Large-cell neuroendocrine carcinoma of the kidney effectively treated by nivolumab and ipilimumab
Abstract
Introduction
Neuroendocrine tumors originating in the kidney are rare, and standard treatments are not established.
Case presentation
An 80-years-old man was referred to our hospital with renal dysfunction and a left renal mass. Based on CT and bone scintigraphy results, he was diagnosed as having a large left renal tumor with a thrombus in the inferior vena cava, harboring lymph node, liver, lung, and left iliac bone metastasis. The renal biopsy indicated a large-cell neuroendocrine carcinoma. Treatment with nivolumab + ipilimumab was introduced. The local and metastatic tumors had shrunk. Subsequently, treatment with nivolumab has remained effective for >2 years.
Conclusion
This case demonstrates the efficacy of treatment with the immune-checkpoint inhibitors against large-cell neuroendocrine carcinoma of the kidney.
Abbreviations & Acronyms
-
- CRP
-
- C-reactive protein
-
- CT
-
- computed tomography
-
- HPF
-
- high-power fields
-
- IVC
-
- inferior vena cava
-
- LCNECs
-
- large-cell neuroendocrine carcinomas
-
- MRI
-
- magnetic resonance imaging
-
- NETs
-
- neuroendocrine tumors
-
- SCNECs
-
- small-cell neuroendocrine carcinoma
Keynote message
A large-cell neuroendocrine carcinoma of the kidney with a thrombus in the inferior vena cava is described. Treatment with nivolumab + ipilimumab was introduced. The efficacy of immune-checkpoint inhibitors has continued for >2 years.
Introduction
Neuroendocrine tumors are peptide hormone-producing tumors derived from neuroendocrine cells; they used to be referred to as carcinoid tumors. The primary site of origin of neuroendocrine tumors is the gastrointestinal tract in 75% of the cases, the lungs and bronchi in 24%, and the urogenital system in <1%. Regarding the urogenital system, it has been reported that neuroendocrine tumors rarely occur in the kidney.1 Primary renal neuroendocrine tumors are broadly classified into highly differentiated neuroendocrine tumors (NETs), large-cell neuroendocrine carcinomas (LCNECs), and small-cell neuroendocrine carcinoma (SCNECs), according to International Classification of Diseases for Oncology. Surgical resection, radiotherapy, and chemotherapy have respectively been recommended for neuroendocrine tumors originating in the urogenital system. It was reported that immune-checkpoint inhibitors were effective against pulmonary, cervical, and renal LCNEC.2-5 We provide the case details of a patient with a renal LCNEC with multiple metastases that were effectively treated by a combination of the immune-checkpoint inhibitors nivolumab and ipilimumab.
Case report
An 80-years-old Japanese man was referred to our hospital with renal dysfunction and a left renal mass identified during an ultrasonographic examination. He had no specific symptoms. The laboratory findings showed renal dysfunction with the serum creatinine level 2.08 mg/dL, anemia with hemoglobin at 10 g/dL, and mild inflammation with the white blood cell count 9400/μL and C-reactive protein (CRP) at 3.01 mg/dL. No hematuria or pyuria was detected by urine analyses.
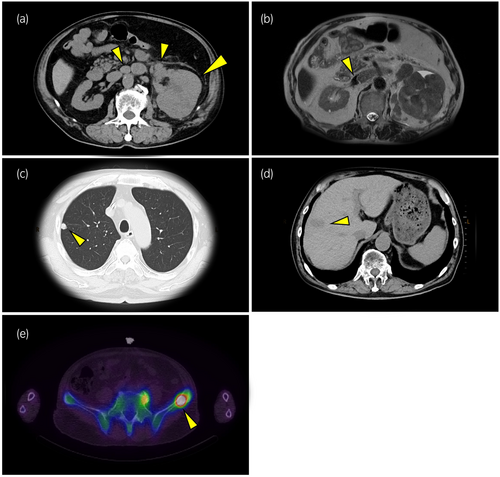
A computed tomography (CT) examination revealed that (i) the patient's left kidney was replaced by a large (108 × 83 × 80-mm) tumor, and (ii) para-aortic lymph nodes were swollen (Fig. 1a). A magnetic resonance imaging (MRI) examination revealed a tumor thrombus in the left renal vein and inferior vena cava (IVC) (Fig. 1b). Distant metastases to the lung and the liver were identified by CT (Fig. 1c,d). Bone scintigraphy revealed left iliac bone metastasis (Fig. 1e). Based on these findings, the patient was clinically diagnosed with a renal cell carcinoma (cT3bN2M1) according to the renal cancer TNM classification (eighth edition) and as at intermediate risk based on his IMDC prognostic risk score. Because of concerns about rapid progress, within 1 day after a needle biopsy of the patient's left kidney was conducted, combination therapy with nivolumab (240 mg/body) and ipilimumab (1 mg/kg, 60 mg/body) administered every 3 weeks was started. We opted the treatment with nivolumab + ipilimumab because we considered it is the most common clear-cell renal cell carcinoma. A subsequent histological examination of a biopsy specimen revealed an undifferentiated tumor that had a nuclear body with an unequal size and irregular shape on hematoxylin and eosin staining (Fig. 2a). These results together with our observation of the positive staining of neuroendocrine markers including INSM1, synaptophysin, chromogranin A, and CD56 in immunohistochemical analyses (Fig. 2b–e) led to the diagnosis of LCNEC of the kidney. The diagnosis of neuroendocrine tumors is based on classic histological features and immunohistochemistry.

