/21 patients), including one complete response and four partial responses, adverse events such as neutropenia, thrombocytopenia, and leukopenia were observed in 10 patients.
The current clinical trials of α-emitters are summarized in Table 3.
| Targeted alpha therapy | Target | Cancer type | Institution |
|---|---|---|---|
| 211At-OKT10-B10 | CD38 | Multiple myeloma | Fred Hutchinson Cancer Center, Seattle, USA (NCT04466475) |
| 211At-OKT10-B10 | CD38 | Multiple myeloma | Fred Hutchinson Cancer Center, Seattle, USA (NCT04579523) |
| 211At-BC8-B10 | CD45 | Hematopoietic cell transplant for non-malignant disease | Fred Hutchinson Cancer Center, Seattle, USA (NCT04083183) |
| 211At-BC8-B10 | CD45 | High-risk acute leukemia | Fred Hutchinson Cancer Center, Seattle, USA (NCT03670966) |
| 211At-BC8-B10 | CD45 | High-risk AML, acute lymphocytic leukemia | Fred Hutchinson Cancer Center, Seattle, USA (NCT03128034) |
| 211At-MABG | Norepinephrine transporter | Ovarian cancer | Fukushima Medical University, Japan |
| 211At[NaAt] | - | Differentiated thyroid cancer | Osaka University Hospital, Suita, Japan (NCT05275946) |
| 225Ac-DOTA-TOC | SSTR2 | Neuroendocrine tumors | Institut Régional du Cancer de Montpellier, France |
| 225Ac-DOTATATE | SSTR2 | Paragangliomas | All India Institute of Medical Sciences, India |
| 225Ac-PSMA-617 | PSMA | Acute myeloid leukemia | St. Vincent's Hospital Research Office-Translational Research Center, Darlinghurst, Australia |
| Steve Biko Hospital-Department of Nuclear Medicine, Pretoria, South Africa (NCT04597411) | |||
| 225Ac-lintuzumab | CD33 | Prostatic neoplasms, castration-resistant | Herbert Irving Cancer Center, USA (NCT03705858) |
| 213Bi-HuM195 | CD33 | Acute myeloid leukemia | Memorial Sloan-Kettering Cancer Center, New York, USA |
| 213Bi-DOTA-SP | NK-1R | Glioblastoma | Medical University of Warsaw, Warsaw, Poland |
| 213Bi-PSMA-617 | PSMA | Metastatic castration-resistant prostate cancer | Steve Biko Academic Hospital, University of Pretoria, Pretoria, South Africa |
| 212Pb-TCMC-trastuzumab | HER2 | Ovarian cancer colon cancers | University of Alabama at Birmingham, Alabama, United States |
| UCSD Moores Cancer Center, California, United States (NCT01384253) | |||
| 212Pb-DOTAMTATE | SSTR | Neuroendocrine | Excel Diagnostics and Nuclear Oncology Center, Houston, Texas, United States (NCT05153772) |
| 227Th-PSMA-specific monoclonal antibody | PSMA | Metastatic castration resistant prostate cancer | Tulane Medical Center, Louisiana, United States (NCT03724747) |
| 227Th-MSLN targeting antibody | Mesothelin | Epithelioid mesothelioma or serous ovarian cancer | National Cancer Institute, Maryland, United States (NCT03507452) |
| 227Th- HER2 targeting antibody | HER2 | Advanced HER2-expressing/amplified breast, gastric or gastroesophageal cancer | Johns Hopkins Hospital/Health System, Maryland, United States (NCT04147819) |
| 227Th-labeled CD22-targeting antibody | CD22 | Relapsed or refractory CD22-positive Non-Hodgkin's lymphoma | Skanes Universitetssjukhus, Lund, Sweden (NCT02581878) |
- Abbreviations: AML, acute myeloid leukemia; HER2, human epidermal growth factor type 2; SSTR2, somatostatin receptor subtype 2; TCMC, 1, 4, 7, 10-tetramethylcarboformyl-1, 4, 7, 10-tetrazazecyclododecane.
7 CONCLUSION
The therapeutic applications of α-emitters has been a focus of attention in recent years. We have summarized the promising α-emitters and their applications in tumor therapy. The therapeutic cytotoxic efficacy of α-emitters, including 223Ra,211At, 225Ac, 213Bi, 212Pb, and 227Th, has been established by a substantial body of results from in vitro and in vivo tests. With advancements in targeting molecules and chelators, and a deeper understanding of the mechanisms of cancer, the toxicities of TAT can be reduced. The restriction of toxicities to the liver and kidney should be specifically addressed in both preclinical toxicological studies and the design of clinical trials. Applications may be expanded to use a greater variety of targeted agents.
AUTHOR CONTRIBUTIONS
Jiajia Zhang, Shanshan Qin, Mengdie Yang, Xiaoyi Zhang, and Shenghong Zhang wrote the manuscript; Jiajia Zhang revised the manuscript; Jiajia Zhang and Shanshan Qin reviewed and corrected the manuscript; Fei Yu critically reviewed the manuscript; and Jiajia Zhang and Fei Yu conceived the study. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.




