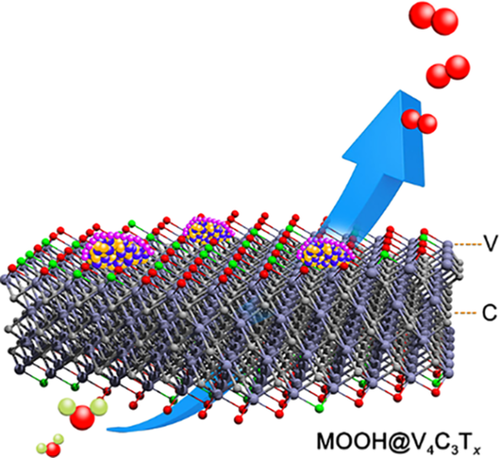
V4C3Tx MXene: A promising active substrate for reactive surface modification and the enhanced electrocatalytic oxygen evolution activity
Funding information: Initiative Postdocs Supporting Program, Grant/Award Number: BX20190281; Ministry of Education of Singapore, Grant/Award Numbers: MOE2017-T2-2-069, MOE2018-T2-1-010; National Natural Science Foundation of China, Grant/Award Number: 51901189; Opening Project of Key Laboratory of Materials Processing and Mold, Grant/Award Number: NERC201903
Abstract
Presented are the synthesis, characterizations, and reactive surface modification (RSM) of a novel nine atomic layered V4C3T x MXene. With the advantages of the multilayered V4C3T x MXene that can simultaneously support the RSM reaction and keep the inner skeleton stable, a series of amorphous Ni/Fe/V-ternary oxide hydroxides thin layer can be successfully modified on the surface of the V4C3T x MXene (denoted as MOOH @V4C3T x, M = Ni, Fe, and V) without disrupting its original structure. Attributed to the in situ reconstruction of highly active oxide hydroxide layer, the nanohybrids exhibited an enhanced oxygen evolution reaction (OER) activity and excellent long-time stability over 70 hours. In particular, a current density of 10 mA cm−2 can be reached by the nanohybrid with the optimized Ni/Fe ratio at an overpotential (η) as low as 275.2 mV, which is comparable to most of the state-of-the-art OER catalysts and better than other MXene-based derivatives. Demonstrated by the tunable physicochemical properties and excellent structural stability of these nanohybrids, we may envision the promising role of the M4X3-based MXenes as substrates for a wide range of energy conversion and storage materials.
With the increasing demands for renewable clean energy, electrocatalytic water splitting techniques that involve both hydrogen evolution reaction and oxygen evolution reaction (OER) have gained growing attention in recent years.1-5 However, due to the sluggish reaction kinetics and the large overpotential, gaining further promotion in OER reactivity is still challenging.6, 7 Recently, some researchers have unlocked the potential of metal oxide hydroxides as an effective catalyst for OER performance.6, 8-10 However, there is still no ground breakthrough for metal oxide hydroxides until now because of their inferior electronic conductivity. Therefore, the integration of metal oxide hydroxides with highly conductive two-dimensional (2D) materials becomes one of the most effective strategies.7, 11 On the other hand, MXenes, as a newly developed 2D metal carbide/nitride family, present attractive alternatives to metal oxide hydroxides for OER thanks to their adjustable chemical composition and abundant surface functional groups.12 Considering their large surface area and excellent electronic conductivity, MXenes have great potential to be used in electrochemical energy conversion and storage applications.13-15 However, MXenes consisting of early transition metals usually possess low intrinsic electrocatalytic OER activities.16-18 One option being researched to compensate the aforementioned intrinsic disadvantages is combining active transition metal oxide hydroxides nanostructures with MXenes. Unfortunately, numerous studies have not proved it to be a straightforward endeavor as this might cause process complications and material deterioration when it is done in an uncontrollable way.
