Experimental African trypanosomiasis: IFN-γ mediates early mortality
Abstract
In this study, we demonstrate that Kupffer cells in the livers of highly susceptible BALB/c mice infected with Trypanosoma congolense were loaded with trypanosomal antigen and appeared highly activated. This was associated with an enlarged capillary bed in the livers and decreased blood pressure of these mice towards the terminal stage. Blocking of murine IL-10 receptor (IL-10R)in vivo shortened the survival time of highly susceptible T. congolense-infected BALB/c mice. Anti-IL-10R treatment decreased the survival of relatively resistant T. congolense-infected C57BL/6 mice dramatically. Blocking of the IL-10R also significantly shortened the survival time of mice infected with T. brucei. The acute death of trypanosome-infected mice treated with anti-IL-10R antibodies in vivo was associated with focal liver necrosis, with significantly increased plasma levels of IL-6, IL-10, IL-12p40 and IFN-γ and enhanced synthesis of IL-6, IL-12p40 and IFN-γ by spleen cell cultures. Anti-IL-10R-induced death of T. congolense-infected C57BL/6 mice could be prevented by administration of a neutralizing antibody specific forIFN-γ. We conclude that phagocytosis of a critical number of trypanosomes by Kupffer cells leads to a systemic inflammatory response syndrome and, depending on the degree of Kupffer cell activation, is followed by death that is mediated by IFN-γ. The role of trypanosome-pulsed macrophages, T cells and genetic influences is discussed in a synopsis.
1 Introduction
African trypanosomes are extracellular hemoprotozoa which infect humans and livestock. Trypanosoma brucei gambiense and T. b. rhodesiense cause sleeping sickness anddeath in humans. T. congolense is the most important pathogen for livestock. Mice are highly susceptible to infections with African trypanosomes. Different strains of mice, however, display different degrees of susceptibility to the disease 1, 2. BALB/c mice are highly susceptible to T. brucei and T. congolense infections, while C57BL/6 mice are relatively resistant, as measured by the levels of parasitemia, immunosuppression and survival time 2–4.
High levels of IFN-γ have been associated with enhanced susceptibility to T. brucei and T. congolense infections, mediating immunosuppression 5, 6. Paradoxically, it also has been shown, by infection of IFN-γ-deficient C57BL/6 mice, that IFN-γ was required for resistance to T. brucei 7, 8. Recently, IL-10-deficient relatively resistant C57BL/6 mice, infected with T. brucei have been shown to have significantly shorter survival than infected wild-type mice, indicating that IL-10 might be crucial for enhanced survival of mice infected with T. brucei 8. Using highly susceptible BALB/c and relatively resistant C57BL/6 mice as a model,we have investigated the role of cytokines in immunosuppression and pathogenesis of T. congolense infections in BALB/c and C57BL/6 mice. Higher levels of IL-4, IL-10 and IFN-γ were detected in the plasma and supernatant fluids of spleen cell cultures of highly susceptible BALB/c mice infected with T. congolense as compared to those found in infected relatively resistant C57BL/6 mice 2, 6, 9. Furthermore, treatment of T. congolense-infected BALB/c mice with anti-IFN-γ mAb dramatically increased the survival time 6. In vitro, anti-IL-10 antibodies markedly decreased the immunosuppressive effect of spleen cells of T. congolense-infected BALB/c mice 9.Administration of monoclonal anti-IL-10 antibodies (of IgM class) to T. congolense-infected BALB/c mice moderately prolonged their survival time 9.
IL-10 is a pleiotropic cytokine 10. Among other functions, IL-10 plays a major role in inhibiting the production of monokines such as IL-1, IL-6, IL-12, TNF-α and in down-regulating IFN-γ synthesis by NK cells and T cells 10. The IL-10 receptor (IL-10R) is a member of the class II interferon receptor-like cytokine receptor family 11. An mAb blocking murine IL-10R was recently generated 12.
Here we report a detrimental effect of blocking the IL-10R in mice infected with African trypanosomes. To our surprise and seemingly in conflict with our previously results obtained with the use of anti-IL-10 antibodies 9, blocking the IL-10R significantly shortened the survival time of BALB/c and C57BL/6 mice infected with T. congolense as well as T. brucei-infected BALB/c and outbred CD1 mice. The acute death of T. congolense-infected mice treated with anti-IL-10R in vivo was associated with focal liver necrosis, elevated levels of IL-6, IL-10, IL-12p40 and IFN-γ in the plasma, and enhanced synthesis of IL-6, IL-12p40 and IFN-γ by spleen cell cultures. Administration of anti-IFN-γ antibody prevented the acute death induced by blocking the IL-10R. Thus, we showed, for the first time, that blocking of the IL-10R in trypanosome-infected mice results in a fatal outcome. More importantly, this is the first direct evidence that IFN-γ mediated the early mortality of infected mice when the functioning of IL-10 was prevented. We have shown that overproduction of IFN-γ killed the host infected with African trypanosomes and that overproduction of IFN-γ was held in check by IL-10 in the resistant, but not the susceptible host. We have demonstrated, for the first time, that abundant parasite antigen was located in the cytoplasm of Kupffer cells in the liver of infected BALB/c mice. Its presence was associated with an apparent state of macrophage activation. In the discussion, we present a synopsis indicating a proposed role of trypanosome-pulsed macrophages, cytokines and trypanosome-specific T cells in the fatal outcome.
