Primary squamous cell carcinoma of the kidney with hepatic invasion
Abstract
Introduction
Primary squamous cell carcinoma of the kidney is rare, with only a few cases reported to date.
Case presentation
A right renal mass was detected in a 73-year-old asymptomatic man. Dynamic contrast-enhanced computed tomography showed a hypodensity mass extending from the upper pole of the kidney to the right lobe of the liver. Renal biopsy revealed that this tumor was squamous cell carcinoma. One month later, computed tomography showed rapid tumor growth. Radical nephrectomy and partial hepatic resection were performed. Pathological analysis indicated that this tumor originated from the tubular epithelium, and the patient was diagnosed with primary squamous cell carcinoma of the kidney.
Following up without adjuvant therapy, he developed retroperitoneal recurrence and multiple lung metastases and expired.
Conclusion
In this case, squamous cell carcinoma of the kidney invaded the liver and progressed rapidly. Considering these observations, surgical resection should be promptly performed in suspected cases.
Keynote message
Primary squamous cell carcinoma of the kidney is very rare. This disease may be invasive and progress rapidly. Considering its rapid progression, surgical resection should be performed as soon as possible in suspected cases.
Abbreviations & Acronyms
-
- CRP
-
- C-reactive protein
-
- CT
-
- computed tomography
-
- FDG-PET
-
- fluorodeoxyglucose-positron emission tomography
-
- GATA3
-
- GATA-binding protein 3
-
- HE
-
- hematoxylin and eosin
-
- PAX8
-
- paired box 8
-
- SCC
-
- squamous cell carcinoma
Introduction
Primary SCC of the kidney is rare, with only a few cases reported to date.1-9 This disease is often detected at an advanced stage with chronic inflammatory reactions like kidney stones and infections, and there are reports of invasion to adjacent organs.8 It is difficult to diagnose only by imaging and blood test, and surgery is the primary treatment option, with chemotherapy and radiotherapy exhibiting limited efficacy.7, 9 We report a very rare case of a primary SCC of the kidney with liver invasion, characterized by rapid progression.
Case presentation
A 73-year-old asymptomatic man, without remarkable family/medical history, was referred to our hospital for further evaluation after an abdominal ultrasonography detected a right renal mass. Physical examination did not reveal abnormalities. Contrast-enhanced CT showed a poorly marginated, inhomogeneous low-density mass with capsular enhancement and internal calcification extending from the upper pole of the right kidney to the right lobe of the liver, measuring approximately 8.0 cm (Fig. 1). There were no distant or lymph node metastases, and no venous thrombosis (cT4N0M0). A CT scan performed 10 months earlier had revealed a renal cyst with internal calcification at the upper pole, but not a right renal mass (Bosniak classification: IIF) (Fig. 1). Blood testing showed elevated levels of CRP (7.57 mg/dL; normal ~0.30 mg/dL) and corrected calcium (12.0 mEq/L; normal 8.8–10.1 mEq/L), and normal liver enzymes.
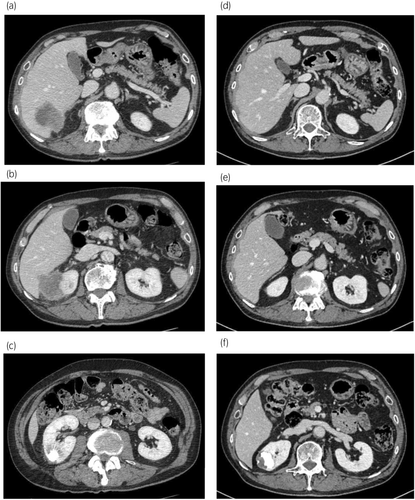
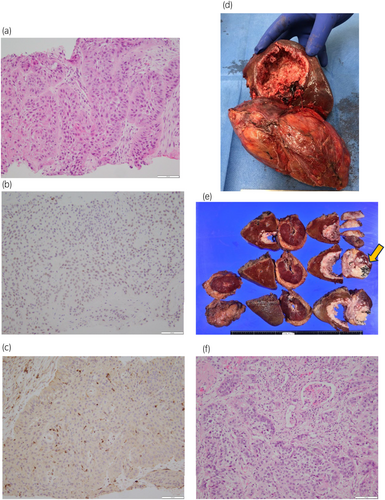
A percutaneous kidney biopsy was performed to identify the origin of the tumor. Histopathological examination revealed SCC forming an infiltrative pavement-like arrangement (Fig. 2). Immunohistochemistry showed positivity for high molecular-weight cytokeratin, p63, almost negativity for GATA3, and negativity for PAX8 (Fig. 2). Nevertheless, this analysis could not determine the origin of this SCC (i.e., urothelial or renal cell).