In recent years, there have been quite a few successful demonstrations of reactive surface modification (RSM) strategy in the preparation of multifunctional MXene-based nanohybrids. For instance, the in situ sulfidation of Mo2TiC2T x MXene has successfully turned the precursor into a MoS2-on-MXene heterostructure by utilizing its abundant surface-exposed metal sites, which demonstrated the great potential of the RSM strategy.19 On the other hand, the RSM process was also deployed for the synthesis of various transition metal sulfide, trichalcogenidophosphates, and alloy modified MXene nanohybrids with additional metal source.20-24 In particular, the strong affinity between the modified species and the surface metal atoms of MXenes can also be observed. Unfortunately, the major drawback of the RSM process is the rapid consumption of the surface-exposed metal atoms on MXenes, make it a stringent requirement for the RSM process. On top of that, the structural stability problem arises from the migration of metal atoms from the inner layer to the surface during the RSM process might screen out most of the frequently used MXenes with general formulas of M2X and M3X2 (M = early transition metals; X = C, N).
Taking the aforementioned considerations into account, we synthesized a novel MXene-based catalyst with nine atomic layers, namely V4C3T x MXene. The multilayered V4C3T x presented excellent structural stability during the RSM process. In addition, the OER performance of the V4C3T x MXene and its RSM derivatives were evaluated. After the in situ surface transformation during OER process, a series of nanohybrids that consist of V4C3T x MXene with Ni/Fe/V-ternary oxide hydroxides thin layer surface modification (denoted as MOOH@V4C3T x, M = Ni, Fe, and V) were obtained. With the optimized Ni/Fe ratio, the nanohybrid delivered the lowest overpotential (η) of 275.2 mV at a current density of 10 mA cm−2, which is comparable to most of the state-of-the-art noble metal-contained or noble metal-free OER catalysts. Finally, with the high OER activity of the surface ternary oxide hydroxide layers and the stable conductive skeleton of the V4C3T x MXene, V4C3T x-based nanohybrid revealed excellent stability over 70-hours long-time OER process.
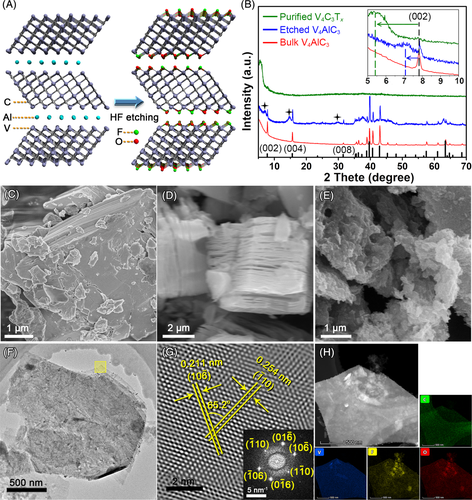
The V4C3T
x MXene was obtained via the HF selective etching method from the bulk V4AlC3 MAX phase (Figure 1A, for detail, see Supporting Information).25 The phase purity of each product was verified by the powder X-ray diffraction (XRD) technique. As shown in Figure 1B, the XRD pattern of the bulk V4AlC3 was in accordance with the corresponding simulated pattern from the single-crystal X-ray data of V4AlC3.26 After HF etching, three new broad peaks of (002), (004), and (008) plane (2θ ≈ 7.0°, 14.6°, and 29.7°, respectively) from V4C3T
x phase appeared. After centrifugation, highly pure V4C3T
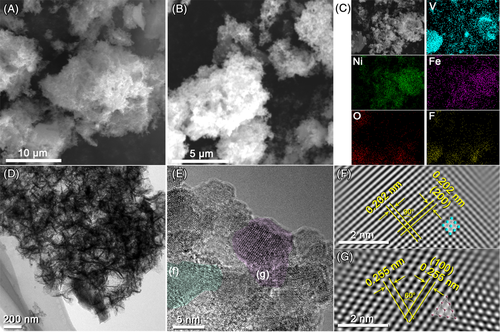
x can be isolated, which was evidenced by seeing only one strong (002) peak located at 2θ ≈ 5.3°.25 The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were then applied to characterize the morphology of the products (Figure 1C-E). From the SEM, the V4AlC3 powder presented a bulk morphology (Figure 1C), while the HF etched V4AlC3 showed a typical accordion-like structure (Figure 1D), indicating the successful removal of interlamellar Al atoms from the V4AlC3 lattice. After ultrasonic exfoliation, the purified V4C3T
x nanosheet can be obtained (Figure 1E). Moreover, the TEM image of an isolated V4C3T
x nanosheet showed the multilayered structure (Figure 1F), which was in accordance with the SEM results. The phase of the V4C3T
x MXene was also confirmed by TEM. The fast Fourier transformation (FFT) pattern and the inverse FFT image of the selected area highlighted in Figure 1F are shown in Figure 1G. From the FFT pattern, single-crystalline nature of the V4C3T
x nanosheets can be confirmed. In addition, a series of lattice planes can be identified from the inverse FFT image. As labeled in Figure 1G, two lattice planes with inter-lattice distances of 0.254 and 0.211 nm can be assigned to the {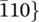 and {10
and {10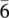 } planes of the V4C3T
x, respectively. The homogeneous distribution of V, C, F, and O in the V4C3T
x nanosheets were also confirmed by the energy dispersive X-ray spectroscopy (EDX) elemental mapping under TEM (Figure 1H).
