Mechanisms of H4/ICOS costimulation: effects on proximal TCR signals and MAP kinase pathways
Abstract
H4/ICOS is a costimulatory molecule related to CD28. Its effects on early TCR signals have been analyzed in mouse CD4+ Th2 cells, expressing H4/ICOS at higher levels than Th1 clones. Anti-H4/ICOS antibodies strongly enhanced CD3-mediated tyrosine phosphorylation of ZAP-70, ζ, or Vav, as well as extracellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK) and p38 MAP kinase activation in these cells. The association of phosphoinositide 3-kinase (PI-3K) to H4/ICOS was enhanced by H4/ICOS cross-linking, and PI-3K inhibitors inhibited ERK and JNK activation andIL-4/IL-10 secretion, but not p38 MAP kinase or ZAP-70 activation. H4/ICOS-mediated activation of JNK, but not ERK or p38, is partially dependent on the expression of CD4 by the cells, whereas H4/ICOS costimulation is partially independent on CD28 expression. Cytochalasin D, an inhibitor of actin polymerization, inhibited ZAP-70, MAP kinase activation, or IL-4/IL-10 secretion. Neither cyclosporin A nor inhibitors of PKC produced detectable inhibition of ZAP-70 phosphorylation or MAP kinase activation in these Th2 cells. Cyclosporin A strongly inhibited IL-4, but not IL-10 secretion. ERK or JNKinhibitors partially inhibited IL-4 and IL-10 secretion, while PKC or p38 inhibitors had no significant effects on IL-4 or IL-10 secretion. Taken together, our data show clear similarities of costimulation mechanisms between H4/ICOS and CD28 during the early steps of TCR activation.
Abbreviations:
-
- MAP kinase:
-
Mitogen-activated protein kinase
-
- ERK:
-
Extracellular signal-regulated kinase
-
- JNK:
-
Jun N-terminal kinase
-
- p38:
-
p38/HOG, stress-activated protein kinase 2
-
- PI-3K:
-
Phosphoinositide 3-kinase
1 Introduction
Optimal activation of T lymphocyte effector functions and antigen-specific clonal expansion requires signals from cell surface costimulatory molecules in addition to signals delivered through the TCR/CD3 complex 1–3. Recently, a new costimulatory molecule termed ICOS (for inducible costimulator), homologous to CD28 and CTLA-4 (CD152) has been identified and cloned in the human, mouse, and rat 4–6. ICOS is the same molecule as H4, a mouse and human costimulatory protein, which we previously described using a hamster monoclonal antibody (7–9, reviewed in 10).
H4/ICOS is a disulfide-linked homodimeric glycoprotein of 50–65 kDa, whose expression is characteristically induced upon activation of peripheral resting T lymphocytes 4, 7, 8. Like CD28, H4/ICOS enhances TCR/CD3-induced proliferation of peripheral resting T lymphocytes 4, 5, 7. However, H4/ICOS costimulation produces no increase of IL-2 secretion, yet enhances the secretion of cytokines including IL-4, IL-10 and IFN-γ 4, 5. H4/ICOS ligand (B7h, B7RP-1) is homologous but distinct to the CD28/CTLA-4 ligands CD80 (B7.1) and CD86 (B7.2) 5, 11, 12. B7h is constitutively expressed by Blymphocytes and macrophages 5, 11, and its expression can be induced in cells from different tissues by TNF-α or LPS 11.
Manipulation of ICOS-B7h interactions "in vivo" and "in vitro" shows that H4/ICOS is a major costimulator of activated T cells 13, important to reactions primarily mediated by Th1 as well as Th2 T cells, including secondary DTH immune responses, experimental allergic encephalomyelitis, allograft rejection, and allergic lung inflammation 5, 14–16, or Ig responses 13. Blockade or loss of H4/ICOS-B7h interactions induces diminished IL-4, IL-10 or IL-13 production and Th2-mediated lung inflammation 13, 17–19, as well as enhanced IFN-γ production and incidence of EAE 18, 19 or lower IFN-γ production 13, 17. ICOS-deficient mice have reduced antibody responses as well as CD40-mediated antibody class switching to foreign antigens 19–21.
The precise mechanisms of H4/ICOS costimulation are not known. However, its functional and sequence homology with CD28 7, 8, 13, and particularly the conservation of a Tyr-Met-X-Met sequence motif in the cytoplasmic domain suggests that H4/ICOS and CD28 might use overlapping signaling pathways.
Several mechanisms of TCR/CD3/CD28 signal integration have been proposed, including the activation of ZAP-70, Vav, and MAP kinases 22–26. To determine if some of these mechanisms of signal integration extend to H4/ICOS, we have used mouse T cells constitutively expressing H4/ICOS to analyze its effect on early TCR/ CD3 signaling and MAP kinase pathways. Our results show that H4/ICOS strongly synergizes with CD3 for tyrosine phosphorylation of TCR/CD3 ζ chains, ZAP-70 and Vav, as well as for the activation of the MAP kinases ERK, JNK and p38. Inhibition of actin polymerization inhibit these effects, while inhibition of phosphoinositide 3-kinase (PI-3K) inhibits activation of the ERK and JNK MAP kinases but not ZAP-70 phosphorylation, showing that the integration of TCR/CD3 and H4/ICOS signals can occur at different levels in the early steps of T cell activation by PI-3K- and actin polymerization-dependent mechanisms.
