Muscle invasive bladder cancer treated with gemcitabine plus cisplatin as neoadjuvant chemotherapy during the second trimester of pregnancy
Abstract
Introduction
Muscle invasive bladder cancer during pregnancy is scarcely reported.
Case presentation
A 41-year-old ex-smoker Asian female, managed with clean intermittent catheterization for 3 years due to neurogenic bladder caused by spinal cord disease, presented with gross hematuria at 10 weeks of gestation. The patient was diagnosed with muscle invasive bladder cancer, cT3aN0M0. Maternal treatment was prioritized and gemcitabine plus cisplatin as neoadjuvant chemotherapy was started at 17 weeks of gestation. Cesarean section was performed at 32 weeks of gestation, followed by robot-assisted radical cystectomy 3 weeks later. Histopathological examination revealed urothelial carcinoma, G3, ypT3b. Postoperative adjuvant nivolumab followed by enfortumab vedotin was not successful and the patient died 8 months after robot-assisted radical cystectomy. Unfortunately, the premature infant with congenital heart disease died of necrotizing enterocolitis at 1-month-old.
Conclusion
Gemcitabine plus cisplatin chemotherapy in the patient with muscle invasive bladder cancer during pregnancy was safe but ultimately resulted in unfavorable outcomes in this case.
Abbreviations & Acronyms
-
- BCa
-
- bladder cancer
-
- CIC
-
- clean intermittent catheterization
-
- GC
-
- gemcitabine plus cisplatin
-
- HE
-
- hematoxylin and eosin
-
- MIBC
-
- muscle invasive bladder cancer
-
- MRI
-
- magnetic resonance imaging
-
- NAC
-
- neo-adjuvant chemotherapy
-
- RARC
-
- robot-assisted radical cystectomy
-
- SD
-
- stable disease
-
- UC
-
- urothelial carcinoma
Keynote message
Bladder cancer during pregnancy is extremely rare. To our knowledge, this is the first case report of muscle-invasive bladder cancer treated with gemcitabine plus cisplatin during pregnancy. Gross hematuria during the gestational period should raise concerns for bladder cancer.
Introduction
BCa is a highly prevalent disease in older males.1 However, BCa during pregnancy is extremely rare. Moreover, MIBC is scarcely reported.2 In a recent review article, Maggen et al. reported 18 cases of non-bilharzial BCa during pregnancy, from 1999 to 2019, and only five cases were MIBC.2
Cancer incidence during pregnancy, such as breast cancer, cervical cancer, and melanoma, has been increasing.3 Prenatal care should follow the standard of care as much as possible to optimize maternal prognosis, always taking into account the health of the fetus.3 During pregnancy, life-saving chemotherapy for the maternal body is a life-threatening concern for the developing fetus.4 Chemotherapy must be avoided in the first trimester and should also be avoided after 35 weeks of gestation for maternal and fetal bone marrow recovery; however, the safe use of chemotherapy in the second and third trimesters (13–26 and 27–35 weeks, respectively) has been reported.3, 4
To our knowledge, this is the first case report of MIBC treated with GC as NAC during pregnancy.
Case presentation
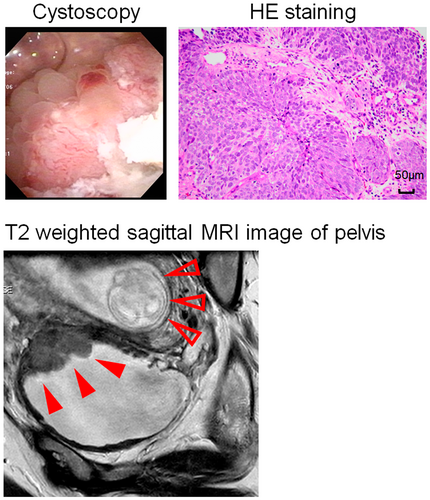
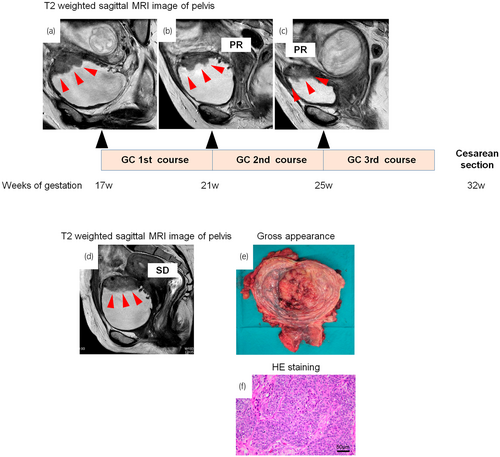
A 41-year-old ex-smoker Asian female, who had been managed with CIC for 3 years due to neurogenic bladder caused by spinal cord disease, presented to the hospital with gross hematuria at 10 weeks of gestation. This was her first pregnancy after fertility treatment. Cystoscopy revealed a 3 cm size bladder tumor located at the left lateral wall of bladder, high-grade UC was diagnosed via biopsy (Fig. 1). Pelvic MRI and whole-body computed tomography revealed a diagnosis of MIBC, cT3aN0M0 (filled red arrows) (Fig. 1). After consultation with the obstetrician, maternal treatment was prioritized and GC as NAC was started at 17 weeks of gestation with the hospital's approval (Fig. 2a). The regimen for GC was gemcitabine 1000 mg/m2 on days 1, 8, and 15 plus cisplatin on 70 mg/m2 day 2. The treatment efficacy was assessed with pelvic MRI after one course of treatment, revealing stable disease with a 21% decrease in maximum tumor size (Fig. 2b), but there was no tumor shrinkage thereafter (Fig. 2c). Pelvic MRI at the end of the third treatment course showed that the primary tumor was increasing (Fig. 2d), so the fourth course was not performed. During NAC, the patient had grade 1 nausea and alopecia, and grade 2 anemia and constipation according to CTCAE ver 5.0. Cesarean section was performed at 32 weeks of gestation, and RARC with intracorporeal ileal conduit5 accompanied by hysterectomy and salpingectomy with preservation of the ovaries was performed 3 weeks later. The tumor was located at the bladder body, with clear borders and irregular mucosa (Fig. 2e). Histopathological examination revealed UC, G3, and ypT3b (Fig. 2f). Lymph node dissection was performed yielding 57 nodes without metastasis. The premature infant (1683 g) had heart disease with ventricular septal defect and patent ductus arteriosus. The patient started adjuvant nivolumab therapy on postoperative day 43 and developed pelvic recurrences 79 days after starting nivolumab, resulting in progressive disease. Then, the treatment was changed to enfortumab vedotin. However, the patient's condition worsened and she died 8 months after RARC. Unfortunately, the infant died of necrotizing enterocolitis at 1 month.