2 Results
2.1 Kupffer cells of mice infected with T. congolense were loaded with parasite antigen and were greatly enlarged
Immunohistochemical staining showed that, on day 6 post-infection, parasite antigen was located in the cytoplasm of large elongated mononuclear cells in livers of infected BALB/c mice treated with PBS (Fig. 1B, 1H) or anti-IL-10R (data not shown). Parasite antigen was also observed in the cytoplasm of elongated mononuclear cells in the livers of infected C57BL/6 mice treated with PBS or anti-IL-10R (data not shown). By immunofluorescent staining, the parasite antigen-containing cells were shown to carry the macrophage marker F4/80 (Fig. 1J) and thus were identified to be macrophages of the liver (Kupffer cells). In contrast, there was no positive staining for T. congolense antigen in livers of uninfected BALB/c mice (Fig. 1A, E, G). Immunohistochemical staining also revealed that the macrophages in the livers of infected BALB/c mice were greatly enlarged, in some areas almost clogging the capillaries (Fig. 1B, D). In contrast, there were less macrophages in the livers of uninfected normal mice and the macrophages in the livers of normal mice were much smaller compared to those of infected mice (Fig. 1C, F).
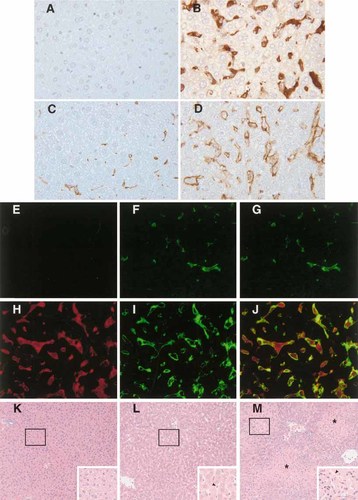
Liver sections of three groups of BALB/c mice: uninfected (A, C, E, F, G, K); T. congolense-infected on day 6, treated with phosphate-buffered saline (PBS) (B, D, H, I, J, L); T. congolense-infected on day 6, treated with anti-IL-10R (see text) (M). Liver sections were stained: for trypanosomal antigen (A, B) or macrophage marker F4/80 (C, D) by immunoperoxidase technique (A–D); for trypanosomal antigen (E, H) or macrophage marker F4/80 (F, I) or both (G, J) by immunofluorescence technique (E–J); with hematoxylin & eosin (K–M). (A, B) No trypanosomal antigen in uninfected (A), but present in infected (B) mice. (C, D) F4/80+ Kupffer cells are more abundant and larger in infected (D) than in uninfected (C) mice. (E, H) No trypanosomal antigen (red) in uninfected (E), but present in infected (H) mice. (F, I) Less and smaller F4/80+ (green) Kupffer cells in uninfected (F) than in infected (I) mice. (G, J) Most of trypanosomal antigen (red) is located within F4/80+ (green) Kupffer cells of infected liver sections (J). (K–M) Uninfected (K), infected and treated with PBS (L), infected and treated with anti-IL-10R (M) mice. Infected mice showed enlarged capillaries (arrows, L, M) and sections from infected mice treated with anti-IL-10R showed foci of necrosis (*, M). Original magnification for (A–J) ×400, for (K–M) ×100, for insets (K–M) ×200.
2.2 Acute death of T. congolense-infected BALB/c mice was linked to a significant decrease of blood pressure
As described below, enlarged capillaries were observed in the liver sections of T. congolense-infected BALB/c mice. Based on this observation, we speculated that the blood pressure of infected mice during the acute death might decrease. Thus, we measured the blood pressure of uninfected and T. congolense-infected BALB/c mice. The blood pressure of infected BALB/c mice decreased significantly on days 7, 8 and 8.5. Thus the blood pressure started to decline about 1 to 2 days prior to the death of the mice (Fig. 2). Concomitantly with development of hypotension, the infected mice showed a drop of body temperature (not shown), hypomotility and piloerection, clinical signs also observed in bacterial endotoxic shock 13.