2 Results
2.1 H4/ICOS expression in Th1 and Th2 mouse T cell lines: association of PI-3K
To determine adequate experimental models to analyze H4/ICOS costimulation, we checked several CD4+ T cell lines for H4/ICOS expression. Although, as described previously 7, all mouse T cell lines examined expressed H4/ICOS, lines of Th2 phenotype expressed higher levels of H4/ICOS than Th1 cell lines (Fig. 1A). These data are in agreement with recent reports 13, 18. In view of these results, the SR.D10 subclone of the D10.G4.1 Th2 cell line was chosen for our study.
It has been suggested that H4/ICOS constitutively associates with PI-3K regardless of cross-linking 13. Fig. 1B shows that immunoprecipitation of H4/ICOS from the cell surface of unstimulated D10 cells recruited a small amount of the 85-kDa regulatory subunit of PI-3K. However, the amount of H4/ICOS-associated PI-3K was enhanced more than 30-fold by pervanadate activation of the cells (Fig. 1B). These results suggest a difference between our system, involving cell lines naturally expressing H4/ICOS, and those used by Coyle et al. 13, using ICOS-transfected Jurkat.
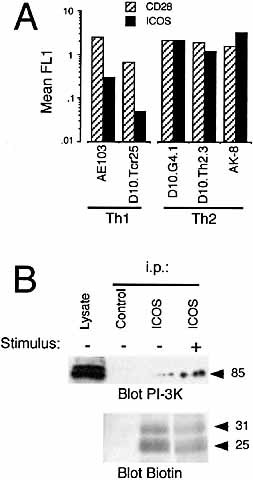
Differences in H4/ICOS expression between Th1 and Th2 mouse T cell clones, and activation-dependent association of PI-3K to H4/ICOS. (A) H4/ICOS and CD28 were analyzed by flow cytometry in resting cells from the Th1 mouse T cell clones AE103 and D10.Tcr25 or the Th2 cell clones D10.G4.1, D10.Th2.3, and AK-8. Results show the mean fluorescence above background for each molecule. (B) Analysis by immunoblot of PI-3K co-precipitation in H4/ICOS immunoprecipitates (i.p.) from the mouse Th2 cell line D10 activated (+) or not (–) with pervanadate. H4/ICOS in immunoprecipitates was detected by blotting with streptavidin-peroxidase using biotin-labeled cells. The presence of PI-3K in lysates from 2×105 cells was also determined, as indicated. Molecular mass is given in kDa.
2.2 H4/ICOS synergizes with TCR/CD3 for early T cell activation
H4/ICOS-mediated costimulation was analyzed using antibody-coated latex microspheres essentially as described in Viola et al. (27, see Methods). Several MAP kinase pathways involved in TCR-mediated activation and CD28 costimulation were analyzed by determining the activation (i.e. dual phosphorylation) of downstream target kinases including ERK1/2, JNK, and p38. The results shown in Fig. 2 indicate that joint ligation of CD3 and H4/ICOS strongly synergized for the activation of all three kinases. Ligation of CD3 or H4/ICOS alone induced a low level of ERK and p38 activation (which usually could only be detected upon long exposures, data not shown); however, under the activation conditions used, JNK activation was only observed upon ligation of both CD3 and H4/ICOS. Thus, while all MAP kinase pathways were targets for H4/ICOS costimulation, the JNK/SAPK pathway might be a specific target for TCR-H4/ICOS signal integration, as previously shown for CD28 23, 26.
Data on CD28 suggest that signal integration of costimulatory molecules can occur upstream in the TCR activation cascade, pointing to ZAP-70, Vav, and even TCR ζ chains as key molecular targets in this process 24, 25, 28. Consequently, we checked for upstream activation targets for H4/ICOS (Fig. 2B). CD3 ligation alone induced low level tyrosine phosphorylation of the tyrosine kinase ZAP-70 in the conditions of the assay. However, H4/ ICOS clearly synergized with CD3 for ZAP-70 phosphorylation. Furthermore, H4/ICOS and CD3 coligation induced enhanced ζ chain and Vav tyrosine phosphorylation (Fig. 2C). A low level of tyrosine phosphorylation of Vav could be observed by H4/ICOS cross-linking alone (Fig. 2C), a phenomenon previously described for CD28 29, 30. Similar results were obtained in other Th2 clones analyzed (data not shown).