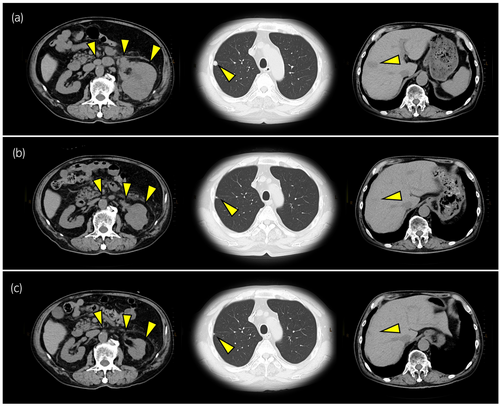
Although the combination therapy with nivolumab + ipilimumab is a standard treatment for intermediate- or poor-risk renal cell carcinoma, this therapy's efficacy for renal LCNECs has not been established. However, we decided to continue the nivolumab + ipilimumab until the first-time radiological evaluation in order to determine the efficacy of the treatment. The CT examination performed 11 weeks after the start of the nivolumab + ipilimumab treatment demonstrated that the local tumor in the patient's left kidney and the metastatic tumors in lymph node, lungs, and liver had shrunk significantly (Fig. 3a,b). The total tumor reduction rate was 46% and no new lesions appeared, thus enabling a partial response according to RECIST ver. 1.1. After four courses of nivolumab + ipilimumab, we switched the patient's treatment to nivolumab-alone based on the standard schedule for metastatic renal cell carcinoma. The patient's hypothyroidism at 12 weeks due to the treatment was improved by levothyroxine sodium (100 μg/day). The adrenal insufficiency with anorexia that was observed at 22 weeks was improved by hydrocortisone (20 mg/day). The radiological evaluation conducted after 5 months of treatment revealed that the patient's left renal tumor and metastatic lesions had reduced in size (Fig. 3c). No adverse events occurred, and the efficacy of nivolumab has continued for >2 years as of this writing.
Discussion
Renal cell carcinomas originate from the renal epithelium and accounts for >90% of kidney cancers. The clear-cell subtype is the most common type of renal cell carcinoma.6 Primary renal neuroendocrine tumors are included among other renal cancers and have been reported infrequently. The pathogenesis of primary renal neuroendocrine tumors is not clear, since intrinsic neuroendocrine cells have not been identified in normal kidney.1 Romero et al. described prognostic factors of primary renal neuroendocrine tumor in their review: tumors >4 cm in size, patient age > 40 years, extrarenal extension, metastasis, purely solid tumors, and a mitotic rate higher than 1/10 high-power fields (HPF) were listed as factors associated with poor prognosis.1 Although our patient had four of these factors (tumor size, age, metastasis, and solid tumor), the treatment with nivolumab + ipilimumab was effective and he has achieved a long survival time. The Clinical Practice Guidelines for Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-MEN) 2019 state that the standard treatment regimen for undifferentiated neuroendocrine carcinomas is cisplatin + etoposide or cisplatin + irinotecan. In our patient's case, the treatment with nivolumab + ipilimumab had already been started before the pathological diagnosis of LCNEC, and the efficacy of chemotherapy including cisplatin was not known. Takagi et al. described a renal LCNEC that was effectively treated by nivolumab as a second-line therapy after chemotherapy with cisplatin and etoposide.5 Kato et al. reported the case of a pulmonary LCNEC that was effectively treated with nivolumab; they noted that the patient's tumor tissue had high PD-L1 expression in immunohistochemical staining.7
Unfortunately, we did not perform an immunohistochemical analysis for PD-L1 expression in our patient's case. In the CheckMate 214 study evaluating the efficacy of nivolumab for renal cell carcinoma, PD-L1 expression levels were not associated with the treatment efficacy.8 Immunohistochemical analyses of the PD-L1 expression in renal cell carcinomas is thus not usually performed for predicting the efficacy of nivolumab. Further case series and studies are necessary to determine the efficacy of immune-checkpoint inhibitors for the treatment of renal cell carcinomas including LCNECs. For locally advanced renal cell carcinomas with a tumor thrombus, cytoreductive surgical resection of the local tumor had been a standard treatment even for metastatic disease. Deferred cytoreductive surgery has recently become a treatment option for metastatic renal cell carcinoma.9 However, the results of the CARMENA study indicated that cytoreductive surgery was not effective against metastatic renal cell carcinoma under sunitinib treatment.10 There is no clear evidence that cytoreductive nephrectomy following immune-checkpoint inhibitor therapy is effective.
At the beginning of his treatment, we did not consider the cytoreductive nephrectomy. In our patient's case, the tumor thrombus progressing to the IVC was eliminated by the treatment with nivolumab + ipilimumab, and thus cytoreductive surgery was not performed.
In conclusion, combination treatment with nivolumab + ipilimumab can be effective against renal LCNECs with multiple metastases. Further research is necessary to elucidate the efficacy of immune-checkpoint inhibitors for neuroendocrine cancers including LCNECs.
Author contributions
Nodoka Okubo: Writing – original draft; writing – review and editing. Takashi Kabuto: Supervision. Hisato Kobayashi: Supervision. Junya Kimura: Investigation. Yoshiaki Imamura: Supervision. Masaya Seki: Supervision. So Inamura: Supervision. Minekatsu Taga: Supervision. Masato Fukushima: Supervision. Naoki Terada: Supervision.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board
Not applicable.
Informed consent
Informed consent was obtained from the patient.
Registry and the Registration No. of the study/trial
Not applicable.




