Recent advances in designing conductive hydrogels for flexible electronics
Qiongyao Peng and Jingsi Chen contributed equally to this study.
Funding information: Canada Foundation for Innovation; Canada Research Chairs; Natural Sciences and Engineering Research Council of Canada
Abstract
Flexible electronics have emerged as an exciting research area in recent years, serving as ideal interfaces bridging biological systems and conventional electronic devices. Flexible electronics can not only collect physiological signals for human health monitoring but also enrich our daily life with multifunctional smart materials and devices. Conductive hydrogels (CHs) have become promising candidates for the fabrication of flexible electronics owing to their biocompatibility, adjustable mechanical flexibility, good conductivity, and multiple stimuli-responsive properties. To achieve on-demand mechanical properties such as stretchability, compressibility, and elasticity, the rational design of polymer networks via modulating chemical and physical intermolecular interactions is required. Moreover, the type of conductive components (eg, electron-conductive materials, ions) and the incorporation method also play an important role in the conductivity of CHs. Electron-CHs usually possess excellent conductivity, while ion-CHs are generally transparent and can generate ion gradients within the hydrogel matrices. This mini review focuses on the recent advances in the design of CHs, introducing various design strategies for electron-CHs and ion-CHs employed in flexible electronics and highlighting their versatile applications such as biosensors, batteries, supercapacitors, nanogenerators, actuators, touch panels, and displays.
1 INTRODUCTION
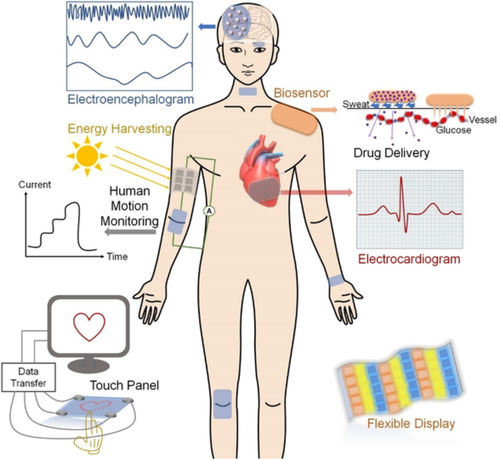
Flexible electronics have attracted enormous attention as multifunctional interfaces bridging soft tissues of human bodies and hard surfaces of conventional monitoring or diagnosing medical instruments.1-4 Various biological signals, such as body temperature and corresponding distribution, electrocardiogram, electroencephalogram, blood glucose and pressure, perspiration, and thyroid hormones, continuously fluctuate in human body and are employed as critical indicators to monitor human health.5, 6 Flexible electronics, especially biosensors, have emerged as a promising approach to directly and effectively collecting such information for health diagnosis.7, 8 Flexible electronics are not only composed of materials that can easily access soft organs or tissues with biocompatibility, mechanical flexibility, and durability, but also integrated with electronic components via different assembly methods to achieve diverse functionalities.9, 10 In the meantime, rapid advances in biosensors impel flexible electronics to be wearable and portable, which can greatly enhance their applicability.11, 12 In addition, stimuli-responsive properties are desirable in flexible electronics, expanding their practical applications in various fields, such as energy harvesting devices, biosensors, smart actuators, artificial muscles and skins, drug delivery systems, wearable displays, and touch panels (Figure 1).7
The conventional designs of flexible electronics usually involve in insulated organic compliant base materials, islands of active devices, and exquisitely designed conductive interconnects.13, 14 Because the first “Sensitive Skin Workshop” held in Washington, DC in 1999 researchers have devoted much effort to fabricating flexible electronics, and significant progress has been made.15 For example, to reduce the cost of packaging, organic semiconductor transistor circuits were directly deposited on flexible printed wiring boards through an upside-down configuration combining interconnect metal, gate metal, source/drain metal, and dielectrics, which integrated transistors at large scale with a long lifetime.16 By adhering gold nanofilm stripes on prestretched dielectric elastomeric membranes of polydimethyl siloxane (PDMS), Suo and coworkers fabricated spontaneous wrinkled metal strips to act as stretchable electrical interconnects to fabricate complete circuits in flexible electronics.17 In order to tolerate extreme mechanical conditions such as large deformation and foldability, a novel configuration of silicon complementary metal-oxide semiconductor integrated circuits (ICs) was designed by Rogers and coworkers, in which a sacrificial poly(methylmethacrylate) layer served as a temporary support for the build-up of ICs on a thin polyimide or PDMS layer and then the ICs were sandwiched by another thin layer of polyimide or PDMS.18-20 The primary limitation of metal film stripes is their low critical strain, and to address this issue, metal connections have been replaced by elastic conductors such as single-walled carbon nanotubes (CNTs) and polymer composites to enhance the stretchability of the devices.21 Bao's group developed flexible organic field-effect transistor devices based on microstructured dielectric elastomer PDMS films, which could readily change the capacitance in response to pressure and mimic the sensing property of natural skin.22 Up to now, multilayer configuration is one of the most straightforward and developed strategies for the fabrication of flexible electronics, and polymeric elastomers can serve as good carrier matrices for the integration of conducting materials. However, elastomers are generally lack of mechanical and biological compatibility with human tissues, and the assembling of surfaces with mechanical mismatching, especially stretchability, usually lead to delamination and defects at the interfaces.1, 23, 24 In the past two decades, conductive hydrogels (CHs) have attracted much attention for the development of flexible electronics, especially in the field of biosensors, due to their excellent biocompatibility, tunable mechanical flexibility, good conductivity, and multiple stimuli-responsive properties.1, 8
Hydrogels are three-dimensional (3D) cross-linked porous polymer networks with high water content and have emerged as promising candidates for various applications including tissue engineering, controlled drug delivery, sensors, and actuators.25-27 Combining conductivity with the mechanical and responsive properties hydrogels have promoted the advances in several emerging areas such as health diagnosing, electronic skins, soft robotics, and energy conversion.28 In nature, conductivity originates from either electron or ion transport. For instance, inorganic materials like metals usually conduct current via electrons, while living organisms like human beings and animals generally deliver electrical information through ions.29 Therefore, the design of CHs is based on the introduction of electron conductors and/or ions into hydrogel matrices. The past two decades witness extensive achievements in developing electron-CHs and/or ion-CHs, whose conductive mechanisms, corresponding components, conductivities, optical properties, and potential applications are summarized in Table 1. Generally speaking, electron-CHs are widely employed in bioengineering applications such as biosensors, tissue engineering systems, and drug delivery devices, because they display better compatibility with biological tissues compared to ion-CHs that suffer from diffusion and leakage of ions to the surrounding environment. Meanwhile, ion-CHs possess ion gradients and transparency, which are highly desirable for energy storage and conversion apparatus like actuators and nanogenerators as well as see-through equipment such as displays and touch panels.