Since CD4 co-localizes with H4/ICOS upon cross-linking 7, 8, it is involved in early TCR/CD3 signals in D10 cells 31–33, and mutual modification of CD4- and CD28-mediated signals have been described 34–36, the role of CD4 in H4/ICOS costimulation was also analyzed. Fig. 2 shows that CD3-CD4 coligation enhanced CD3 signals, inducing strong tyrosine phosphorylation of ZAP-70 (Fig. 2B) as well as ERK, p38 and JNK activation in these cells (Fig. 2A). Using D10 cells expressing or not CD4, we observed that H4/ICOS costimulation of ERK activation is largely independent of CD4 expression, whereas JNK activation is clearly stronger in cells expressing CD4 (Fig. 3A). Intriguingly, costimulation of p38 activation is stronger in cells lacking CD4 (Fig. 3A).
The role of CD28 in H4/ICOS costimulation was also analyzed in CD4+ T cells from CD28-deficient mice. Since resting T lymphocytes express negligible levels of H4/ICOS, CD4+ T cell blasts were used. H4/ICOS expression in cells from CD28–/– mice was about one fourth of that of cells from congenic normal mice (Fig. 3B), confirming previous data showing CD28 dependence of optimal H4/ICOS expression 18, 37, 38. This difference was not observed in other cell surface markers, including CD3 (Fig. 3B) or CD4 (data not shown). Despite the low-level H4/ICOS expression, ZAP-70 phosphorylation was enhanced by CD3 and H4/ICOS co-ligation in these CD28-deficient cells (Fig. 3C), although H4/ICOS-costimulation of ZAP-70 phosphorylation was stronger in cells from CD28+/+ mice (Fig. 3C). These differences in costimulation extended to MAP kinase activation, and were particularly clear in the case of ERK (Fig. 3C). Whether these differences are due to the differences in ICOS expression between CD28+/+ and CD28–/– cells, or there is some degree of functional dependence of ICOS costimulation on CD28 expression, needs to be further ascertained.
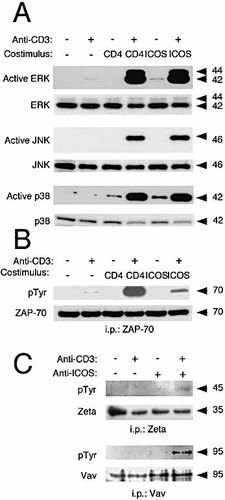
H4/ICOS-mediated costimulation of early TCR activation. (A) Immunoblot of cell lysates of SR.D10 cells incubated for 5 min with polystyrene beads coated with anti-CD3 (1 μg/ml), anti-CD4 (5 μg/ml) or anti-H4/ICOS antibody (5 μg/ml). Parallel samples of cell lysates were analyzed by immunoblot for the presence of active forms of the MAP kinases ERK, JNK, and p38 using antibodies specific for dually phosphorylated kinases. The same blots were then stripped and re-probed with kinase-specific antibodies to check for protein loading. (B) Lysates from the cells shown in (A) (5×106 cells/determination) were immunoprecipitated with anti-ZAP-70 antibodies coupled to Sepharose. The immunoprecipitates were separated by electrophoresis, transferred to PVDF membranes and then analyzed by immunoblotting with anti-pTyr antibody, stripped and re-probed with anti-ZAP-70 antibody. (C) Lysates form cells activated as described in (A) were immunoprecipitated with anti-ζ or anti-Vav antibodies, as indicated. After electrophoresis, the immunoprecipitates were analyzed by immunoblot with anti-phosphotyrosine, stripped, and re-probed with anti-ζ or anti-Vav antibodies.
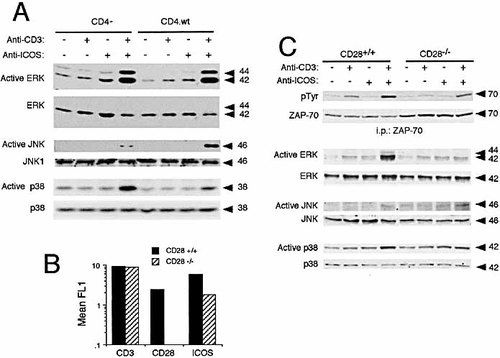
Role of CD4 or CD28 expression onH4/ICOS costimulation. (A) Cells expressing (CD4.wt) or not (CD4–) were activated as described in Fig. 2. Cell lysates were analyzed by immunoblot to detect active forms of ERK, JNK, or p38, then stripped and re-probed for protein loading with kinase-specific antibodies. (B) Surface expression of CD3, CD28, and H4/ICOS in CD4+ T cell blasts from CD28-deficient (CD28–/–) or normal (CD28+/+) C57BL.6 mice, as determined by flow cytometry. (C) Effect of H4/ICOS costimulation on ZAP-70 tyrosine phosphorylation and MAP kinase activation determined by immunoblot of ZAP-70 immunoprecipitates or cell lysates from CD28+/+ or CD28–/– CD4+ T cell blasts, as described in Fig. 2.