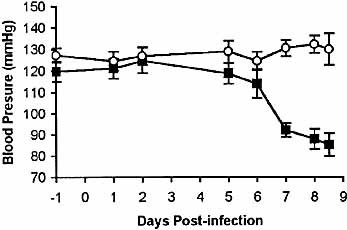
Blood pressure of T. congolense-infected mice decreased significantly prior to death. Groups of six BALB/c mice (▪) were infected with 104 T. congolense. Six normal, uninfected BALB/c mice (○) were used as controls. Blood pressure was measured as described in Sect. 4. Data are presented as means ± SE. The results presented are representative of two experiments.
2.3 Administration of anti-IL-10R shortened the survival time of highly susceptible T. congolense-infected BALB/c mice
The infected BALB/c mice received either 200 μg purified anti-IL-10R mAb or 200 μg rat IgG in PBS on days 3 and 5 post-infection. Statistical analysis showed that the survival time of the T. congolense-infected mice treated with anti-IL-10R was significantly (Fig. 3A; p<0.05) shorter than that of infected control BALB/c mice treated with rat IgG, i.e. 7.3±0.2 days versus 8.5±0.3 days. Uninfected mice given anti-IL-10R did not exhibit any detrimental effects.
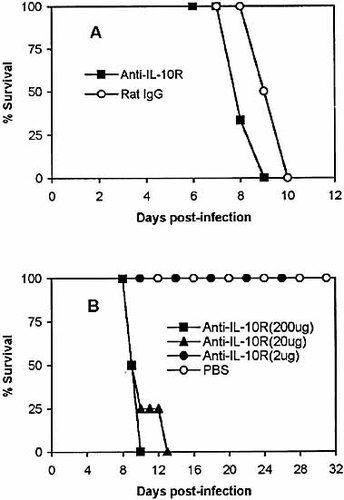
Administration of anti-IL-10R significantly shortens the survival time of highly susceptible BALB/c and relatively resistant C57BL/6 mice infected with T. congolense. Groups of six BALB/c mice (A) were injected i. p. with either 200 μg of anti-IL-10R in 200 μl PBS or 200 μg of rat IgG in 200 μl of PBS on days 3 and 5 post-infection. Groups of four to six C57BL/6 mice (B) were injected i.p. with either 200 μg, 20 μg, or 2 μg of anti-IL-10R in 200 μl of PBS or 200 μl of PBS alone on days 3 and 5 post-infection. Mice were monitored for 31 days. The results presented are representative of three experiments.
2.4 Administration of anti-IL-10R dramatically shortened the survival time of relatively resistant T. congolense-infected C57BL/6 mice
As shown above, anti-IL-10R treatment significantly, but not markedly, shortened the survival time of highly susceptible BALB/c mice. Previous data showed that relatively resistant C57BL/6 mice survived 163±12 days after infection with T. congolense VAT TC13 4. We next infected C57BL/6 mice with 103 organism of T. congolense VAT TC13 and treated the mice either with 200 μg, 20 μg, 2 μg of anti-IL-10R in PBS or PBS alone on days 3 and 5 post-infection. As shown in Fig. 3B, treatment of infected C57BL/6 mice with 200 μg or 20 μg caused significant reduction of the survival time compared to the control infected mice (p<0.001). Infected C57BL/6 mice treated with 2 μg of anti-IL-10R did not die earlier than infected control mice injected with PBS. They were killed on day 31 post-infection (Fig. 3B). The infected control mice were also killed on day 31 post-infection.
2.5 Administration of anti-IL-10R shortened the survival time of T. brucei-infected mice
We next infected BALB/c and outbred CD1 mice with 103 organisms of T. brucei, strain 10–26, and then treated the mice with 100 μg of anti-IL-10R or equal amounts of rat IgG (as control) on days 3 and 5 post-infection. The survival time of both BALB/c and CD1 mice infected with T. brucei was significantly shortened by anti-IL-10R treatment (Fig. 4).
Administration of anti-IL-10R significantly shortens the survival time of inbred BALB/c (p<0.001) and outbred CD1 (p<0.001) mice infected with T. brucei strain 10–26. Groups of four inbred BALB/c mice (A) and outbred CD1 mice (B) were injected i.p. with either 100 μg of anti-IL-10R in 200 μl PBS or 100 μg rat IgG in 200 μl PBS on days 3 and 5 post-infection.
2.6 Effect of anti-IL-10R treatment on parasitemia
The difference of parasitemia between infected mice treated with anti-IL-10R in PBS and infected mice treated with PBS alone was not statistically significant in both BALB/c mice and C57BL/6 mice (data not shown).