| CHs | Conductive components | Materials | Conductivity (S/m) | Optical property | Application |
|---|---|---|---|---|---|
| Electron-CHs | Metallic nanoparticles/nanowires | Ag30-33/Au34-38/Cu39 nanoparticles | 0.057 to (2.2 × 107) | Transparent or semitransparent | Biosensor drug delivery, tissue engineering |
| Carbon-based materials | CNTs40-44 | 0.072-8.2 | Mostly black or semitransparent | Strain sensor, tissue engineering, biosensor, supercapacitor, drug delivery | |
| GO/rGO/graphene45-54 | 1.56 × 10−5 to (662 ± 41) | ||||
| Conducting polymers | Polyaniline55-68 | (1 ± 0.2) × 10−3 to (43 ± 1.9) | Mostly black and rarely transparent | Tissue engineering, biosensor, drug delivery, supercapacitor, bioelectrode, strain sensor | |
| Polypyrrole65, 66, 69-72 | 0.3-70 | ||||
| PEDOT:PSS73-76 | 1.5 × 10−3 to 4000 | ||||
| Hybrid | Pt77/Ag78/(GO+rGO)79/SWCNTs80+polyaniline | 13.64-21 | Usually black or semitransparent | Biosensor, battery, fuel cell, supercapacitor, tissue engineering, pressure sensor, strain sensor | |
| Au81/(Fe + Co)82/Fe3O483/CNTs84+polypyrrole | 34.93 | ||||
| Graphene/GO+Ni/Ni(OH)285-87 | - | ||||
| GO+PEDOT:PSS88 | 1250 | ||||
| GO+CuS + carbon dot89 | - | ||||
| Ion-CHs | Acids | H2SO490, 91/H3PO492 | 8.2 | Transparent | Supercapacitor |
| Metallic salts | LiCl93-104/Na+105-110/Ca2+111, 112/Al3+113/Fe3+114-119/Tb3+120/K+121-125 | 1.25 × 10−3 to 185 | Transparent or semitransparent | Supercapacitor, solar cell, nanogenerator, actuator, electronic eel/fish, displays, touch panels, pressure sensor, strain sensor, biosensor | |
| Na2S + sulfur+NaOH126 | 11.53-31.35 | ||||
| Ionic liquids | 1-Ethyl-3-methylimidazolium chloride127 | 0.04-2.32 | Semitransparent | Supercapacitor | |
| Electron and ion-CHs | Electron-conductive components and ions | Na++Au nanoparticles128 | 0.45-2.92 | Usually black or opaque | Supercapacitor, biosensor, pressure sensor, battery strain sensor, tissue engineering |
| HCl129, 130/HClO491/(Na++SWCNTs)131 + polyaniline | - | ||||
| H2SO4132/Na+133 + PEDOT | 880 | ||||
| Na++Ca2++SWCNTs134 | - | ||||
| Fe3++polypyrrole135, 136/rGO137 | 9.16 × 10−5 to 7000 | ||||
| Mg2++PEDOT:PSS + polypyrrole138 | - | ||||
| Zn2+139/CuPcTs140+polypyrrole | 12-780 |
- Abbreviations: CHs, conductive hydrogels; CNTs, carbon nanotubes; CuPcTs, copper phthalocyanine-3,4′,4″,4‴-tetrasulfonic acid tetrasodium salt; GO, graphene oxide; PEDOT:PSS, poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate); rGO, reduced GO; SWCNTs, single-walled CNTs.
In this mini review, we first introduce the design strategies of electron-CHs, from the perspective of constructing polymer networks with various electron-conducting materials. Then the fabrication of ion-CHs is discussed, as well as their unique applications as compared to electron-CHs. Different designs of hydrogels with the combination of electronic and ionic conductivity are also presented, focusing on applications under special conditions. Current challenges and future research perspectives of CHs are proposed, which aims to bring new insights to the development of flexible electronics.
2 Electron-CHs
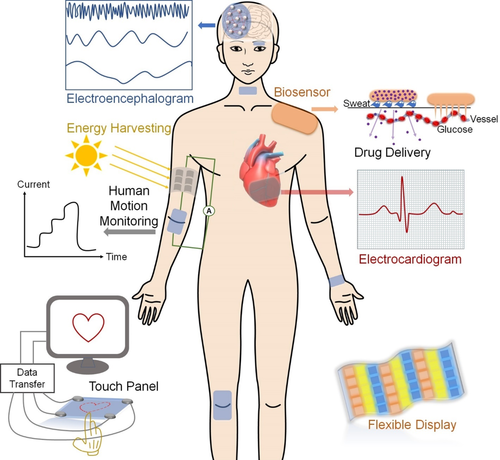
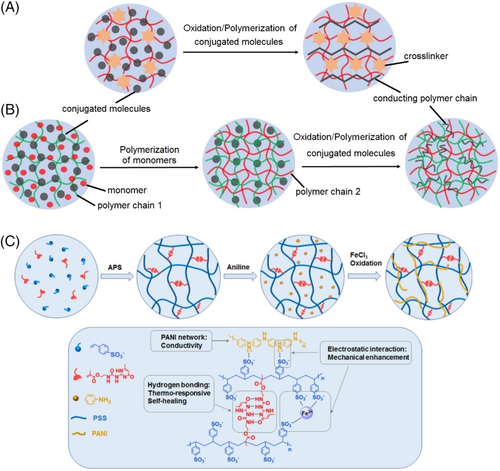
Electron-conducting materials are generally classified into three categories: metallic (Au, Ag, Cu, etc.) nanoparticles/nanowires; carbon-based materials (eg, carbon grease, carbon nanoparticles/carbon nanowires/CNTs, graphene, graphene oxide [GO], reduced GO [rGO] nanosheets), and conducting polymers (eg, polyaniline, polypyrrole, poly(phenylene vinylene), polythiophene, poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) [PEDOT:PSS]).141 The most widely used method to prepare CHs is directly doping or blending the electron-conducting components into hydrogel matrices. However, due to the high specific surface energy of nanomaterials, the simple mixing method usually suffers from heterogeneous phase separation or aggregation of the conducting fillers, leading to reduced electrical conductivity and compromised mechanical properties. A rational design of polymer networks can optimize the distribution of the dispersed phase via introducing specific molecular interactions between the conductive agents and polymer matrix, where both covalent bonds (eg, phenylboronic ester complexation, dynamic imine bond, disulfide bond, Diels-Alder reaction) and noncovalent interactions (eg, hydrogen bonding, hydrophobic interaction, host-guest interaction, electrostatic interaction) are employed.25, 142-149
2.1 Single network electron-CHs
2.1.1 Metal nanomaterial-based electron-CHs
Metallic nanomaterials have been widely used for fabricating CHs owing to their unique characteristics including high electrical conductivity, high specific surface energy, as well as optical, magnetic, and catalytic properties. It is well known that silver possesses the highest electrical conductivity among pure metals, and Ag nanomaterials have been demonstrated to show excellent electron transport ability. To avoid the agglomeration of Ag nanoparticles, Xiang and Chen anchored Ag+ with the deprotonized carboxylic acid groups of the hydrogel network, and then the Ag+ ions were reduced into Ag nanoparticles toward a CH displaying pH-responsive electrical property (Figure 2A).30 Similarly, Devaki and coworkers reported an electron-CH with a homogeneous network through combining in situ polymerization of acrylic acid and in situ reduction of Ag+.31 With the development of 3D printing, photoreduction of Ag+ and photopolymerization of polyethylene glycol diacrylate (Figure 2B) were integrated in one system to produce 3D structures with complexity and electrical property at high speed.32, 33 Au nanomaterials have also been extensively exploited as building blocks for electron-CHs, taking advantage of their facile surface modification via thiol chemistry. Au nanoparticle-containing electron-CHs with thermoresponsiveness and tunable conductivity were obtained by direct photopolymerization of acrylamide or N-isopropylacrylamide (NIPAM) in an aqueous solution with gold nanodispersion.34 Willner and coworkers reported a “breathing” mechanism to construct Au-loaded hydrogels, where the polyacrylamide (PAM) hydrogel could breathe in Au particles via swelling in an aqueous solution containing Au nanoparticles and the following shrinking in acetone immobilized the nanoparticles in polymer network.35 The repeatable swelling and shrinking process could lead to hydrogels with solvent-switchable electronic properties. An electron-CH with thermoswitchable electronic property was obtained by Zhao et al, via copolymerizing vinyl-functionalized Au nanoparticles with NIPAM.36 Chitosan-based thermosensitive CH with homogeneously dispersed Au nanoparticles and Au nanorods-incorporated gelatin-based CH were separately prepared for applications in cardiac tissue engineering.37, 38 CHs incorporated with other kinds of metals were also developed. For example, Cu nanoparticles were in situ generated from the reduction of Cu2+ within a poly(vinyl alcohol) (PVA) network grafted with PAM branches, and the prepared CH could work as a vapor sensor.39 The direct doping of metallic nanomaterials into hydrogel networks usually shows little effect on their mechanical properties, and this kind of CHs are widely employed as sensors in response to various environmental changes including stress, temperature, pH, and oxidation.