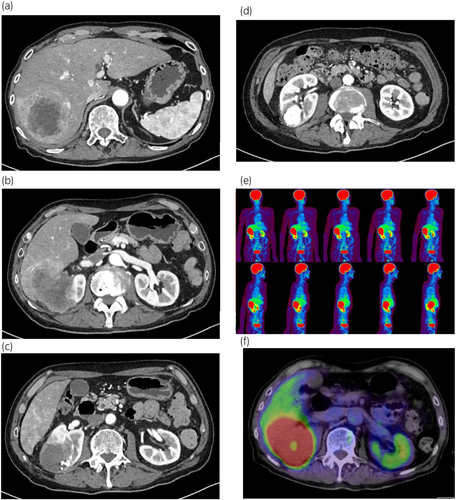
Two weeks after the initial visit, the patient developed chronic fever and worsening general malaise. Repeated contrast-enhanced CT at 1 month after initial presentation showed rapid tumor growth and progression to hepatic invasion. There were no distant or lymph node metastases observed (Fig. 3). FDG-PET/CT confirmed the absence of metastasis (Fig. 3). Blood testing showed further elevation of CRP and corrected calcium levels (CRP: 9.64 mg/dL; calcium: 14.64 mg/dL).
Radical nephrectomy and hepatic resection of the posterior segment by laparotomy were urgently performed. The right kidney and part of the liver were removed as a single lump with a reversed L-shaped incision (Fig. 2). No lymph node enlargement or venous thrombosis was observed, but mild adhesions were noted. Total operative time was about 10 h, total blood loss was 1850 mL. Almost the entire upper pole of the kidney had been replaced by the tumor, and a calcified renal cyst had been filled with tumoral tissue, including numerous small black stones (>100) (Fig. 2). The tumor had invaded the Gerota's fascia, right adrenal gland, and liver. Histopathological analysis indicated negative resection margins and revealed a pure SCC arranged in a multilayered squamous-like sheet, with focal or fenestrated structures and infiltrative growth (Fig. 2). Tumor cells were growing and migrating within the tubules and along the glomerular structures. Immunohistochemistry showed positivity for high molecular-weight cytokeratin, p63, and negativity for GATA3 and PAX8. Although differentiation was not possible with immunostaining, the findings suggesting an origin from the cyst wall led to the determination that it derived from the renal tubules. These findings led us to conclude that the final diagnosis was primary SCC of the kidney (pT4N0, moderate-to-well differentiated).
The patient was discharged 12 days after surgery without major complications. He did not receive any adjuvant treatment. Two months after surgery, he developed right retroperitoneal recurrence and multiple lung metastases. The patient expired of multiple organ failure due to cancer progression 4 months after surgery.
Discussion
SCC of the urinary tract rarely originates from the kidney, and the etiology of renal SCC remains unclear. This is one of the few cases of renal primary SCC (Table 1). We present a case of invading the liver and exhibiting extremely rapid tumor growth.