} planes of the V4C3T
x, respectively. The homogeneous distribution of V, C, F, and O in the V4C3T
x nanosheets were also confirmed by the energy dispersive X-ray spectroscopy (EDX) elemental mapping under TEM (Figure 1H).
While for the RSM-V4C3T x nanoprecursors, facile self-assembly, and thermal reduction process were applied (for detail, see Supporting Information).21, 22 For nanohybrids with varied Ni/Fe ratios in precursors, their contents were further confirmed by inductively coupled plasma-atomic emission spectrometry (ICP-AES) analysis. The atomic ratio of Ni, Fe, and V4C3T x in each sample was calculated (Table S1), which were in accordance with those starting materials. According to the ICP analysis, the RSM-V4C3T x nanoprecursors with different alloy compositions were denoted as M1@V4C3T x, M2@V4C3T x, M3@V4C3T x, and M4@V4C3T x. The phase purity of RSM-V4C3T x nanoprecursors was also confirmed by XRD. As shown in Figure S1A, the nanocomposites consisted of Ni metal phase (JCPDS No. 87-0712) and V4C3 phase (JCPDS No. 89-5055). Obviously, with the increase of Fe content, the peaks of (111) and (200) planes arise from alloy were weakened and broadened, whereas the (111) and (220) planes arise from V4C3T x remained nearly unchanged. The XRD patterns indicated a decrease in the size of alloy with the increasing content of Fe. While for the control samples that were synthesized without V4C3T x MXene (Figure S1B), peaks of (111) and (200) planes arise from the alloy became much sharper, indicating a drastic increase in grain size after thermal reduction. Figure 2A,B showed the SEM images of the M3@V4C3T x nanoprecursor before and after thermal reduction, respectively. From the SEM, the porous morphology of the nanoprecursor was fully maintained. EDX elemental mapping was also applied to verify the homogeneous distribution of each element in the sample (Figure 2C). The TEM and high-resolution TEM (HRTEM) images of the nanoprecursor are shown in Figure 2D,E, respectively. As shown in Figure 2D, the layered structure can be clearly observed at low magnification. From the HRTEM image, the grains of alloy with a diameter of around 10 nm were randomly attached on the surface of the V4C3T x nanosheets (Figure 2E). The corresponding inverse FFT images of the V4C3T x substrate and the alloy grain were shown in Figure 2F,G, respectively. As labeled in Figure 2F, the lattice planes with inter-lattice distances of 0.202 nm can be identified. The two lattice planes were ascribed to the {200} planes of the NiO phase (JCPDS No. 78-0643), which might be originated from the surface oxidation of the highly active alloy nanoparticles. In addition, for the inverse FFT images of the V4C3T x substrate shown in Figure 2G, the lattice planes with inter-lattice distances of 0.255 nm corresponded to the {100} plane of V4C3T x, which confirmed the presence of V4C3T x MXene and was consistent with the XRD results.