2.7 Acute death of T. congolense-infected mice treated with anti-IL-10R was associated with focal liver necrosis
Generally, the capillaries of the livers of T. congolense-infected BALB/c mice (Fig. 1L, 1 M) were much wider than the liver capillaries of uninfected mice (Fig. 1K). On day 6 post-infection, focal necrosis was observed microscopically in almost the whole area of liver sections of infected BALB/c mice treated with anti-IL-10R in vivo (Fig. 1M). No necrosis was seen in close vicinity of either portal triads or central veins, coinciding with lack of trypanosome-laden Kupffer cells. No necrosis was observed in livers of normal uninfected BALB/c mice (Fig. 1K) as well as infected mice treated with PBS on day 6 post-infection (Fig. 1L). On day 8 post-infection, enlarged capillaries and liver necrosis were also observed in infected C57BL/6 mice treated with anti-IL-10R but not the infected C57BL/6 mice treated with PBS (data not shown).
2.8 Acute death of T. congolense-infected mice treated with anti-IL-10R was associated with enhanced cytokine synthesis in vivo
We next asked the question how the anti-IL-10R treatment might influence the balance of cytokine levels in T. congolense-infected mice. Thus, we measured the plasma levels of TNF-α, IL-6, IL-10, IL-12p40 and IFN-γ in infected BALB/c (on day 7 post-infection) and C57BL/ 6 mice (on day 8 post-infection) treated with anti-IL-10R on days 3 and 5 post-infection. There were no significant differences between TNF-α plasma concentrations of anti-IL-10R-treated and PBS-treated BALB/c mice (60.0±31.8 pg/ml versus 64.1±37.5 pg/ml for mice treated with anti-IL-10R and PBS, respectively). Anti-IL-10R treatment of T. congolense-infected BALB/c and C57BL/6 mice significantly enhanced the plasma levels of IL-6, IL-10, IL-12p40 and IFN-γ by 3- to 12-fold compared to T. congolense-infected mice treated with PBS (Fig. 5).
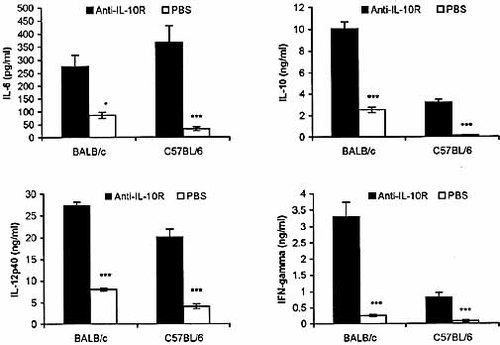
Administration of anti-IL-10R Ab significantly enhanced the plasma levels of IL-6, IL-10, IL-12p40 and IFN-γ in T. congolense-infected BALB/c and C57BL/6 mice. Groups of six infected BALB/c mice and C57BL/6 mice were injected i.p. with either 200 μg of anti-IL-10R in 200 μl PBS or 200 μl PBS alone on days 3 and 5 post-infection. Plasma samples were collected on days 7 and 8 post-infection from BALB/c and C57BL/6 mice, respectively. Cytokines were measured by ELISA as described in Sect. 4. Data are presented as means ± SE. The results presented are representative of two experiments. *p<0.05; ***p<0.001.
2.9 Acute death of T. congolense-infected BALB/c and C57BL/6 mice treated with anti-IL-10R in vivo was associated with increased cytokine synthesis by spleen cells cultured in vitro
Splenocytes were collected from T. congolense-infected BALB/c (day 7 post-infection) and C57BL/6 (day 8 post-infection) mice treated with anti-IL-10R or PBS, and cultured in vitro for 48 h. The levels of IL-6, IL-10, IL-12p40 and IFN-γ in the culture supernatant fluids were determined using a sandwich ELISA. The production of IL-6, IL12p40 and IFN-γ by splenocytes cultured in vitro were significantly elevated by 3- to 35-fold for infected BALB/ c and C57BL/6 mice following treatment with anti-IL-10R mAb (Fig. 6). IL-10 levels were not significantly enhanced (data not shown).
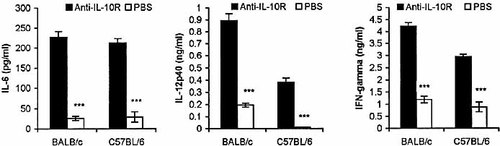
Administration of anti-IL-10R in vivo to T. congolense-infected mice enhances the synthesis of IL-6, IL-12 and IFN-γ by spleen cells cultured in vitro. Groups of six BALB/c mice and C57BL/6 mice were injected i.p. with either 200 μg of anti-IL-10R in 200 μl PBS or 200 μl PBS alone on days 3 and 5 post-infection. Spleen cells collected on day 7 post-infection from BALB/c mice and on day 8 post-infection from C57BL/6 mice were cultured at concentration of 5×106 cells/ml in 96-well-plates (200 μl/ well) for 48 h, and the supernatant fluids were assayed for IL-6, IL-12p40 and IFN-γ, as described in Sect. 4. Data are presented as means ± SE. The results presented are representative of two experiments. ***p<0.001.