2.1.2 Carbon material-based electron-CHs
Carbon materials such as CNTs and graphene provide new opportunities to build 3D-conductive networks within polymer matrices, which not only establish pathways for electron transport through the π-conjugated structure but also enhance the mechanical properties of the materials due to their high specific surface area and abundant surface functionalities.7 The typical design strategies of CNT/GO/rGO-based CHs are either dispersing conductive components in hydrogel matrices or constructing 3D networks based on cross-linking of GO nanosheets, as shown in Figure 2C. Adhesive CHs were prepared by in situ polymerization of acrylic acid and acrylamide in water/glycerol solutions in the presence of polydopamine-decorated CNTs, which exhibited antifreezing and antiheating properties and were used as long-lasting strain sensors under extreme conditions (Figure 2D).40 Polydopamine-coated CNTs dispersion liquid could be also added into a solution of gelatin-grafted-dopamine and chitosan to generate CHs through oxidative coupling of dopamine. The obtained CHs were employed as would dress to enhance regeneration of infected skin.41 Besides, CNTs were introduced into a self-healing hydrogel composed of tetrafunctional borate-cross-linked PVA. It possessed a high stretchability up to 1000% to fulfill human-motion detection.42 When CNTs were dispersed in an alginate hydrogel, the prepared CH could simultaneously facilitate the growth of bacteria colonies and collect the electrons produced by the cells, which was applied to monitor microbial electroactivity.43 When CNTs and laponite nanoclays were doped into poly(N-isopropyl acrylamide) hydrogels, thermal- and photothermal-responsive CHs with high stretchability, self-healing, adhesiveness, and 3D printability were acquired, holding great potential for human motion sensing application.44
Graphene and GO nanosheets are of great interest for the fabrication of electron-CHs. Self-assembly of 2D graphene or GO nanosheets can lead to the construction of 3D networks through π- π stacking and hydrogen bonding. For example, Xu et al reported that by heating the mixture of GO and hydroquinone aqueous solution, a functionalized graphene hydrogel could be facilely prepared as a supercapacitor electrode, in which hydroquinone not only played a role in reducing GO to graphene but also performed as a pseudocapacitive component via π-π interaction within the graphene frameworks.45 However, the hydrogel prepared from pure GO dispersion suffered from poor mechanical properties, especially stretchability, for practical applications such as strain sensors.46 A CH was developed from an aqueous mixture of PVA, polyethylene glycol, and GO, where GO served as cross-linkers to connect PVA and polyethylene glycol polymer chains through hydrogen bonds. The mechanical strength of the CH was enhanced by freezing-thawing process of PVA and it was successfully utilized as human electrocardiogram electrode.47 Another approach to fabricate graphene/GO-dispersed CHs is in situ polymerization of monomers of the polymer matrix. For instance, graphene sheet-incorporated poly(acrylic acid) led to a pH-responsive CH as a potential drug delivery platform.48 Annabi and coworkers also fabricated a human protein-based CH for tissue engineering and regenerative medicine applications by introducing GO particles as cross-linkers into covalently bonded domains of methacryloyl-substituted tropoelastin.49 Moreover, mussel-inspired dopamine chemistry has been applied to reduce GO for the fabrication of CHs. For example, GO and rGO reduced by polydopamine were combined with in situ polymerization of acrylamide to generate self-adhesive, self-healable tough CHs, which could be exploited as cell stimulators, implanted electrodes, and motion sensors.50 Similarly, polydopamine-coated GO/rGO dispersion liquid with enhanced hydrophilicity could interact with dopamine-grafted hyaluronic acid to form CHs by oxidative coupling of catechol groups of dopamine. The CHs behaved well in promoting skin regeneration during wound healing especially owing to excellent conductivity, antibacterial, and radical-scavenging abilities.51 A chitosan/GO composite hydrogel with self-healing and adhesive properties was prepared for tissue engineering, where chitosan and GO interacted with each other via electrostatic interactions and the hydrogel was formed based on the polymerization of dopamine (Figure 2E).52
2.1.3 Conducting polymer-based electron-CHs
Conducting polymers have gained increasing attention for the fabrication of CHs in recent years. Compared to other conducting materials, they possess several advantages such as adjustable electronic conductivity, biocompatibility, flexibility, and versatile solubility in the reduced form.150, 151 Conducting polymers possess unique π-conjugated structure for electron transport, and among them, polyaniline, polypyrrole, and PEDOT:PSS are most frequently investigated. Schematic illustrations of basic designs of conducting polymer-based electron-CHs are shown in Figure 3.