In order to get further insight into the feature of chemical bonding on the surface of the V4C3T x MXene and RSM-V4C3T x nanoprecursors, X-ray photoelectron spectroscopy (XPS) of the V4C3T x MXene were performed (Figure 3A-D). For the V4C3T x MXene, the high-resolution XPS signals of V 2p spectrum can be deconvoluted into five peaks. As shown in Figure 3A, the peak centered at 513.3 eV was related to the binding energy of V-C species.27 The other four peaks were related to the V2+ (514.0 and 521.1 eV) and V3+ (516.0 and 523.1 eV) species, respectively.27, 28 In the C 1s spectrum (Figure 3B), the binding energy at 282.4 eV confirmed the presence of C-V species.27, 29 While the other three peaks centered at 284.7, 286.3, and 287.1 eV corresponding to the adventitious contaminations.27 For the O 1s spectrum (Figure 3C), four peaks centered at 529.6, 530.5, 532.3, and 534.8 eV can be deconvoluted. For the peak deconvolution of the O 1s spectrum, peaks centered at 530.5 and 532.3 eV can be assigned to the C-V-OH and C-V-O x species.27 The peak at 529.6 eV was related to the surface metal oxide species,30 whereas binding energy of 534.8 eV might be related to the surface adsorbed water.31, 32 The F 1s spectrum can be deconvoluted into two peaks (Figure 3D), which might arise from the surface C-V-F x (683.8 eV) and other F contained species (686.4 eV).20
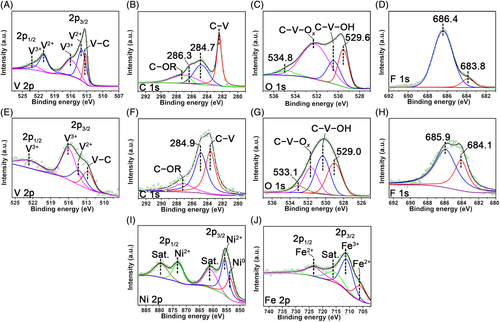
In comparison, the high-resolution XPS signals of V 2p, C 1s, O 1s, and F 1s spectra from the RSM-V4C3T x nanoprecursor are also shown in Figure 3. For the deconvoluted V 2p peak, the binding energy of 512.8 eV corresponded to the V-C species, indicating the retention of V4C3T x skeleton. Compared with the V 2p spectrum of the V4C3T x MXene, the intensity of the two peaks related to V3+ (516.0 and 522.0 eV) increased while the peak related to V2+ (514.4 eV) decreased (Figure 3E). The results indicated that the surface V species were also involved in the RSM. For the C 1s spectrum (Figure 3F), the deconvoluted peak centered at 283.6 eV can be assigned to the binding energy of C-V species.27, 29 Similarly, the other two peaks at 284.9 and 287.2 eV were related to the adventitious contaminations.27 The O 1s spectrum can also be deconvoluted into four peaks (Figure 3G). The first three peaks still related to the surface metal oxide species (529.0 eV), C-V-OH (530.4 eV) and C-V-O x species (531.7 eV), respectively.27 And the peak at 533.1 eV was corresponded to the surface adsorbed water.31, 33 Similarly, the F 1s spectrum can be deconvoluted into two peaks (Figure 3H), which belonged to the surface C-V-F x component (684.1 eV) and other F contained species (685.9 eV).20 Additionally, the XPS spectra of Ni 2p and Fe 2p from the nanoprecursor were also acquired. As shown in Figure 3I, the Ni 2p spectrum can be deconvoluted into five peaks. The peak at 853.8 eV was assigned to the 2p3/2 core level of Ni0,22 whereas the peaks at 855.7 and 873.2 eV were related to the Ni2+ species.34 The two peaks at 861.2 and 879.7 eV were the satellite peaks of the Ni 2p1/2 and 2p3/2 core levels, respectively.34, 35 For the Fe 2p spectrum (Figure 3J), the two deconvoluted peaks located at 706.4 and 723.4 eV were related to the Fe2+ species.34 While the peak at 711.3 eV indicated the presence of Fe3+ species.11, 36 The satellite peak of Fe 2p3/2 core level was located at 716.2 eV.35
As demonstrated by some in situ studies, the surface of electrocatalyst will go through a reformation, thus forming the surface metal hydroxides/oxide hydroxides (MOOH) species which might be the indeed OER active species during OER process.8-10, 37 For the RSM-V4C3T x nanoprecursors, the surface alloy nanoparticles were also expected to be the sacrificial templates, which can undergo in situ generation of MOOH species anchoring on the surface of V4C3T x MXene. Therefore, cyclic voltammetry (CV) was first applied to evaluate the surface reformation behaviors of the RSM-V4C3T x nanoprecursors and V4C3T x MXene under high applied potential. As shown in Figure S2, all the RSM-V4C3T x nanoprecursors exhibited enhanced OER activities after CV cycles in 1 M KOH solution. And the broad redox peaks observed in the first CV cycle (related to the V4C3T x MXene) gradually decreased as the Fe content increased. The stable redox peaks observed from all the CV curves related to RSM-V4C3T x nanoprecursors were similar to other reported Ni/Fe containing OER catalysts,38, 39 which suggested the surface transformation during OER process and generation of surface MOOH layers.