2.3 PI-3K dependence of H4/ICOS-mediated costimulus
The conservation of a PI-3K association motif and the association of this kinase to H4/ICOS suggest a prime role of this kinase in the biological effects of H4/ICOS. Pre-treatment of SR.D10 cells with the PI-3K inhibitors LY294002 (LY, 20 μM) or Wortmannin (0.1 μM) clearly inhibited ERK-1/2 and JNK activation, but not p38 activation induced by combined anti-CD3 plus anti-H4/ ICOS antibodies (Fig. 4A). However, some differences were noted, as Wortmannin blocked both ERK and JNK activation (i.e. ≥90% inhibition, Fig. 4), whereas LY-induced inhibition was partial under the same conditions (Fig. 4A, top).
LY294002 or Wortmannin did not inhibit tyrosine phosphorylation of ZAP-70 induced by anti-CD3 plus anti-H4/ ICOS, indicating that optimal ZAP-70 recruitment and activation is independent on PI-3K activity under these conditions. Both LY294002 and Wortmannin strongly inhibited IL-4 or IL-10 secretion in SR.D10 cells activated by anti-CD3 plus anti-H4/ICOS (Fig. 4C). This suggests that p38 activation is not relevant to IL-4 or IL-10 secretion by these cells. On the other hand, the data show that LY294002 is as effective as Wortmannin in inhibiting lymphokine secretion (Fig. 4C), whereas Wortmannin inhibits ERK or JNK activation more efficiently (Fig. 4A). On the other hand, the ERK inhibitor U0126 (U0, 5 μM) or the JNK inhibitor L-JNKI1 (JNKI, 20 μM) partially inhibited IL-4 and IL-10 secretion, whereas SB203580, a p38 inhibitor (SB, 2 μM) did not produce significant effects (Fig. 5). Taken together, these data suggests that PI-3K is involved in ERK and JNK activation as well as in additional pathways relevant to lymphokine secretion.
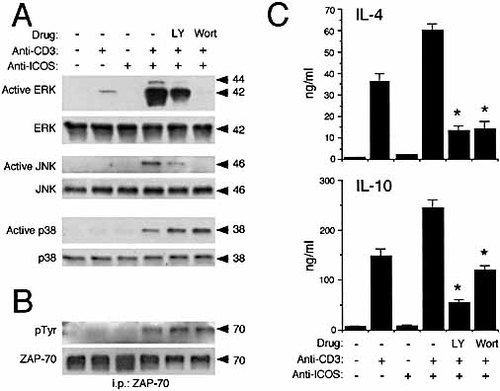
Effect of PI-3K inhibitors on H4/ICOS costimulation. (A) Lysates of SR.D10 cells activated as in Fig. 2 with or without the PI-3K inhibitors LY 294002 (LY, 20 μM) or Wortmannin (Wort, 0.1 μM) were probed by immunoblot using antibodies specific for active forms of the ERK, JNK and p38 kinases. These blots were stripped and re-probed with antibodies specific for each kinase, as indicated. (B) Blots of ZAP-70 precipitates of the same cell lysates were probed with anti-phosphotyrosine antibodies (pTyr), stripped, and blotted with anti-ZAP-70 antibodies. (C) IL-4 and IL-10 secretion by cells activated for 5 h in the presence of different drugs, as determined by capture ELISA in culture supernatants. Data represent the mean ±SE of triplicate cultures. Significant differences (p<0.05) between samples with or without drug are indicated by asterisks.
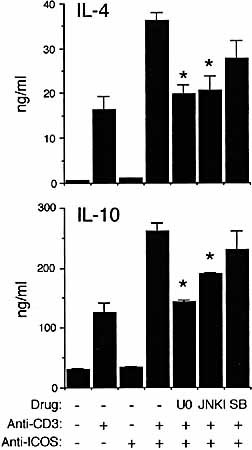
Effect of the ERK inhibitor U0126 (U0, 1 μM), the JNK inhibitor L-JNKI1 (JNKI, 20 μM), or the p38 inhibitor SB203580 (SB, 2 μM) on IL-4 and IL-10 secretion by SR.D10 cells. Activation as described in Fig. 4C.
2.4 Role of actin cytoskeleton, PKC and calcineurin in H4/ICOS costimulation
Further experiments were performed to analyze the importance of actin cytoskeleton, calcineurin, and protein kinase C on H4/ICOS-mediated costimulation of TCR/CD3 signals in D10 cells (Fig. 6). Activation of ERK and ZAP-70 tyrosine phosphorylation were strongly inhibited by cytochalasin D, an inhibitor of actin polymerization. Cytochalasin D also blocked the appearance of the activated 46-kDa form of JNK, possibly the low molecular mass product of JNK1, 39, 40. Intriguingly, a strong phosphorylated form of 48 kDa appeared. This might correspond to the low molecular mass form of JNK2, whose dual phosphorylation was occasionally detectable as a faint band upon activation of untreated cells (Fig. 6A). The functional meaning of the phosphorylation of this JNK isoform is obscure, as it did not induce activation of lymphokine secretion, as shown below. Cytochalasin D also induced a strong inhibition of ZAP-70 phosphorylation (Fig. 6B), and blocked IL-4 and IL-10 secretion (Fig. 6C). Taken together, these results show that actin polymerization is required for H4/ICOS costimulation, acting upstream of ZAP-70 phosphorylation.