| Reference number | Author | Sex | Age | Presentation | Location | Treatment | Tumor extent | Adjuvant treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Terada (2010) | M | 73 years | Hematuria and lumbago | Bladder, left ureter, and left kidney | Cystectomy and nephroureterectomy | Replace the entire kidney | Absent | Alive and disease free after at 3 months after surgery |
| 2 | Kulshreshtha (2012) | F | 60 years | Weight loss for 3 months | Mid and lower pole of the left kidney | Radical nephrectomy with lymph node dissection |
6.5 × 5.5 cm Gerota fascia invasion and para-aortic lymph node metastasis (pT4N1) |
Absent | Alive and disease free after at 13 months after surgery |
| 3 | Ghosh (2014) | M | 51 years | Dull and intermittent flank pain for 5 months | Lower pole of the right kidney | Radical nephrectomy | 5.8 × 5.5 cm (pT1bN0) | Absent | Alive and disease free after at 12 months after surgery |
| 4 | Sahoo (2015) | F | 50 years | Right abdomen pain for 6 months | Upper pole of the right kidney | Radical nephrectomy | 8.0 × 6.0 cm (pT2aNx) | Absent | Alive and disease free after at 6 months after surgery |
| 5 | Wang (2016) | M | 61 years | Hematuria and lumbago for 2 months | Right kidney | Radical nephrectomy | Gerota fascia invasion (pT3aNx) | Absent | Alive and disease free after at 1 month after surgery |
| 6 | Zhang (2020) | F | 61 years | Intermittent flank pain for 2 months | Lower pole of the right kidney | Radical nephrectomy | Gerota fascia invasion (pT3aNx) | Absent | Alive and disease free after at 3 months after surgery |
| 7 | Fotovat (2021) | F | 41 years | Flank pain and dysuria for 3 months | Lower pole of the left kidney | Radical nephrectomy | Gerota fascia invasion and para-aortic lymph node metastasis (pT3aN1) | Adjuvant chemotherapy with cisplatin and gemcitabine | Ovarian metastasis 8 months after surgery, then death |
| 8 | Cheol (2022) | M | 61 years | Flank pain and weight loss for 2 months | Lower pole of the right kidney | Radical nephrectomy with right hemicolectomy |
9.0 × 8.0 cm Ascending colon invasion (pT4N0) |
Absent | Alive and disease free after at 6 months after surgery |
| 9 | Liang (2023) | M | 52 years | 1 week of renal cyst found in physical examination | Upper pole of the right kidney | Robot-assisted partial nephrectomy | 8.3 × 8.2 × 8.1 cm (pT2aNx) | Absent | Alive and disease free after at 6 months after surgery |
| 10 | Present | M | 73 years | Renal mass on echo | Upper pole of the right kidney | Radical nephrectomy with partial hepatectomy |
9.0 × 9.0 cm Adrenal and hepatic invasion (pT4) |
Absent | Retroperitoneal recurrence and multiple lung metastases appeared 2 months after surgery, then death |
SCC tends to invade surrounding organs, while urothelial carcinoma is associated with distant metastases.10 Hepatic invasion is rare in renal cell carcinoma, and combined renal-hepatic resection is recommended as curative treatment.11 Invasion of the ascending colon by SCC of the kidney has been previously reported.8 Nevertheless, invasion of other organs, including the liver, has rarely been reported thus far.
Most previous cases of SCC of the kidney developed following chronic stresses (e.g., kidney stones and hydronephrosis).1, 2, 4-9 SCC in various organs is thought to be associated with chronic inflammation.12, 13 In this case, a calcified renal cyst was observed several years before the onset of this cancer, and calcified content might irritate the epithelium of the wall. Histopathology showed the cyst was filled with tumoral tissue and tumor cell grew within the tubules, and immunohistochemistry showed no diagnostic information of urothelial and renal cell carcinoma. Thus, we thought that the epithelium of the wall was most likely the origin of this cancer.
Unlike in this case, disease progression was not regularly monitored (e.g., on a monthly basis) in previous cases. According to our observations, SCC of the kidney could progress rapidly within weeks or months. Therefore, prompt surgical intervention for renal tumors that develop due to chronic stress could be an appropriate treatment option.
Platinum-based chemotherapy has been used to treat SCC of the kidney, renal pelvis, and ureter.7, 14 Nonetheless, this treatment has demonstrated limited effectiveness, and including this case, recurrent cases often result in death. Chemotherapy with cisplatin, methotrexate, and vinblastine is effective in treating SCC of the urinary tract.15 However, this option has not been established as standard treatment for SCC of the kidney. Despite the low responsiveness of SCC of the urinary bladder to standard chemotherapy regimens, the use of preoperative radiotherapy has been associated with improved patient survival.10 Further research is required to evaluate the effectiveness of newly developed treatments (e.g., immune checkpoint inhibitors) as well as chemotherapy and radiotherapy strategies.
Conclusion
We reported the case of renal SCC with invasion of the liver, treated by combined renal-hepatic resection. Renal SCC is very rare, could progress very rapidly, and should be suspected in cases where chronic stress has precipitated the renal malignancy. Furthermore, considering the risk of exceedingly rapid progression of this disease, surgery should be performed as soon as possible.
Author contributions
Masato Takanashi: Writing – original draft; writing – review and editing. Miho Asaoka: Validation. Masashi Imano: Validation. Azumi Fujioka: Validation. Yuka Oishi: Validation. Goro Matsuda: Validation. Sawako Chiba: Writing – review and editing. Kotaro Hirai: Supervision; writing – review and editing.
Conflict of interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board
Not applicable.
Informed consent
Written informed consent was provided by the patient for the publication of this case report and any accompanying images.
Registry and the Registration No. of the study/trial
Not applicable.