Based on the morphology and CV analyses, the electrocatalytic OER performance of the V4C3T x MXene and the series of MOOH@V4C3T x nanohybrids were carefully evaluated by linear sweep voltammetry (LSV) in 1 M KOH solution (Figure 4A, Figures S3A,3B). The Ohmic potential drop was also corrected. As plotted in Figure 4A, the pristine V4C3T x MXene showed nearly no OER activity in the potential window being tested. However, the surface-modified V4C3T x MXenes all showed an enhanced OER activity. Particularly, the M3OOH@V4C3T x nanohybrid only needed an overpotential (η) of 275.2 mV to reach a current density of 10 mA cm−2, which depicted the lowest overpotential among all the tested MOOH@V4C3T x samples including M1OOH@V4C3T x (η = 303.9 mV), M2OOH@V4C3T x (η = 290.6 mV), M4OOH@V4C3T x (η = 286.5 mV), and the commercial noble metal-based IrO2 catalyst (η = 311.4 mV). For the bare MOOH and MOOH obtained from Ni/Fe alloy (denoted as MOOH@M), the same Ni/Fe ratio as the corresponding MOOH@V4C3T x nanohybrids, LSV data were also collected. As plotted in Figure 4A, the MOOH@V4C3T x nanohybrids showed a lower η than most of the control samples, whereas the M3OOH@M3 and M4OOH@M4 present the comparable η with the M3OOH@V4C3T x. Evidenced by the SEM analysis, the 2D structures of the four MOOH@V4C3T x nanohybrids were still maintained after the OER process (Figure S3C-D). In order to probe the OER kinetics of the serial samples, Tafel plots were calculated from the corresponding LSV curves. As shown in Figure 4B and Figure S3B, the M1OOH@V4C3T x, M2OOH@V4C3T x, M3OOH@V4C3T x, and M4OOH@V4C3T x presented the Tafel slopes of 51.6, 50.0, 51.4, and 51.9 mV dec−1, respectively, which were all lower than the bare V4C3T x MXene (122.9 mV dec−1), commercial IrO2 catalyst (71.9 mV dec−1), MOOH@M and bare MOOH (>100 mV dec−1). From the Tafel slopes, all of the MOOH@V4C3T x nanohybrids presented faster OER kinetics than the samples without V4C3T x MXene. In order to get a further insight into the role of V4C3T x MXene in promoting the OER activity, electrochemical impedance spectroscopy was performed on the V4C3T x MXene, MOOH@V4C3T x nanohybrids, and the MOOH@M samples. As shown in Figure 4C, evidenced by the Nyquist plots, the samples with V4C3T x MXene all presented a reduced radius of the semicircle at the high-frequency region, indicating a lower charge transfer resistance (Rct). Therefore, the enhanced OER activity of MXene-containing nanohybrids can be partly ascribed to the rapid charge transfer kinetics of the highly conductive network constructed by MXene nanosheets. Since the critical Fe3+ content in NiOOH matrix was around 25% to keep the homogeneous Ni1 − xFe xOOH structure,40 the best OER performance of M3OOH@V4C3T x nanohybrid might be ascribed to the rich Fe substituted site with a homogeneous structure and the fast charge transfer network of MXene substrate. Noteworthy, the low overpotential and Tafel slope of the as-prepared MOOH@V4C3T x nanohybrid is comparable or even better than some of the state-of-the-art noble metal-contained or non-noble metal-based OER electrocatalysts, such as N-Co3O4@NC-2 (η = 266 [email protected] mV dec−1),41 Raney NiO x (η = 304 mV@53 mV dec−1),42 Ir1@Co/NC (η = 260 mV@163 mV dec−1),43 and CoCo-LDH 2D nanomesh (η = 319 mV@42 mV dec−1).44 When compared with the recent reported MXene-based OER electrocatalysts, for example, FeNi-LDH/Ti3C2-MXene (η = 298 mV@43 mV dec−1),45 peeled off NiFe-LDH/MXene powder (η = 314 mV@67 mV dec−1),46 NiCoS/Ti3C2T x (η = 365 [email protected] mV dec−1),47 and the Co/N-CNTs@Ti3C2T x (η = 411 [email protected] mV dec−1),48 the nanohybrid reported in this study also delivers lower overpotential and faster OER kinetics.