Neither the calcineurin phosphatase inhibitor cyclosporin A, nor the PKC inhibitors RO-31-8220 (RO) and GF 109203X (GF) produced detectable changes in ZAP-70 phosphorylation or in ERK and JNK activation (Fig. 6A, B). On the other hand, cyclosporin A blocked IL-4 secretion, and it did not produce significant changes of IL-10 secretion. As shown in Fig. 6C, the PKC inhibitors RO and GF produced no significant effect on IL-4 or IL-10 secretion in this experimental system.
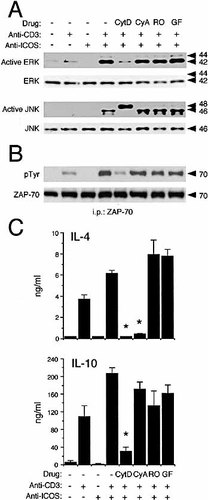
Effect of the actin polymerization inhibitor cytochalasin D (CytD, 10 μM), the calcineurin inhibitor cyclosporin A (CyA, 1 μM), or the PKC inhibitors RO-31–8220 (RO, 0.5 μM) and GF109203X (GF, 0.5 μM) on (A) the activation of the ERK and JNK MAP kinases, as determined by immunoblot of lysates from cells activated as described in Fig. 2, using antibodies specific for active forms of the kinases, or (B) by immunoblot of immunoprecipitated ZAP-70 with phosphotyrosine-specific antibodies. (C) IL-4 and IL-10 secretion by cells activated for 5 h in the presence of different stimuli and drugs, as indicated. Conditions, as described in Fig. 4.
3 Discussion
H4/ICOS is an activation-induced molecule homologous to the T cell regulatory proteins CD28 and CTLA-4 4–6. Its ligand (Bh7) is also homologous to the CD28/CTLA-4 ligands B7.1 and B7.2 5, 11, 12. Although its function is not fully understood, experiments performed using H4/ ICOS-specific antibodies show a costimulatory effect of H4/ICOS ligation, enhancing T cell proliferation and altering the pattern of lymphokine secretion (4, 5, 7, reviewed in 10). Particularly, H4/ICOS might be important for IL-4-and IL-10-mediated responses including the homeostasis of inflammatory reactions, the balance of Th1/Th2 differentiation, or B lymphocyte differentiation to plasma cells 13, 17–21.
CD28 and H4/ICOS share a Y-M-x-M sequence motif in their cytoplasmic domain, which, in CD28, can efficiently bind PI-3K upon phosphorylation of the tyrosine residue. Unlike one recent report showing that PI-3K association to ICOS is largely independent on activation 13, we have observed that it is strongly enhanced by cell activation (Fig. 1).
Our results show that H4/ICOS produces a strong enhancement of early TCR/CD3-mediated signals, including enhanced tyrosine phosphorylation of TCR zeta, ZAP-70, and Vav (Fig. 2–4, 6). The mechanisms involved in enhanced tyrosine phosphorylation of substrates by H4/ICOS are not clear, although similar findings have been observed in CD28-mediated costimulation 24, 25.
It is intriguing that inhibitors of events thought to occur later in the activation process, like the inhibitor of actin polymerization cytochalasin D, can also inhibit events as early as ZAP-70 phosphorylation. One possible explanation is a positive feedback mechanism whereby H4/ICOS favors TCR-mediated activation of Vav and Rho, producing enhanced TCR/CD3 recruitment and proximity by an actin polymerization-dependent mechanism (reviewed in 41), perhaps by the reorganization of membrane microdomains as recently observed in CD28 27. CD28 is needed for efficient induction of H4/ICOS surface expression (Fig. 3B, 18, 37, 38), and hence H4/ICOS signaling is at least indirectly dependent on CD28 expression (Fig. 3C). The process of reorganization of membrane molecules would also help in localizing in close range key activating enzymes like the tyrosine kinase Lck, as well as ZAP-70 substrates including Vav itself and the Vav-associated scaffolds LAT and SLP-76. The role of these molecules in H4/ICOS-mediated costimulation needs to be further ascertained.
In addition, H4/ICOS ligation synergized with TCR/CD3 signals for activation of the ERK and p38 MAP kinases, although a low-level activation was detected by separate ligation of TCR/CD3 or H4/ICOS in some experiments (Fig. 2, 3, and data not shown). JNK activation was clearly observed by coligation of TCR/CD3 plus H4/ ICOS, but was never detected using either stimulus alone (Fig. 2–4, 6). This phenomenon resembles previous data on the mechanisms of CD28 costimulation, pointing to JNK as a key molecule in the integration of TCR and CD28 signals for secretion of lymphokines like IL-2 23, 26, although recent data challenges JNK as the sole integration point of TCR and CD28 39, 40, 42. Furthermore, integration of TCR and CD28 signals can also occur at Vav and ZAP-70, upstream in the activation cascade 22, 24, 25, 28. These data suggest several integration points between TCR and CD28 signals, and possibly between TCR and H4/ICOS, depending on the cell and the phenomenon considered.