2.10 Acute death of T. congolense-infected C57BL/6 mice treated with anti-IL-10R in vivo was prevented by administration of anti-IFN-γ antibody
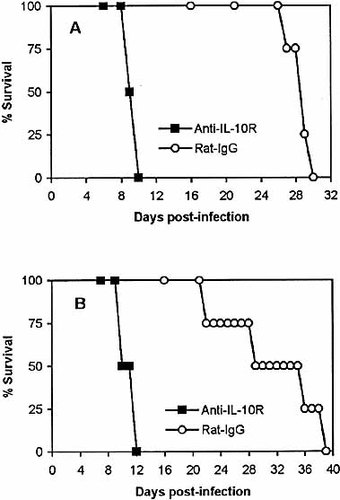
We next tried to elucidate the mechanism(s) that may be involved in the early death of infected mice treated with anti-IL-10R. We did find that the acute death of infected mice treated with anti-IL-10R was associated with elevated production of IL-6, IL-10, IL-12p40 and IFN-γ. Of these cytokines, IL-10 could not exert any function since the IL-10R was blocked. We have previously shown that treatment of T. congolense-infected BALB/c mice with anti-IFN-γ could increase the survival of those mice 6. Therefore, we speculated that the excessively high levels of IFN-γ in the plasma, after blocking of the IL-10R, might be a major factor mediating the acute death of infected mice treated with anti-IL-10R. Accordingly, we administered anti-IFN-γ mAb to T. congolense-infected C57BL/6 mice whose IL-10R were blocked by anti-IL-10R. As control, we also treated infected C57BL/6 mice with anti-IL-10R only or with equivalent amounts of rat IgG. The infected C57BL/6 mice treated with anti-IL-10R alone succumbed to the infection between day 9 and day 13 post-infection, with an average survival time of 11.8±0.95 days (Fig. 7). In contrast, all of the infected mice treated with both anti-IL-10R and anti-IFN-γ mAb, as well as the infected mice treated with rat IgG alone survived (Fig. 7). These mice were killed on day 39 post-infection. Thus, the condition leading to acute death of T. congolense-infected C57BL/6 mice treated with anti-IL-10R in vivo could be reversed by administration of anti-IFN-γ mAb.
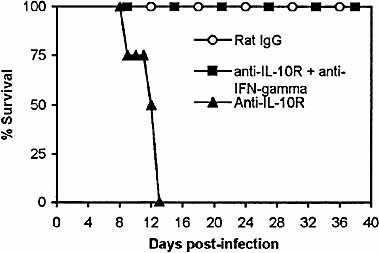
Acute death of T. congolense-infected C57BL/6 mice treated with anti-IL-10R in vivo could be prevented by administration of anti-IFN-γ antibody. Groups of four to five C57BL/6 mice were infected with 103 T. congolense. The infected mice were treated with anti-IL-10R (▴), anti-IL-10R and anti-IFN-γ antibodies (▪) or rat IgG (○) as described in Sect. 4. The mice were monitored daily for survival until day 39 post-infection. The results presented are representative of two experiments.
3 Discussion
How can we put the above data into perspective? We have tried to demonstrate a synopsis by two diagrams shown in Fig. 8. We believe that these diagrams, although simplistic and incomplete, represent a view that is consistent with our present knowledge.
The events of steps 1–2 (Fig. 8 A, B) are well documented: trypanosomes opsonized by antibody to variant surface glycoprotein (VSG) are phagocytosed by macrophages. Antibodies to the VSG do mediate phagocytosis by macrophages in vitro 14. Anti-VSG antibody of IgM class is as efficient as antibody of IgG2a isotype in causing phagocytosis of T. congolense in vitro 15. In mice infected with T. brucei, 60–80 % of the opsonized T. brucei end up in the liver and less, but a considerable number, in the spleen 16, 17. We have shown here that Kupffer cells of T. congolense-infected BALB/c mice are loaded with trypanosomal antigen (Fig. 1). Thus, we provided direct evidence in vivo that macrophages engulf trypanosomes in the liver of infected mice.