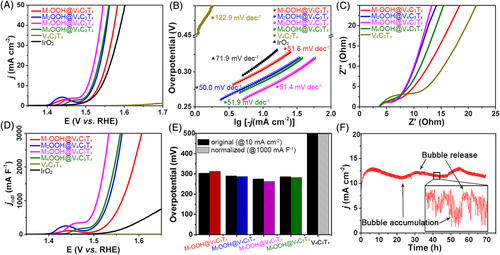
 of V4C3T
x MXene and the series MOOH@V4C3T
x nanohybrids. F, The chronoamperometry curves of the M3OOH@V4C3T
x nanohybrid at a static potential with current density of around 10 mA cm−2 over 70 hours. OER, oxygen evolution reaction
of V4C3T
x MXene and the series MOOH@V4C3T
x nanohybrids. F, The chronoamperometry curves of the M3OOH@V4C3T
x nanohybrid at a static potential with current density of around 10 mA cm−2 over 70 hours. OER, oxygen evolution reactionIn order to explore the intrinsic property variations of the MOOH@V4C3T x nanohybrid, NiOOH monolayer (001)-surface adsorbed on O-terminated V4C3O2 monolayer was taken as the model structure for density functional theory simulation (Figure S4A). As a result, the optimized interlayer spacing was only 1.464 Å with a binding energy of −0.079 eV per unit cell (the area of a supercell is 32.60 Å2), which indicated an appreciable electronic coupling between NiOOH and V4C3O2 layers. As confirmed by the total density of states (TDOS) results (Figure S4B), the NiOOH, V4C3O2, NiOOH@V4C3O2, Fe-doped NiOOH@V4C3O2, and V-doped NiOOH@V4C3O2 nanohybrids were all present continuous TDOS near the Fermi levels, revealing the intrinsically metallic features thus possessing fast charge transfer and showing higher electron conductivity. Additionally, as shown in Figure S4B, the peak from low electrical states of bare NiOOH shifted to the Fermi level after NiOOH stacked with V4C3O2, suggesting an enhancement on electrical states at the Fermi level and a stronger carrier density.
It should be pointed out that the electrocatalytic activity is usually proportional to the surface area. Therefore, the electrochemical double-layer capacitance (C
dl) of the V4C3T
x MXene and MOOH@V4C3T
x nanohybrids were determined to evaluate their electrochemically effective surface area. As shown in Figures S5 and S6, the pristine V4C3T
x MXene presented the highest C
dl area (25.12 mF cm−2) among all the tested samples, which was around 2- to 8-fold larger than the MOOH@V4C3T
x nanohybrids. For four MOOH@V4C3T
x nanohybrids, the M3OOH@V4C3T
x gave the smallest C
dl area (2.18 mF cm−2). Hence, the LSV curves of all the samples were normalized by the corresponding C
dl area in order to evaluate their real OER activities. As shown in Figure 4D and Figure S6B, the normalized LSV curves presented a similar tendency as shown in Figure 4A and Figure S3A. In order to better evaluate the relationship between C
dl area and OER activities, the normalized overpotential for each MOOH@V4C3T
x nanohybrid to reach the 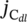 of 1000 mA F−1 was chosen as the standard. Interestingly, it was found that, although the normalized overpotentials (
of 1000 mA F−1 was chosen as the standard. Interestingly, it was found that, although the normalized overpotentials (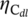 ) of the M2OOH@V4C3T
x, M3OOH@V4C3T
x, and M4OOH@V4C3T
x were all lower than the original ones, the
) of the M2OOH@V4C3T
x, M3OOH@V4C3T
x, and M4OOH@V4C3T
x were all lower than the original ones, the  of M1OOH@V4C3T
x was higher than the original value (Figure 4E). The estimated turnover frequencies of the MOOH@V4C3T
x, MOOH@M, and IrO2 catalysts also confirmed the higher intrinsic OER activities of the MOOH@V4C3T
x nanohybrids (Figure S6C). These results implied that the enhancement of OER performance after Ni/Fe RSM on V4C3T
x MXene might be related to the higher intrinsic activity of the suitable Ni/Fe ratio and the synergy of the V4C3T
x MXene substrate.