It has been reported that, in some models, CD28 activation can be only achieved in T cells with abundant free Lck, i.e. lacking CD4 36. In addition, the results shown in Fig. 2 suggest that CD4 and H4/ICOS might have overlapping costimulatory mechanisms in SR.D10 cells. The analysis of H4/ICOS costimulation in D10 cells expressing or not CD4 indicates that CD4 expression is not an absolute need for H4/ICOS costimulation, although MAP kinase activation can be higher (p38) or lower (JNK) in the CD4– cells (Fig. 3).
In this study, IL-4 and IL-10 where chosen as two lymphokines showing positive, but distinct, responses to H4/ICOS costimulation 4, 5, and being under different mechanisms of transcription control 43–46. Our results support the contention that efficient lymphokine secretion and H4/ICOS costimulation is partially dependenton ERK and JNK activation (Fig. 4, 5). So, ERK and JNK inhibitors partially inhibited IL-4 and IL-10 secretion (Fig. 5). Although p38 activation was enhanced by H4/ICOS costimulation, this was not relevant to IL-4 or IL-10 secretion by these Th2 cells (Fig. 2–5). Nevertheless, enhanced p38 activity might be important to the blocking effect of anti-ICOS reactions in Th1-mediated immune reactions 15, 16.
The calcineurin inhibitor cyclosporin A had no effect on ZAP-70 or MAP kinase activation (Fig. 6). However, as expected from the importance of NFAT in IL-4, but not in IL-10 transcription, cyclosporin A blocked IL-4 secretion while leaving IL-10 secretion unaffected (Fig. 6), in agreement with previous data 47. Also in agreement with previously published data using Th2 cells 47, inhibitors of protein kinase C barely modified IL-4 or IL-10 secretion induced by TCR plus H4/ICOS (Fig. 6). The elements involved in the H4/ICOS-mediated costimulation of IL-10 secretion are obscure, since IL-10 transcription is primarily under the control of the ubiquitous Sp1 and Sp2 transcription factors 44, 46. Furthermore, previous data indicate that basal IL-10 mRNA in unstimulated D10 cells is very low 47, making mRNA changes 48 an unlikely IL-10 regulatory mechanism in this system. JNK activation might exert a positive role on IL-10 secretion through Sp1 and Sp2 regulation 49. In fact, IL-10 secretion is inhibited by JNK-specific inhibitors (Fig. 5) or by PI-3K inhibitors that block JNK activation (Fig. 4).
It is know that the IL-4 promoter possesses several AP-1 and NF-AT sites 50. It is not surprising, then, that ERK and JNK inhibitors induce significant inhibition of IL-4 secretion (Fig. 5). Interestingly, PI-3K inhibitors are strong inhibitors of IL-4 and IL-10 secretion. This might be due to inhibition of ERK and JNK activation (Fig. 4A). In addition, PI-3K might activate other pathways relevant to lymphokine secretion. For instance, one important target of PI-3K is Akt-1, whose activation is strongly enhanced by H4/ICOS costimulation 51. However, recent data indicates that Akt is not involved in the regulation of Th2 cytokines 52. Another possible target is the activation of PLC-γ mediated by the Tec family kinase Itk, as recently observed in CD28 costimulation 53.
There are clear differences between H4/ICOS and CD28. One major difference is their constitutive (CD28) or inducible (H4/ICOS) expression in resting peripheral T cells, the differences in expression between Th1 and Th2 CD4+ T cell clones (Fig. 1, 13, 18), and the expression patterns of their ligands. These differences might influence the expansion of T cells in different activation stages, the differentiation of T and B cells and/or favor the secretion of particular sets of lymphokines. In addition, differences in the cytoplasmic Y-M-x-M motif (YMNM in CD28, YMFM in ICOS) or the C-terminal region of the cytoplasmic domain might affect the association of molecules, like Grb-2, involved in certain CD28 downstream signaling pathways 13, 30, 54. Although the molecular mechanisms underlying these differences need to be further ascertained, our results usingTh2 cell lines suggest that they are not relevant to at least some mechanisms of signal integration between TCR/CD3 and the costimuli, involving ZAP-70 and MAP kinases.