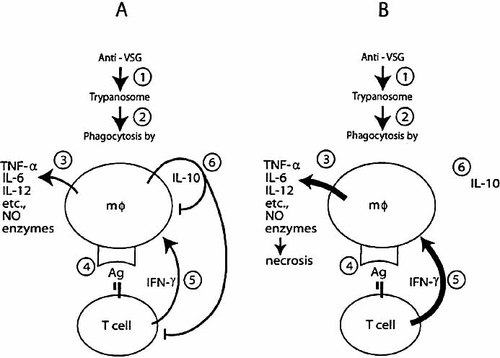
T. congolense infections: a simplistic view of the cascade of events. (A) Regular infection leading to shock-like syndrome and early death in susceptible mice. (B) Infection and subsequent treatment with anti-IL-10 receptor leading to shock-like syndrome and earlier mortality in susceptible and resistant mice. (See Sect. 3 for further explanations).
There is good evidence for step 3 in vivo and in vitro: macrophages that have engulfed trypanosomes are highly activated. Macrophages isolated from T. brucei-infected mice during the first wave of parasitemia have an increased ability to secrete plasminogen activator, anion superoxide, H2O2 18, nitric oxide (NO) 19, and IL-10 20. Even stimulation of macrophages with homogenates of T. brucei or trypanosomal glycosylphosphatidylinositol (GPI) induces synthesis of cachectin/TNF-α and IL-1 21–23. Although not shown to be derived from macrophages, T. congolense-infected mice have elevated plasma levels of TNF-α, IL-6, IL-10 and IL-12p40 (Fig. 5 and 6, 9) at a time when Kupffer cells are pulsed with trypanosomal antigen and when these cells are in much greater numbers and their volumes much more enlarged than Kupffer cells in livers of uninfected mice (Fig. 1). Macrophages that have engulfed T. congolense or have been stimulated with T. congolense homogenates in vitro are induced to secrete TNF-α, IL-6, IL-10, IL-12 and NO 15, 24, 25.
Although we presently do not know whether there is any significant interaction between T cells and Kupffer cells in the liver, we have provided evidence for steps 4 and 5 to occur in the spleen: the synergistic action of trypanosome-specific T cells and trypanosome-pulsed macrophages induce IFN-γ production in T. congolense-infected BALB/c mice. We have shown that an unusual population of matrix-adherent T cells (adherent to plastic, nylon wool and Sephadex G-10) in synergy with trypanosome-pulsed macrophages produced a great amount of IFN-γ. The T cells were trypanosome-specific and have not been found in normal mice 26. We have extended this study by direct immunocytochemistry with double labeling. These IFN-γ-producing T cells are present at very low prevalence (<1% of total spleen cells) and are TCRγ δ– and NK 1.1–. They are Thy 1.2+, CD3+, TCRβ+, about 75% CD4+, 21% CD4–8– and 4% CD8+ (Shi et al, unpublished). We also have in vitro evidence that cytokine production by trypanosome-pulsed macrophages is enhanced by addition of IFN-γ 24, 25. In context of steps 4 and 5, the IFN-γ proposed to increase the activation of trypanosome-pulsed Kupffer cells might be predominantly produced in the spleen (Fig. 6) and disseminated via the circulation (Fig. 5). Because of present lack of knowledge, the potential production of IFN-γ by trypanosome-specific T cells in the liver cannot be excluded.
There is also evidence for step 6: trypanosome-pulsed macrophages produce IL-10. T. congolense-pulsed macrophages produce IL-10 24, 25 and macrophages isolated from T. brucei-infected mice have been shown to produce IL-10 20.
We believe that the scenario shown in Fig. 8A is sufficient for leading to the death of highly susceptible BALB/c mice due to a systemic inflammatory response syndrome (SIRS) 27. Anti-IL-10R treatment simply accelerates the process. The similarity of SIRS produced by bacterial endotoxin 13 to the acute death of T. congolense-infected BALB/c mice is shown by the involvement of macrophages (Fig. 1), cytokine release syndrome (Fig. 5), enlarged capillary bed (Fig. 1), decrease of blood pressure (Fig. 2), and drop of body temperature (not shown), hypomotility and piloerection at the terminal stage. In the scenario of Fig. 8B, blocking of the IL-10R leads to lack of any function of IL-10. Thus, anti-IL-10R treatment of infected C57BL/6 mice results in enhanced synthesis of IFN-γ by trypanosome-specific T cells (Fig. 6). It also results in an absence of a down-regulatory effect of IL-10 on the trypanosome-pulsed macrophages, leading to a tremendous enlargement of Kupffer cells and an up-regulation of synthesis of inflammatory cytokines, such as IL-6 and IL-12p40 (Fig. 5, 6). Altogether, the outcome is an early death of the otherwise relatively resistant C57BL/6 mice (Fig. 3).