of M1OOH@V4C3T
x was higher than the original value (Figure 4E). The estimated turnover frequencies of the MOOH@V4C3T
x, MOOH@M, and IrO2 catalysts also confirmed the higher intrinsic OER activities of the MOOH@V4C3T
x nanohybrids (Figure S6C). These results implied that the enhancement of OER performance after Ni/Fe RSM on V4C3T
x MXene might be related to the higher intrinsic activity of the suitable Ni/Fe ratio and the synergy of the V4C3T
x MXene substrate.
Finally, the chronoamperometry curve of the optimized M3OOH@V4C3T x nanohybrid was recorded at a static potential with current density of around 10 mA cm−2 over 70 hours to evaluate the stabilities of the nanohybrid (Figure 4F). After the 70-hours OER process, the current density remained almost the same. As observed in the XRD pattern, the tested sample presented an amorphous nature (Figure S7). Therefore, TEM and HRTEM images of the nanohybrid after 70-hours OER process were taken for further verification. As shown in Figure S8, the lattice pattern of the V4C3T x substrate can still be identified from the HRTEM and inverse FFT image, indicating the good stability of the V4C3T x MXene. However, many thin amorphous patches were covered on the surface of V4C3T x MXene, which can be ascribed to the surface MOOH species. To characterize the variation in chemical bonding of the nanohybrid and verify the products, XPS was performed again on the materials after 70-hours OER process. As shown in the XPS spectra (Figure S9), after the long-time OER process, the signals of C-V and C-V-F x components can still be detected from the C 1s and the F 1s spectra. While for the O 1s spectrum, signals of surface metal oxide species (529.3 eV), C-V-OH (530.2 eV), C-V-O x (531.1 eV) species, and surface adsorbed water (532.0 eV) were still detected, respectively.27, 31 And a new deconvoluted peak located at 528.4 eV appeared, which is possibly related to the surface MOOH species. For Ni 2p, the deconvoluted peak of Ni0 disappeared and only Ni2+ signal can be detected. In addition, in V 2p and Fe 2p spectra, the deconvoluted peaks for V3+ (516.2 and 522.9 eV) and Fe3+ (712.1 and 734.1 eV) became stronger,11, 49 indicating that V3+ and Fe3+ species might exist in the MOOH surface layers as dopant after the surface oxidation reaction.
In summary, a novel nine atomic layered V4C3T x MXene was obtained via the HF etching method, for which the multilayered structure endowed it with excellent structural stability during RSM and OER processes. By utilizing the reactive surface alloy modified V4C3T x MXene as nanoprecursor, a series of MOOH@V4C3T x (M = Ni, Fe, and V) nanohybrids were successful obtained via in situ surface transformation during the OER process. Owing to the high OER activity of the surface ternary oxide hydroxide layers and the stable conductive skeleton of the V4C3T x MXene, these nanohybrids present excellent OER activity and durability. With an optimized Ni/Fe ratio, the nanohybrid delivered a very low η of 275.2 mV, and excellent stability over 70-hours long-time OER process. Demonstrated by the tunable physicochemical properties and excellent structural stability of these nanohybrids, the multilayered M4X3-based MXenes show great promise as efficient substrates for future energy conversion and storage applications.
ACKNOWLEDGMENTS
The calculation was carried out at National Supercomputer Center in Tianjin, and performed on TianHe-1(A). The authors gratefully acknowledge the financial support from Singapore MOE Tier 2 MOE2017-T2-2-069 and MOE2018-T2-1-010. The National Natural Science Foundation of China (No. 51901189), the “Shuimu Tsinghua Scholar Program” and the Opening Project of Key Laboratory of Materials Processing and Mold (No. NERC201903). The authors also acknowledge the Facility for Analysis, Characterization, Testing and Simulation (FACTS), Nanyang Technological University, Singapore, for use of the XPS Kratos AXIS Supra facilities; the Analytical & Testing Center of Northwestern Polytechnical University, for use of the SEM Helios G4 CX microscope; and the Shaanxi Materials Analysis and Research Center for use of the TEM Talos F200X facilities.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.