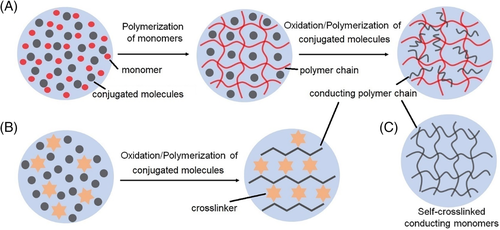
Polyaniline has been demonstrated to exhibited good biocompatibility, and most polyaniline-involved CHs are utilized for tissue engineering or metabolism monitoring. For example, an electrostatic adduct of emeraldine-polyaniline was prepared by in situ polymerization of aniline in poly(2-acrylamido-2-methyl-1-propane sulfonic acid) aqueous solution and then cross-linked by poly(ethylene glycol) diglycidyl ether. The resultant redox CH could be used for glucose electrooxidation catalysis.55 Similarly, the in situ precipitation of polyaniline was also induced by the oxidization of aniline monomers in poly(ethylene glycol) diacrylate solution, and the following UV irradiation resulted in the formation of a CH for nerve regeneration.56 Due to the aromatic structure, polyaniline usually shows poor solubility in aqueous solutions, and a general way to address this problem is introducing hydrophilic moieties into the polymer backbone. For instance, aniline tetramers were grafted onto hydrophilic chitosan to increase their solubility, and the polymer was then cross-linked by dibenzaldehyde-terminated poly(ethylene glycol) through Schiff-base reaction. The developed injectable and self-healing CH could serve as cell delivery carrier for cardiac cell therapy.57 Recent studies reported the fabrication of several multifunctional CHs by grafting polyaniline onto amine groups-bearing quaternized chitosan followed by subsequent buildup of polymer networks via dynamic Schiff-base bonds with aldehyde groups-contained polymers. Corresponding CHs generally possessed multifunctionality such as injectable, antibacterial, self-healing, degradable properties, which were conducive to applications of cutaneous wound healing, drug release, myoblast cell therapy, and muscle regeneration.58-60 Li et al designed a boronate-bearing polyaniline that interacted with PVA for the fabrication of CHs. The microstructure and mechanical properties of the CHs were modulated by freeze-thaw process, and the hydrogels were employed as electrodes for high-performance flexible supercapacitors.61 It should be noted that the incorporation of insulating polymers tends to impair the conductivity of CHs, and several strategies have been proposed to prepare hydrogels with excellent mechanical properties as well as high conductivity. Bao and coworkers used phatic acid as cross-linker and dopant during the in situ polymerization of polyaniline, leading to a mesh-like conducting polymer hydrogel showing an electronic conductivity of 0.11 S·cm−1. This hierarchical nanostructured CH possessed excellent electrode performances when employed as a glucose enzyme sensor, owing to the continuously connected 3D-conductive structure (Figure 4A).62 Guo and coworkers prepared a self-cross-linked polyaniline CH via direct oxidative coupling reaction of aniline hydrochloric salt, which served as excellent electrodes for electrochemical energy storage.63 Besides, a peptide-based molecule N-fluorenylmethoxycarbonyl diphenylalanine could interact with polyaniline via electrostatic interactions and hydrogen bonding, which acted as hydrogelator for the formation of CHs. The self-assembled CHs were demonstrated to show pressure-sensitive conductivity and high cell viability.64
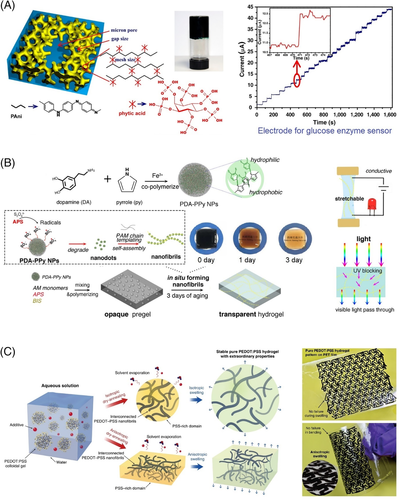
Compared to aniline, pyrrole molecules show lower solubility in aqueous solutions. Although the solubility of pyrrole can be enhanced by increasing temperature, the high temperature is unfavorable for commonly used oxidizing agent such as ferric chloride and ammonium persulfate. Hur and coworkers utilized copper chloride (CuCl2·2H2O) as the oxidizing agent for the polymerization of pyrrole monomers within a melted agarose gel, yielding a stretchable, self-healable, thermal-moldable CH as paintable bioelectrodes on human skin.69 Shi et al reported an organic/aqueous interfacial polymerization method for the preparation of polypyrrole-based CH, where pyrrole molecules were dissolved in isopropanol alcohol and the aqueous solution contained ammonium persulfate and phytic acid cross-linkers. The polymerization occurred at isopropanol alcohol/water interface, and the prepared CH behaved well as supercapacitor electrodes.70 Most of the developed carbon material-based and conducting polymer-based CHs are optically opaque, because the average sizes of these conducting fillers are usually more than half of the shortest wavelength of visible light. Driven by applications in see-through surgical operations, Han and coworkers designed a transparent polypyrrole-containing CH through the in situ formation of polydopamine-polypyrrole nanofibrils from polydopamine-polypyrrole nanoparticles in a PAM matrix (Figure 4B). This transparent and adhesive CH served as human motion strain sensors and wearable electrodes for collecting electrocardiography and magnetocardiography signals.71
As the most famous derivative of polythiophene, PEDOT has gained enormous attention because of its outstanding conductivity and exceptional electrochemical stability in the oxidized state. Although pure PEDOT is immiscible with water, the complex of PEDOT and PSS is water-soluble, expanding applications of the conducting polymer. The most straightforward method to prepare PEDOT:PSS-incorporated CHs is direct doping PEDOT:PSS colloidal particles into a hydrogel matrix. PVA has been extensively exploited because the mechanical properties of PVA hydrogels can be easily modulated via the cyclic freeze-thaw process. Rong et al and Gotovtsev et al incorporated PEDOT:PSS as conductive components into PVA to fabricate CHs for flexible strain sensors, where ethylene glycol and iota-carrageenan were used, respectively, to reinforce the intermolecular interactions.73, 74 Besides the simple blending method, Mawad and coworkers functionalized PEDOT with carboxyl groups and pendant groups bearing double bonds. By copolymerizing the modified PEDOT with acrylic acid and poly(ethylene glycol) diacrylate, the developed CHs were suitable for cell adhesion, proliferation, and differentiation.75 Very recently, Lu and coworkers fabricated pure PEDOT:PSS hydrogels with interconnected networks of PEDOT:PSS nanofibrils, which were formed with the addition of dimethyl sulfoxide (DMSO) as well as the subsequent controlled dry-annealing and rehydration. This kind of hydrogel showed superior electrochemical stability during the charge storage and injection processes in wet physiological environments and it could be patterned into complex geometries (Figure 4C).76
2.1.4 Electron-CHs with hybrid conducting materials
A large number of electron-CHs integrate different kinds of conducting materials in one system to achieve desirable conductive and mechanical properties. Among them, CHs bearing metallic nanoparticles and conducting polymers are prevalent. For example, Yu and coworkers fabricated a homogenous high-density Pt nanoparticles-loaded polyaniline hydrogel as sensitive glucose sensors and human metabolites (eg, uric acid, cholesterol, and triglycerides) detectors.77 Similarly, Das et al designed a photo-responsive hybrid CH, in which polyaniline chains were cross-linked by folic acid, and Ag nanoparticles were formed via the reduction of Ag+.78 The electrical deposition of Au nanoparticles on a phytic acid-cross-linked polypyrrole hydrogel resulted in an amperometric immunosensor.81 A polypyrrole CH acting as electrocatalyst for oxygen reduction reaction, oxygen evolution reaction, and hydrogen evolution reaction was obtained via the introduction of Fe and Co species into the polypyrrole hydrogel. This trifunctional CH provided inspirations for the design of rechargeable metal-air batteries, fuel cells, and water electrolyzers.82 Fe3O4 nanoparticles were also utilized as the dispersed phase within a polypyrrole CH cross-linked by phytic acid and copper (II) phthalocyanine tetrasulfonate salts, which performed as electrodes for high-performance lithium-ion batteries.83
The combination of graphene and nickel-based materials is also popular, owing to the porous and robust microstructures of graphene hydrogels and the versatile nanostructures of nickel-based materials. Chen et al reported a facile way to prepare graphene hydrogel/nickel foam composite as the electrode for high-rate electrochemical capacitors, where graphene hydrogel was deposited in the micropores of nickel foam.85 Besides, graphene-nickel hydroxide hydrogel was developed by in situ growth of Ni(OH)2 nanoplates from nickel nitrate within a graphene hydrogel via hydrothermal treatment.86 Furthermore, Ni(OH)2 nanoflowers were added into a GO dispersion for the preparation of graphene-based composite hydrogels. Owing to large accessible surface area contributed by the nanoplates/nanoflowers, the performances of the corresponding supercapacitors were enhanced and reinforced with elevated rates of ion diffusion and charge transport.87