Although we believe our view is consistent with the presently known facts, we are very aware that the painted picture is incomplete. How do the high levels of IL-10 observed in infected BALB/cmice (Fig. 5) fit into the synopsis discussed above? This observation could be interpreted to mean that our synopsis is incorrect. Our present answer, however, is this. Anti-IL-10R treatment very profoundly elevates IFN-γ levels in T. congolense-infected BALB/c mice (Fig. 5, 6), indicating that IL-10 does have a down-regulatory effect on IFN-γ synthesis of highly susceptible BALB/c mice. However, IL-10 appears to be unable to sufficiently down-regulate IFN-γ in infected BALB/c mice even without anti-IL-10R treatment (Fig. 5, 6, 9). We think there are genetic factors, which are still unknown, that make this to happen. In genetic analyses of T. congolense infections of BALB/c versus C57BL/6 mice, five different quantitative trait loci that control resistance/susceptibility have been identified 28, 29. The genes in these loci are still unknown. We suggest that the function(s) of one or more than one of these still unknown gene products in T. congolense-infected BALB/c mice is exerting an IFN-γ-inducing effect that is overriding the down-regulatory function of IL-10. Thus, in T. congolense-infected BALB/c mice, IL-10 is down-regulating the production of IFN-γ (Fig. 5), albeit insufficiently. Thus, a critical level of IFN-γ in trypanosome-infected mice appears to be lethal.
What is the nature of the interaction of T cells and trypanosome-pulsed macrophages that results in the crucial IFN-γ production? Considering the observed cytokine pattern discussed above,we think this interaction might not be MHC-restricted. Although we have no direct evidence, we speculate that trypanosomal GPI, the membrane anchor of the VSG, might be the major antigen involved. If this is correct, a CD1-dependent antigen presentation is conceivable 30, 31.
We suggest that early mortality of mice infected with T. congolense is due to SIRS 27 in which IFN-γ produced by trypanosome-specific T cells (26, Fig. 6) is a major mediator, similar to mechanisms observed in bacterial endotoxemia 32. Although most, but not all, of our studies have been carried out with T. congolense infections, we suggest that similar mechanisms operate in mice infected with T. brucei (Fig. 4).
In summary, we have presented a coherent, although still incomplete, hypothesis to explain the early death of mice infected with African trypanosomes.
4 Materials and methods
4.1 Mice
Female, 8–10-week-old BALB/c AnNCrlBR (BALB/c) mice and 5–6-week-old female outbred Swiss white mice (CD1) were purchased from the Animal Resource Center of the University of Saskatchewan (Saskatoon, Canada). The 8–10-week-old female C57BL/6NCrlBR (C57BL/6) mice were obtained from Charles River Laboratories (St. Constant, Quebec). The mice were kept in polycarbonate cages on sawdust, andallowed free access to food and water throughout the experiments, according to the recommendations of the Canadian Council of Animal Care.
4.2 Parasites
T. congolense, Trans Mara strain, variant antigenic type (VAT) TC13 was used in this study. The origin of this parasite strain has been previously described 33. T. brucei, strain 10–26, was obtained from Dr. Terry Pearson, University of Victoria, Victoria, Canada. Frozen stabilates of parasites were used for infecting CD1 mice immunosuppressed with cyclophosphamide, and passages were made every third day as described previously 33. The parasites purified from the blood of infected CD1 mice by DEAE-cellulose chromatography 34 were used for infecting of BALB/c, C57BL/6 and CD1 mice.
4.3 Antibodies
The rat hybridoma 1B1.3a (blocking mouse IL-10R), XMG 1.2 (neutralizing mouse IFN-γ) and MCAP497 (specific for mouse macrophage antigen F4/80) were purchased from the American Type CultureCollection (ATCC, Manassas, VA). We produced a polyclonal antiserum against T. congolense: two rabbits were infected intravenously with 106 T. congolense, and cured by intravenous injections of Berenil (7 mg/kg) on days 7 and 8. At 4.5 months later, the rabbits were subcutaneously injected, at four sites, with 0.1 ml of an emulsion containing 2×108 T. congolense, 25 mg Berenil and 0.3 ml of complete Freund's adjuvant. At 2 weeks later, they were subcutaneously injected, at four sites, with 0.1 ml of an emulsion containing 2×108 T. congolense, 25 mg Berenil and 0.3 ml of incomplete Freund's adjuvant. Blood samples were collected from the rabbits 10 days after the last injection. The quality of the antiserum was checked by Western blot using T. congolense homogenate.