4 Materials and methods
4.1 Cells and plasmids
The SR.D10 subclone of the mouse Th2 clone D10.G4.1 was used and was maintained in Click's EHAA medium supplemented with 10% heat-inactivated FCS (culture medium) containing 5 U/ml mouse IL-2, 10 U/ml mouse IL-4, and 25 pg/ml mouse IL-1α 55. To obtain the clones CD4–.Neo.2H2, and CD4FL.4F3, the CD4–.F1 mutant of SR.D10 32 was transfected by electroporation with pSRαNeo, or with the same vector containing mouse wild-type CD4 (FL.4F3). These cells were maintained as described for SR.D10 cells. The Th1 clones AE103 56 and D10.Tcr25 57 and the Th2 clones D10.Th2.3 57 and AK-8 58 were grown as previously described 59.
4.2 Antibodies and other reagents
The following monoclonal antibodies were used: YCD3–1 (rat IgG2b anti-mouse CD3 60); M1/70 (rat IgG2b anti-mouse CD11b 61); GK1.5 (rat IgG2b anti-mouse CD4 62); C398.4A (hamster IgG anti-mouse and human H4/ICOS 7, 8; and 37.51 (hamster IgG anti-mouse CD28 63). They were used asprotein A- or protein G-purified preparations of hybridoma supernatants. Horseradish peroxidase coupled anti-phosphotyrosine monoclonal antibody PY-20 was obtained from Amersham Pharmacia Biotech (Little Chalfont, GB). A mouse IgG1 monoclonal antibody specific for the PI-3K 85 kDa subunit was from Upstate Biotechnology (Upstate, NY, cat. no. 05-212).
Rabbit anti-ZAP-70 antibodies were specific for a fusion protein of GST and residues 266 and 344 of mouse ZAP-70. Rabbit anti-ζ antibodies were specific for residues 132–144 of mouse ζchains conjugated to Ovalbumin. Anti-ZAP-70 and anti-ζ antibodies were purified by affinity chromatography over the immunizing protein or peptide coupled to Sepharose. For immunoprecipitation, purified anti-ZAP-70 or anti-ζ antibodies were coupled to CNBr-Sepharose. Rabbit anti-Active ERK antibodies were from New England Biolabs, (Beverly, MA). Rabbit anti-active JNK, and anti-active p38 antibodies were from Promega (Madison, WI). Rabbit anti-ERK, anti-JNK1, and anti-p38 were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-Vav serum (301-S) was a gift of Dr. Xose Bustelo (Salamanca, Spain). FITC-conjugated goat anti-hamster IgG was from Southern Biotechnology (Birmingham, AL). Horseradish peroxidase-conjugated anti-mouse Ig or anti-rabbit Ig antibodies were from Sigma. Normal Armenian hamster IgG was obtained from Armenian hamster sera by protein A-Sepharose affinity chromatography.
Cyclosporin A and LY 294002 were from Sigma Chemical Co. (St. Louis, MO); cytochalasin D, Wortmannin, SB 203580, U0126, GF 109203X (GÖ 6850, bisindolylmaleimide) and RO-31–8220 were from Calbiochem (San Diego, CA). The JNK-specific inhibitor L-JNKI1 was obtained from Alexis Biochemicals (San Diego, CA).
4.3 Cell culture and activation
For activation, the antibodies were adsorbed to polystyrene beads (5-μm diameter, Polysciences) by overnight incubation at 4°C in PBS (2×107–4×107 beads/ml plus1 μg/ml anti-CD3 plus 5 μg anti-H4/ICOS or the filling antibodies M1/70 and normal Armenian Ig, respectively). Cells (108 cells/ml) were resuspended in culture medium and mixed 1:1with antibody-coated polystyrene beads. After 5 min at 37°C, 1 ml of ice-cold PBS, 500 μM EDTA, 200 μM sodium orthovanadate was added, and the cells were spun for 2 min at 1,000×g before lysis. Where indicated, cells (107/ml in culture medium) were incubated with metabolic inhibitors for 30 min at 37°C, washed, and then activated as described. For lymphokine determinations, 3–5 μl of cells plus beads were taken after the 5 min activation and incubated for further 5 h at 37°C in 0.2 ml of complete culture medium supplemented with metabolic inhibitors were appropriate.
For the experiments described in Fig. 1B, SR.D10 cells (2×107/point) were incubated for 30 min with anti-H4/ICOS or control antibody (10 μg/ml, 2 ml) inice-cold PBS plus 5% FCS. To detect surface H4/ICOS, each sample included 5×106 cells that had previously been biotinylated by incubation (15 min in the cold) with freshly prepared sulfo-NHS biotin (Pierce) in phosphate-buffered saline, pH 8.0 (50 μg/ml biotin, 107 cells/ml) and washed. The cells were washed and resuspended in culture medium and kept in the cold. One sample was activated for 3 min at 37°C with freshly prepared 0.5 mM pervanadate. Then, all the samples received rabbit anti-mouse Ig, cross-reactive with hamster Ig (50 μg/ml). After further 5 min, the activation was stopped by adding ice-cold PBS, 500 μM EDTA, 200 μM sodium orthovanadate, and lysed as described above.