Other Electron-CHs associating two different electron-conducting components are reported. For instance, the polymerization of anilines could be induced within a PVA-H2SO4 hydrogel in the presence of single-walled CNTs (SWCNTs), leading to an all-in-one configured flexible supercapacitor. The corresponding supercapacitor showed a better capacitive performance of 15.8 mF cm−2 at a current density 0.044 mA cm−2, compared with the counterparts with pure SWCNTs (0.16 mF cm−2) or polyaniline (3.85 mF cm−2).80 GO dispersion and phytic acid-cross-linked polyaniline hydrogel could self-assemble into a CH with subsequent postreduction of GO to rGO. The CHs could be shaped into fibers in a parallel but un-contact configuration and coated with PVA-H2SO4 hydrogel as electrolyte, yielding an all-hydrogel-state supercapacitor with moldability.79 Via solution casting and the subsequent solvent-removing and rehydration, mixtures of linear polyurethane, PEDOT:PSS and liquid crystalline GO were formulated for the production of CH films which served as matrices for the growth and differentiation of human neuron stem cell into neurons.88
Electron-CHs containing three electron-conducting materials were also investigated. In order to enhance the specific capacitance, cyclic stability and energy density of CuS or GO-based supercapacitors, GO CHs decorated with carbon dot-supported copper sulfide were fabricated by hydrothermal reaction and were employed as positive electrodes of asymmetric supercapacitors with rGO serving as negative electrodes.89
2.2 Double networks electron-CHs
The first double networks (DN) hydrogel was reported by Gong et al in 2003, aiming to improve the mechanical properties of hydrogels with appropriate elasticity, high mechanical strength, and low surface friction for the replacement of connective tissues such as articular cartilage, semilunar cartilage, tendons, and ligaments. The first network of the DN hydrogel was a relatively rigid polyelectrolyte poly(2-acrylamido-2-methylpropanesulfonic acid), serving as the sacrificing network to dissipate energy upon damage, while the second network was composed of flexible PAM to withstand large deformation. However, the sacrificing network would be permanently fractured due to the covalently bonded structure.152 Suo and coworkers used noncovalent bonds to build a recoverable sacrificing network, based on the electrostatic interaction between the carboxylic groups of alginate and Ca2+.27 The incorporation of nanomaterials or micromaterials was also proved to improve the strength of DN hydrogels. As presented in Figure 5A,B, all these strategies can be applied to the rational design of DN electron-CHs to render the hydrogels unique properties, including mechanically robust, stimuli responsiveness, adhesiveness, self-healing, and long-term durability for on-demand applications.
Stimuli-responsive hydrogels with conductive property and proper mechanical characteristics are promising candidates for the fabrication of electrochemical sensors, electricity and/or environment-dependent actuators. Thermoresponsiveness is one of the most frequently investigated properties, because the temperature of a certain environment can be easily controlled. Poly(N-isopropylacrylamide) (polyNIPAM) has been the most intensively studied thermoresponsive polymer, owing to its temperature-dependent coil-to-globule transition near the body temperature. PolyNIPAM directly synthesized within a superelastic graphene aerogel, leading to a mechanically robust and thermoresponsive electron-CH.53 An alternative strategy is to introduce electron-conductive components within a polyNIPAM matrix. For example, aniline or pyrrole monomers could be absorbed into a polyNIPAM hydrogel due to its temperature-driven swelling, and the monomers were in situ polymerized into the continuous second network cross-linked by phytic acid. This kind of DN electron-CHs held great promise in developing switcher of thermo-responsive electronic devices.65, 66
Self-healing is an intrinsic property of human skin, which is highly desirable for enhancing the durability of CHs-based wearable flexible electronics and has been achieved by the design of self-recoverable polymer networks.153 For example, acryloyl-β-cyclodextrin, NIPAM, and (poly(ethylene glycol)-b-poly(propylene glycol)-b-poly(ethylene glycol)/CNT were copolymerized to form a covalently connected network assisted by reversible host-guest interactions between β-cyclodextrin and NIPAM. Subsequent in situ polymerization of pyrrole monomers within the previous polymer network resulted in a second interconnected network consisting of CNT and polypyrrole nanoaggregates. This CH could recover to the original state within 100 seconds after a 400% elongation, which successfully served as pressure-dependent sensor, large-scale human motion monitoring sensor and self-healable electronic circuit.84 By introducing negatively charged poly(sodium p-styrenesulfonate hydrate) and positively charged poly(1-methyl-3-[4-vinylbenzyl] imidazolium chloride) in PVA, the electrostatic interactions contributed to the self-healing property. After the reduction of HAuCl4 into Au nanoparticles, a CH was obtained and the reversible metal-ligand coordination between Au nanoparticles and OH groups of PVA further improved the self-healing property. The hydrogel could heal a cut in only 10 seconds without any stimuli.128 Recently, Chen and coworkers reported a self-healing strain-sensitive CH in which 2-ureido-4[1H]-pyrimidinone functionalized methacrylate and 4-styrenesulfonate (SS) were copolymerized to be the first network and the second polyaniline network interacted with SS moieties via electrostatic interaction (Figure 5C). The dynamic and reversible dissociation/association process of the quadruple hydrogen bonds contributed to the energy dissipation and network reconstruction of the hydrogel, which offered the hydrogel stretchable and self-healing properties. After damage, the CH could reconstruct the network immediately to return to the initial state within 30 seconds.67
DN electron-CHs with other functionalities have also been developed. The synergy of the phytic acid-cross-linked polyaniline network and the micelle-cross-linked poly(acrylic acid) network resulted in an extremely stretchable CH, which possessed a large tensile deformation of 1160% and a wide strain sensing range of 0-1130%.68 Tough CHs with fracture energy up to 12 000 J m−2 were designed by generating polypyrrole nanorods in hydrogel matrices consisting of PAM and chitosan.72 CHs with superior metal adhesion were prepared by adding GO sheets as interlamellar bridges into a hydrogel based on synthetic polymers and starch, holding promises as smart electronic device adhesives.54
3 ION-CHs
Hydrogels are water-abundant polymer networks with porous structure, allowing water molecules to freely move across polymer networks.29 The high mobility of ions in water paves the way to the design of ion-CHs, which are commonly fabricated with the direct introduction of free ions. Materials with the capability to generate free ions in water can generally be divided into three categories: acids (eg, HCl, H2SO4, H3PO4); metallic salts (eg, NaCl/Na2SO4, KCl, LiCl, LiClO4, FeCl3/FeNO3, CaCO3/CaCl2, TbCl3, AlCl3); ionic liquids (eg, 1-ethyl-3-methylimidazolium chloride). Compared to electron-CHs, ion-CHs show several advantages and the unique characteristics expand their applications in flexible electronics. First, unlike most electron-CHs showing dark colors, transparency can be easily achieved in ion-CHs, offering them advantages for applications requiring high transparency such as see-through touch panels. Second, mobile ions in ion-CHs can form electric double layer with surfaces rich or deficient in electrons (Figure 6A), therefore, ion-CHs can serve as electrolytes of solid batteries and play critical role in actuators. Besides, some ion-CHs possess water retention capability or anti-freezing property, which improves the durability and environment adaptability of flexible electronics. In this section, ion-CHs designed for specific applications are highlighted.