4.4 Infections and treatment of mice with anti-IL-10R mAb or anti-IFN-γ mAb
Groups of four to six BALB/c or C57BL/6 mice were infected i. p. with 103 organisms of T. congolense VAT TC13. These mice were injected i. p. on days 3 and 5 post-infection either with 200 μg, 20 μg or 2 μg of purified anti-IL-10R mAb in 200 μl of PBS or as controls, equal amounts of rat IgG in 200 μl PBS or PBS alone. Groups of four BALB/c or CD1 mice were infected i. p. with 103 organisms of T. brucei. These mice were injected i. p. on days 3 and 5 post-infection with either 100 μg of purified anti-IL-10R mAb or 100 μg of rat IgG (as control). To test the potential effect of concomitant treatment with anti-IL-10R and anti-IFN-γ, T. congolense-infected C57BL/6 mice were injected i. p. on days 3 and5 post-infection with either 50 μg of purified anti-IL-10R mAb or equal amount of rat IgG as control. The infected C57BL/6 mice treated with anti-IL-10R mAb were injected with either 200 μgpurified anti-IFN-γ mAb or 200 μg rat IgG on days 4 and 6. The infected C57BL/6 mice treated with rat IgG (control) on days 3 and 5 were injected with additional 200 μg rat IgG on days 4 and 6. For estimation of parasitemia and survival time, all infected mice were monitored daily for parasitemia and mortality. For determination of the effect of in vivo injections of anti-IL-10R mAb, cytokines were measured in the plasma and supernatants of spleen cell cultures. For this purpose, anti-IL-10R-treated BALB/c mice and PBS-treated BALB/c mice were euthanized with CO2 on day 7 post-infection and, C57BL/6 mice on day 8. The plasma of the mice were collected after centrifuging the blood at 1,000×g for 30 min, and stored at –35°C until used.
4.5 Estimation of parasitemia and survival time
A drop of blood was taken from the tail vein of each infected mouse. The parasitemia was estimated by counting the number of parasites present in at least 10 fields at ×400 magnification by phase-contrast microscopy. The survival time was defined as the number of days post-infection that the infected mice remained alive.
4.6 Histopathology and immunohistochemistry
Liver tissues were taken from T. congolense-infected mice treated with anti-IL-10R or PBS on day 6 post-infection as well as from uninfected mice. Tissues were fixed in 10% neutral buffered formalin for 24 h at 4°C, embedded in paraffin and cut into 5-μm sections. Sections were stained with hematoxylin and eosin for histological examination. Immunohistochemical staining for parasite antigen was performed according to Sudarto et al. 35.
4.7 Blood pressure measurement
Group of six BALB/c mice were infected with 104 organisms of T. congolense. Six normal, uninfected BALB/c mice were used as controls. All mice were trained four times to get them accustomed to the measurement environment before the measurements began. The measurements of blood pressure were performed on days –1, 1, 2, 5, 6, 7, 8 and 8.5 post-infection by using the BP-2000, Blood Pressure Analysis System (Visitech Systems, Apex, NC).
4.8 Splenocyte cultures for measurement of cytokine synthesis
Splenocytes from T. congolense-infected BALB/c and C57BL/6 mice treated with either anti-IL-10R in PBS or PBS alone in vivo were cultured at 5×106 cells/ml (200 μl/well) in 96-well tissue culture plates (Falcon, Becton Dickinson and Company, Franklin Lakes, NJ) in a humidified incubator containing 5% CO2 in the air. The culture supernatant fluids were collected after 48 h and centrifuged at 1,500×g for 10 min, and the supernatant fluids were stored for cytokine assays at –35°C until used.
4.9 Cytokine assays
Recombinant murine cytokines (IL-6, IL-10, IL-12p40, TNF-α and IFN-γ) and antibodies to these cytokines for use in ELISA were purchased from PharMingen (San Diego, CA). The levels of IL-6, IL-10, IL-12p40, TNF-α and IFN-γ in plasma and culture supernatant fluids were determined by routine sandwich ELISA using Immulon-4 plates (Dynax Technologies INC, Chantilly, VA), according to the manufacturer's protocols. Each sample of plasma and tissue culture fluid was tested for each cytokine in triplicate. The detection limits of the ELISA assays were 16 pg/ml for IL-6 and IL-12p40, and 31 pg/ml for IL-10 and IFN-γ.
4.10 Statistical analysis
Data are represented as means ± standard error (SE). Significance of differences was determined by Student's t-test or Analysis of Variance (ANOVA) using StatView SE 1988 Software (Abacus Concepts, Berkeley, CA).
Acknowledgements
This work was supported by a research grant from the Canadian Institutes of Health Research/Regional Partnership Program (to H. T.) and a postdoctoral fellowship from the Health Services Utilization and Research Commission of Saskatchewan (to M. S.). We thank DNAX, Palo Alto, CA for the permission to use the hybridoma 1B1.3a (anti-murine IL-10 receptor). We also thank Terry Pearson, University of Victoria, Victoria, BC for providing us with the T. brucei strain 10–20, Juliane Deubner, Western College of Veterinary Medicine for drawing the diagrams of the synopsis and Hui Di Wang, Department of Pharmacology, University of Saskatchewan for assisting us in the use of the blood pressure analysis system.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