Discussion
We experienced the first case of a patient with MIBC who was treated with GC as NAC during the second trimester of pregnancy. The use of GC chemotherapy in the second trimester was safe, but ultimately resulted in unfavorable outcomes. In this case, BCa may have been detected later because of the sensory insensitivity caused by the spinal cord disease, but gross hematuria during the gestational period should raise concerns for BCa.
There have been few reports on the details of MIBC treatment during pregnancy, and only one case has been published using NAC with carboplatin and adriamycin.6 The following treatment options were considered: (i) immediate radical cystectomy and abortion, and (ii) NAC followed by cesarean section and subsequent radical cystectomy (the optimal cesarean section time is over 24 weeks of gestation when the fetal survival rate is about 90% in Japan7). The second option was chosen because NAC improves the outcome of MIBC8 and the previous report shows the safe use of chemotherapy in the second and third trimesters of pregnancy.4 GC is the most modern chemotherapy as NAC for MIBC,8 but there have been no reports of it being administered to pregnant patients with MIBC. One case of lung cancer9 and one case of adenocarcinoma of the biliary tract10 in which GC was administered during the gestational period have been reported, although the administration resumes were different. In both cases, there were no problems with fetal and infant development. In our case, the association of GC with infant heart disease and necrotizing enterocolitis was not clear, because GC was started in the second trimester, when the main organogenic period had ended.4 Moreover, necrotizing enterocolitis is more likely to occur in premature infants.
The initial symptom of BCa during pregnancy is also painless gross hematuria.11 In addition, urinary symptoms during pregnancy are often underestimated, and hematuria is often mistaken for bleeding of gynecological origin.11 In this case, cystoscopy was performed immediately after the appearance of gross hematuria confirmed the diagnosis, but the BCa might occurred earlier, and the patient's sensory insensitivity caused by the spinal cord disease may have hindered the BCa discovery. Although CIC due to spinal cord disease is known to be a risk factor for BCa,12 in this case, the association between CIC and BCa was not clear. The median time from the start of CIC to the onset of BCa has been previously reported to be 17.3 years,12 which is longer than in this case.
This report has several limitations. First, mothers have a reduced immune response to maintain fetal pregnancy, and this reduced immune response during pregnancy might have a negative impact on cancers. However, the mechanism linking this to the patient's BCa is not clear. Second, it was not clear what the optimal interval between cesarean section and RARC would have been, although 3–4 weeks after delivery has been suggested because of the higher risk of bleeding due to pelvic congestion.2
In conclusion, GC chemotherapy in the patient with MIBC during the second trimester of pregnancy was safe, but ultimately resulted in unfavorable outcomes in this case. Gross hematuria during the gestational period should be considered as a possible sign of BCa in cases of sensory insensitivity and chronic dysuria due to spinal cord disease, as in this case.
Author contributions
Takeshi Sasaki: Writing – original draft; data curation. Ikadai Ryota: Writing – original draft; data curation. Akinobu Ishiyama: Resources. Sho Takakura: Resources. Momoko Kato: Resources. Shunsuke Owa: Resources. Taketomo Nishikawa: Resources. Shinichiro Higashi: Resources. Kouhei Nishikawa: Resources. Takahiro Inoue: Supervision; writing – review and editing.
Conflict of interest
The authors declare no conflict of interest. Takeshi Sasaki is an Editorial Board member of the International Journal of Urology and a co-author of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication.
Approval of the research protocol by an institutional reviewer board
Not applicable.
Informed consent
Informed consent was obtained from the surviving family members (the patient's husband and mother) for the publication of this case report.
Registry and the registration No. of the study/trial
Not applicable.