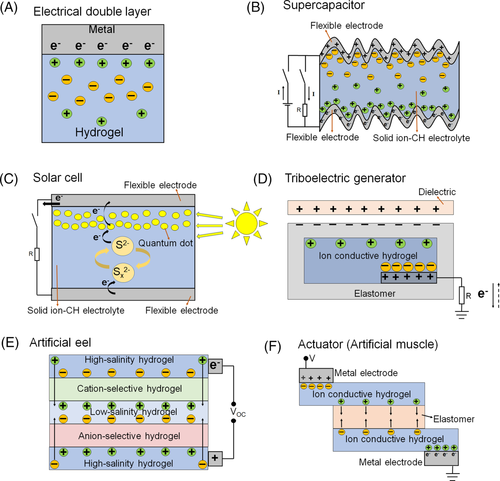
3.1 Energy storage and conversion devices
The development of next-generation electronics requires the devices to be portable, wearable, and implantable. Therefore, the fabrication of portable energy storage and conversion devices (eg, batteries, capacitors, electricity generators, actuators) has become an emerging area, where ion-CHs play an important role. As an energy storage device, supercapacitor possesses high charge-discharge rate and long cyclic service lifetime compared to conventional batteries, in which ion-CHs generally serve as solid electrolytes and separators. The conventional method to fabricate ion-CHs used in supercapacitors is dissolving acid into polymer hydrogels, imparting hydrogels with proton conductivity. For example, H2SO4 was introduced in PVA and polyvinylpyrrolidone hydrogel, respectively, resulting in high-performance solid electrolytes and self-healing supercapacitors.70, 90 H3PO4 was added into a silica nanoparticle-cross-linked PAM hydrogel to fabricate a stretchable and compressible supercapacitor.92 Na2SO4 was introduced into anionic polyurethane acrylates-PAM hydrogel, yielding sticky hydrogel electrolytes for the preparation of stretchable supercapacitors via prestrain-stick-release method (Figure 6B).154 Besides, ionic liquid 1-ethyl-3-methylimidazolium chloride was dissolved into a solution of chitosan and hydroxyethyl methacrylate as both ion-donor and polymerization initiator, leading to a gel electrolyte for supercapacitor with adjustable performance.127
Energy harvesting devices, such as solar cells and triboelectric nanogenerators, have drawn enormous research interest because they are environment-friendly, sustainable, and clean. Compared to liquid electrolytes used in solar cells, electrolytes based on ion-CHs can avoid leakage, volatilization, and combustion of the organic solvents.12 For example, a polysulfide hydrogel electrolyte was fabricated by heating and cooling a mixture of Na2S, sulfur, sodium hydroxide, and 12-hydroxystearic acid toward a quasi-solid-state quantum dot-sensitized solar cell with prominent thermal stability (Figure 6c).126 For dye-sensitized solar cells, a series of ion-CHs were formed through thermal gelation of carboxymethylcellulose and iodide/triiodide redox mediator, which were assembled into sustainable, stable, and transparent solar cells.105 Triboelectric nanogenerators (TENGs) are able to convert biomechanical energy into electricity, based on the well-known triboelectric effect (Figure 6D).93, 94 Benefiting from the transparency, stretchability, and ion transportability of ion-CHs, TENGs can not only combine energy generation with sensing capability but also overcome the incompatibility of the multilayers made from different materials (eg, metals, fabrics, polymers and indium tin oxide). Dielectric film (electrification layer) and elastomer-wrapped LiCl-PAM film were assembled to a single electrode of TENG, which generated alternative current via repeated approach-separation movements. Unlike most stretchable devices showing degradation of performance upon deformation, this kind of soft TENG exhibited abnormal enhanced performance at stretched state (about 210 V at a stretch ratio of 8; about 110 V at a stretch ratio is 1).95 To enhance the interfacial interactions between hydrophilic ion-CH and hydrophobic elastomers, interface modification was realized by the treatment of benzophenone upon ultraviolet irradiation.106 A self-powered energy source inspired by electric eel was developed with a peak electric potential up to 600 V and current of 1 A, which was generated from a series of hydrogels with ionic gradients (Figure 6E).29, 121
Ion-CHs have also been exploited to develop actuators that can convert electrical signals into mechanical responses (eg, deformation, movement).29 A transparent actuator was assembled in a symmetric structure with five layers consisting of electrode, electrolytic elastomer, dielectric elastomer, electrolytic elastomer, and electrode in series, where ion-CHs such as PAM hydrogel containing NaCl/LiCl were employed as the electrolytic elastomer. When voltage was applied, oppositely charged ions were transported from ion-CHs to each side of the dielectric elastomer layer, and the resulted electrostatic interaction would squeeze the dielectric elastomer layer to be thinner and gain areal strain, demonstrating the conversion from electricity to mechanical deformation (Figure 6F).96, 97 The concept of electrostatic interaction-driven deformation was also applied to design a fast-moving soft electronic fish, which could swim at a speed of 6.4 cm/s. A curved LiCl-containing PAM hydrogel was sandwiched between two prestretched dielectric films, and then a high-voltage was applied to the ion-CH and the surrounding aqueous environment. Oppositely charged ions were accumulated on the hydrogel/dielectric film interface and dielectric film/water interface respectively. The electrostatic interaction induced different deformations of the upper and lower surfaces of the dielectric film, leading to controlled movement of the fins.98 Moreover, a transparent loudspeaker was fabricated from stretchable ion-CHs. The high-voltage signals could induce mechanical deformations of the actuator, and the mechanical responses at high and specific frequencies further generated corresponding sounds.107
3.2 Displays and touch panels
Stretchability, foldability, bendability, compressibility, and transparency are prerequisites for the next-generation displays and touch panels, which can benefit from the flexible and transparent ion-CHs. For stretchable and luminescent displays, Ionic-CHs usually serve as ionic conductors to sandwich a luminescent materials-contained elastomer layer. When voltages are applied, electrical signals are delivered through two Ionic-CH conductors and stimulate electroluminescence of the confined layer, whose color varies with different luminescent materials doped in the elastomer (Figure 7A). LiCl-involved PAM (LiCl-PAM) hydrogel is the most frequently used ionic conductor for stretchable and luminescent displays, owing to its high conductivity, strong ionic strength, transparency, hygroscopic property of LiCl, and high toughness of PAM hydrogel. Through photopatterning and transfer printing, Li and coworkers reported a stretchable multicolor display by sandwiching an 8 × 8-pixels-patterned ZnS-silicone light-emitting layer between LiCl-PAM hydrogel electrode layers as shown in Figure 7C. This display could also be used as a touch panel due to its force-dependent capacitance variation of each pixel unit. Furthermore, the ion-CH was exploited for the fabrication of a crawl robot showing a speed of about 4.8 m/hour, by controlling areal strains of different pixel units.99, 100 Suo's team designed an electroluminescent display with giant stretchability of 1500% under high voltages and high frequencies, via sandwiching phosphor particles with dielectric elastomer layers and confining this layered structure between LiCl-PAM hydrogel ionic conductors.101 Then Suo and coworkers employed an acrylic elastomer film doped with circular cholesteric liquid crystal as the dielectric layer. The stretchable liquid crystal electronics possessed opaqueness-transparency transition in response to voltage off-on statuses or to different strains when voltage was on, through rearrangements and realignments of the liquid crystal molecules into the homeotropic state under specific electric field or deformation.102