4.4 T cell blasts
CD4+ T cells were isolated from the spleens of CD28–/– (Jackson, Bar Harbor, ME) or congenic C57BL/6 mice by immunoglobulin-anti-immunoglobulin columns as described 59, 64. These cells were activated 106 cells/ml in culture medium with 2.5 μg/ml concanavalin A, plus 10 U/ml IL-2 and T cell-depleted, mitomycin C-treated spleen cells as feeders (5×105/ml). After 48 h, the cells were washed and separated in a discontinuous Percoll gradient. Blasts from the 0–50% Percoll interface were taken, washed thoroughly, and expanded for further 48 h at 0.4×106 cells/ml in culture medium plus 20 U/ml IL-2. The resulting cells were ≥98% CD3+ CD4+ cell blasts, as determined by flow cytometry, and used in activation experiments with antibody-coated polystyrene beads as described above.
4.5 Flow cytometry
Cells were stained by incubation with antibody at 107 cells/ml in staining buffer (PBS, 0.1% NaN3, 2% heat-inactivated fetal bovine serum). After 30 min at 4°C, the cells were washed and incubated for further 30 min in the cold with FITC-coupled anti-hamster IgG antibodies in staining buffer. The cells were then washed four times with staining buffer and analyzed on an EPICS-XL flow cytometer (Coulter Electronics).
4.6 Cell lysis and immunoprecipitation
Cells were lysed on ice for 30 min at 2×107 cells/ml with 50 mM Tris/HCl, pH 7.6, 10 mM 3-[(3-cholamido propyl)-dimethyl ammonium]-1-propane sulfonate (CHAPS), 150 mM NaCl, 1 mM MgCl2, 1 mM EGTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF, and 1 mM NaVO4. Post nuclear lysates were used for immunoblot, or immunoprecipitated as described 59, 64. For immunoprecipitation of cell surface H4/ICOS, cell lysis and immunoprecipitation was performed as described above, except that cells were lysed at 107 cells/ml, and that immunoprecipitation was carried out using Sepharose-conjugated protein A.
4.7 Immunoblot
For immunoblot of cell lysates, 10 μl (equivalent to 2×105 cells) were mixed V:V with 2× reducing SDS Laemmli sample buffer. Immunoprecipitates were extracted with 1± reducing sample buffer. The samples were boiled for 2 min and separated by SDS-PAGE in 10% acrylamide gels. Proteins were transferred to PVDF membranes (Immobilon-P, Millipore), and blocked for 2 h with 1% BSA in TBST containing 200 μM sodium orthovanadate. The membranes were washed with TBST, incubated overnight in the cold with anti-active MAP kinase antibodies, horseradish peroxidase(HRP)-coupled anti-phosphotyrosine antibodies, or HRP-streptavidin in 1% BSA in TBST, and washed with TBST. Then, the blot was visualized using the Supersignal West Pico chemiluminiscence substrate(Pierce) for phosphotyrosine or biotin determination. For detection of anti-active MAP kinase antibodies, the membranes were incubated for further 2 h at room temperature with HRP-conjugated anti-rabbit IgG in 1% non-fat dry milk in TBST, washed, and developed with chemoluminiscence substrate as above. The membranes were washed, stripped and re-probed with antibodies specific for MAP kinases, ζ, ZAP-70, or Vav. Anti-PI-3K detection in immunoprecipitates or cell lysates was performed as described above, except that membrane blocking and incubation with antibodies were performed in 1% non-fat dry milk in TBST, and HRP-conjugated anti-mouse Ig was used as second antibody.
4.8 Cytokine detection by ELISA
IL-4 and IL-10 were detected in culture supernatants by specific capture ELISA. 11B11 was used for capture and biotinylated BVD6–24G2 (PharMingen) was used for detection of IL-4. JES5–2A5 and biotinylated JES5–16E3 (PharMingen) were used as capture and detection antibodies, respectively, in IL-10 assays. The assays were performed according to the instructions of the supplier and developed using streptavidin-HRP (Amersham) and Sigma-fast o-phenylenediamine substrate (Sigma). Cytokine content was calculated from reference standard curves set up with recombinant cytokines.
Acknowledgements
The authors thank Charles A. Janeway, Jr., for generously providing many cell lines and antibodies used in this study, Dr. Xose Bustelo for anti-Vav antiserum, and Maria Luisa del Pozo for her skillful technical assistance. This work was supported by Fellowships from "Fondo de Investigación Sanitaria" (A.S., and G.C.), I.S. Carlos III (A. J.-P), Ministerio de Educación y Cultura (G.C.), and Comunidad Autónoma de Madrid, (A.S. and M.J.F.), and grants FIS 98/0037-CO2–01 and FIS 98/0037-CO2–02 from "Fondo de Investigación Sanitaria" and ISCIII-01/30 (Ministerio de Sanidad y Consumo, Spain), grant BMC2001–2177 from Ministerio de Ciencia y Tecnología, Spain, and grants from Associazzione Italiana Ricerca Cancro (AIRC, Milan, Italy).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