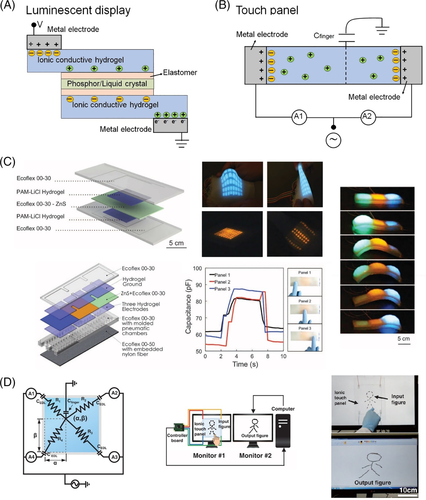
In contrast to the complicated structures of stretchable luminescent displays, the design of stretchable and transparent touch panels is relatively simple, which only requires an ion-CH panel and distributed electrodes (Figure 7B). Kim and coworkers successfully controlled the movement of a computer cursor by using 1D and 2D LiCl-PAM ionic-CHs as touch panels (Figure 7D).103 However, it remains a challenge to build an accurate correlation between the generated currents and input signals. Considering the strong nonlinearity of touch locating using Cartesian coordinates, Zhou and colleagues fabricated a circular LiCl-PAM touch panel to improve the accuracy of touch locating using polar coordinates.104 Moreover, Kweon and coworkers prepared a durable and smart CH touch panel showing stretchable, self-healable, and thermoresponsive properties, by incorporating thermoresponsive polymer PNIPAM into a hybrid CH composed of sodium tetraborate decahydrate-cross-linked PVA and poly(sodium acrylate).108
3.3 Sensors
Various stretchable multifunctional sensors serve as interfaces bridging soft tissues of human bodies and electronic devices, playing important roles in collecting signals, and delivering drugs.1, 7 Human epidermal skins incessantly undergo diverse external stimuli such as pressure, impact, and stretch. Excellent transparency, decent mechanical properties, tunable responsiveness, and biocompatibility of ion-CHs enable those promising candidates both as electronic skins to sense environmental stimuli and as implanted electrochemical sensors for monitoring or regulating metabolism. Up to now, the most well-developed CH sensors are pressure sensors and strain sensors.
In general, there are two types of pressure sensors: one is based on variation of capacitance, while the other is dependent on deformation-caused changes of resistance. For example, Suo and coworkers used CH/elastomer/CH as building blocks to assemble a highly sensitive sensing array, which could detect both the location and the pressure (ranging from 0 to 40 kPa) of touch via recording capacitance variations of the dielectric layer.109 When the LiCl-PAM CH was replaced by a tough DN CH of PAM and Ca2+-cross-linked alginate, the device could be used for applications under extreme environments (eg, temperature as low as −57°C).111 Lei et al employed the same strategy to design an ionic skin using a bioinspired mineral hydrogel as the CH layer, which consisted of amorphous calcium carbonate nanoparticles physically cross-linked by polyacrylic acid and alginate.112 Other functionalities have also been incorporated in the pressure-sensitive ionic-CH sensors. For instance, a self-assembled SiO2 nanofibrous framework was wrapped with Al3+-cross-linked alginate to form an ion-CH with ultrahigh water content, superelasticity and shape-memory performance. This ion-CH sensor possessed a low-pressure regime ranging from 0 to 2.5 kPa with a pressure sensitivity (ratio of the relative variation of current to the difference of loaded pressure) of 0.042 kPa−1. When the pressure was over 2.5 kPa, the pressure sensitivity was increased to 0.24 kPa−1.113 Liu and coworkers designed a PAM hydrogel containing host-guest interactions between 1-benzyl-3-vinylimidazolium and cucurbit[8]uril as a pressure sensor. This hydrogel could withstand an elongation up to 10 000% and support objects with 2000 times weight of itself.155
Tensile deformation increases the average distance between mobile ions, leading to the increase of electrical resistance of ion-CH sensors. Strain sensors generally are designed to sustain large tensile deformation with high sensitivity in order to detect and monitor diverse human motions such as movements of fingers, wrists, elbows, shoulders, knees, throat, eyelids, and face muscles. PVA and poly(vinylpyrrolidone) were covalently cross-linked and further strengthened by Fe3+-cross-linked cellulose nanocrystals to form a self-healing ion-CH as a wearable soft strain sensor.114 Dynamic metal coordination bonds between polyacrylic acid/glutamic acid-based polymers and Fe3+/Al3+ were frequently used for the construction of physically cross-linked polymer networks.115-119 PVA and sodium tetraborate were also employed for the design of ion-CHs due to good mechanical properties originated from dynamic covalent bonds and the conductivity rendered by Na+.110 Tb3+ ions not only served as cross-linking points of the ion-CHs but also exhibited photoluminescence.120 In addition, the adhesive property of polydopamine facilitated the development of adhesive ion-CH strain sensors.110, 122 It should be noted that pressure-sensitivity and tensile-sensitivity usually coexist in ion-CHs but display distinct gauge factors (ratio of relative variation of resistance to applied strain).
Ion-CH-based electrochemical sensors, including immunosensors, thermistors, and gas sensors, have gained increasing attention because they can conveniently detect the physiological signals of human body and monitor the change of environment. For example, a label-free electrochemical immunosensor for anchoring of tumor biomarkers was built on an ion-CH substrate which was cross-linked by 1,3,5-benzenetricarboxylic acid and Fe3+.156 Wu and coworkers used a DN ion-CH composed of PAM and K+-cross-linked carrageenan to fabricate a thermistor and a gas sensor.123, 124 The self-healing thermistor was prepared based on the thermo-reversible helix-coil structure transition of carrageenan, showing a sensitivity of 0.77°C in the range of 25-70°C. The ion-CH was also used to detect NH3 and NO2 gases, because NH3 and NO2 could form substantial hydrogen bonds with oxygenated functional groups of the polymer network and impede the movements of K+ and Cl−, resulting in an increased resistance.
4 ELECTRON-CHs AND ION-CHs
The abovementioned electron-conductive materials and ion-conductive components can be simultaneously integrated into one hydrogel, combining the advantages of electron-CHs and ion-CHs in one system. Synergies between conducting polymers (eg, polyaniline, PEDOT:PSS) and H+ (eg, HCl, HClO4, H2SO4) were commonly employed to generate CH electrodes with high performances. For example, host-guest interactions between α-cyclodextrin-functionalized PAM and hydrophobic polyaniline were utilized to form a semitransparent CH as supercapacitor electrodes, which remained stable up to 35 000 cycles at a current density of 8 A g−1, assisted by the incorporation of HCl in the hydrogel acted as doping agents for polyaniline and provided abundant proton for electrical conduction.129 CH biointerfaces were fabricated based on the electrostatic interactions between negatively charged sulfate ions of heparin and positively charged aniline groups of polyaniline, where the addition of HCl could facilitate the formation of a uniform polyaniline network.130 In situ formed polyaniline hydrogel electrodes were doped with HClO4, wrapping a PVA-H2SO4 hydrogel electrolyte to fabricate an all-in-one flexible supercapacitor with large areal capacitance of 488 mF cm−2.91 Moreover, H2SO4 was demonstrated to change the conformation of PEDOT chain from a random coil to an expanded state, which was used to enhance the interchain interactions of PEDOT through thermal treatment, and the prepared CH exhibited extremely high conductivity of 880 S m−1.132
The addition of metal ions can provide ionic conductivity to CHs, and the introduction of Mg2+, Ca2+, and Fe3+ usually associated with organic ligands via metal-coordination interaction to build polymer networks. When sodium borate was introduced into a PVA and polydopamine network reinforced by polyaniline-functionalized single-wall CNTs, a self-healing and adhesive CH strain sensor was fabricated.131 Besides, sodium SS was combined with a PEDOT-based CH to prepare neural interfaces.133 Pressure sensors based on CH spheres were developed by adding a mixed solution of sodium alginate and carboxyl-functionalized single-walled CNTs into CaCl2 solution.134 Mg2+ was proved to interact with negatively charged PSS chains in PEDOT:PSS aqueous dispersions, acting as ionic cross-linkers for hydrogel formation. Therefore, The Mg2+ cross-linked PEDOT:PSS hydrogel was further combined with electropolymerized polypyrrole, leading to an efficient cathode for high-performance Mg-air battery.138 Fe3+ can not only form complexes with carboxyl groups but also coordinate with multiple catechol groups inspired by mussel. For instance, in a polypyrrole-doped CH, the cross-linking was originated from the interactions between carboxyl groups and Fe3+.135 When Fe3+ was introduced into a solution containing polydopamine-functionalized rGO and acrylic acid, a tough CH with biocompatibility was developed as strain sensors for human motion detection.137 A paintable adhesive CH, serving as therapeutic cardiac patch, was fabricated based on interactions between Fe3+ and carboxyl groups of gelatin chains as well as catechol groups of tethered dopamine molecules.136
Other self-assembled metal-ligand supramolecular structures were exploited with conducting polymers to construct CH networks. For example, 2,2′:6′,2″-terpyridine and Zn2+ assembled into interconnected cubic cages with dynamic association/dissociation. A CH showing high conductivity of 12 S m−1, electrical self-healing, and decent mechanical properties were prepared by introducing this supramolecular structure into a nanostructured polypyrrole hydrogel.139 Copper phthalocyanine-3,4′,4″,4‴-tetrasulfonic acid tetrasodium salt (CuPcTs), a kind of liquid crystal molecules with disc shape, could form quadruple electrostatic interactions with positively charged polypyrrole chains through its tetra-sulfonic acid functional groups. This self-assembly resulted in CHs with a structure of interconnected nanofibers, exhibiting enhanced conductivity of 780 S m−1 compared to 7 S m−1 of pristine polypyrrole hydrogel.140
5 CONCLUSIONS AND PERSPECTIVES
During the past two decades, flexible and CHs have attracted considerable research interest and been extensively explored with the rapid development of flexible electronics, which not only provide guard for our health but also enrich our daily life. The design of electron-CHs is based on incorporating electron-conductive components such as metal nanoparticles, carbon-based nanomaterials and conducting polymers into a flexible polymer network. The hydrogels can be modulated with a wide range of conductivity and are extensively explored as flexible supercapacitors, electrodes for solar cells, biosensors for human health monitoring, and implantable platforms for cell regeneration and drug delivery. Compared to ion-CHs that may suffer from diffusion of ions to surrounding environment, electron-CHs show better biocompatibility, which have been extensively applied in bioelectronics and implanted devices. Up to now, the CH-based drug delivery devices are all developed on electron-CHs. Ion-CHs transmit electrical signals via ion transport within the porous network. Due to their transparency and the capability to form ionic gradients, ion-CHs pave the way to a variety of emerging applications including artificial muscles, electronic skins, nanogenerators, soft robotics, flexible touch panels, and displays. As strain/pressure sensors are dependent on deformation-driven resistance/capacitance variation, they can be fabricated from both electron- and ion-CHs. Besides conductive components, the electrical performances of CHs are also contributed by the design of microstructures, which has been demonstrated to play an important role in energy storage devices.
Despite much progress achieved, some challenging issues are still remained. First, the water evaporation of CHs is undesirable in electronic devices, which not only contaminates the circuits but also impair the mechanical properties of CHs. Up to now, the most effective method is to encapsulate the CH in water-resistant elastomers.109, 157 The water retention capacity of PAM hydrogel has been demonstrated to be improved by introducing highly hygroscopic salt LiCl into the hydrogel.125 However, the concentration of LiCl needs to be high and a facile strategy to enhance the water retention capacity of hydrogels is required. Second, the fabrication of CHs for specific applications usually involves complicated procedures, which is not favorable for massive and low-cost industrial manufacture. The development of fast 2D and 3D printing provide promising approaches to the facile fabrication of flexible electronics. Zhao et al and Tian et al, respectively, used 2D-patterned printing of electron-CH inks on substrates to construct CH films.76, 158 Odent and coworkers reported a fast bottom-up fabrication of complex conductive architectures from photo-polymerizable ionic-CH precursors via 3D printing.159 Moreover, the development of wearable and portable devices demands the integration of electronic units with different functions (eg, energy generator, data collecting unit, feedback system) in one platform. Inspired by the concept of lab-on-skin,160 the assembling of CH-based solar cells, CH sensors, wireless data transmitters and other electronic within a finite space have emerged as a hot area, holding great promise for biomedical applications, soft robotics and smart devices.
ACKNOWLEDGMENT
We gratefully acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Canada Foundation for Innovation (CFI), and the Canada Research Chairs Program (H. Zeng).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Biographies

Qiongyao Peng received her BSc in materials science and engineering from Central South University in 2010. She obtained her MSc in materials science and engineering from Shanghai Jiao Tong University in 2014. She is currently pursuing her PhD program in chemical engineering at University of Alberta. Her research mainly focuses on development of functional hydrogels and coacervation-inspired wet adhesives for bioengineering applications.

Jingsi Chen received her BSc in material science and engineering from Southeast University in 2012 and obtained a PhD degree in material engineering from University of Alberta in 2019. She is currently a postdoctoral fellow at the University of Alberta and her research mainly focuses on the development of functional supramolecular hydrogels toward bioengineering applications.

Hongbo Zeng is a professor in the Department of Chemical and Materials Engineering at the University of Alberta, a Tier 1 Canada Research Chair in intermolecular forces and interfacial science, a fellow of the Canadian Academy of Engineering and a member of the Royal Society of Canada's College of New Scholars. He received his BSc and MSc at Tsinghua University, and obtained his PhD in chemical engineering at University of California, Santa Barbara under the supervision of Prof. Jacob Israelachvili and Prof. Matthew Tirrell. His research interests are in colloid and interface science, functional materials, with a special focus on intermolecular and surface interactions in soft matter and engineering applications. He has published over 280 refereed research journal articles, and edited a book “Polymer Adhesion, Friction and Lubrication” (Wiley). His research has been recognized by many awards such as the Petro-Canada Young Innovator Award, Martha Cook Piper Research Prize, The Canadian Journal of Chemical Engineering Lectureship Award, Hatch Innovation Award of the Chemical Institute of Canada, International Award for Outstanding Young Chemical Engineer and the NSERC E.W.R Steacie Memorial Fellowship.