The impact of the temporal sequence of cranial radiotherapy and platin-based chemotherapy on hearing impairment in pediatric and adolescent CNS and head-and-neck cancer patients: A report from the PanCareLIFE consortium
Abstract
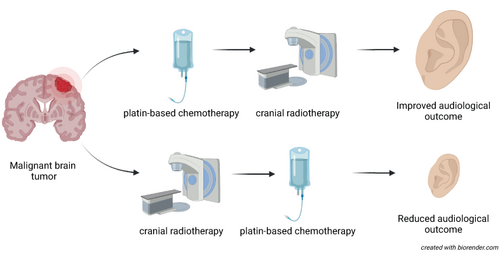
The impact of the temporal sequence by which cranial radiotherapy (CRT) and platin-based chemotherapy (PCth) are administered on sensorineural hearing loss (SNHL) in pediatric and adolescent central nervous system (CNS) and head-and-neck (HN) cancer patients has not yet been studied in detail. We examined the ototoxic effects of sequentially applied CRT and PCth. This study included children and adolescents with CNS and HN tumors who participated in the multicountry PanCareLIFE (PCL) consortium. Audiological outcomes were compared between patients who received CRT prior to PCth and those who received it afterwards. The incidence, degree and posttreatment progression of SNHL, defined as Muenster classification grade ≥MS2b, were evaluated in 141 patients. One hundred and nineteen patients were included in a time-to-onset analysis. Eighty-eight patients received CRT prior to PCth (Group 1) and 53 patients received PCth before CRT (Group 2). Over a median follow-up time of 1.6 years, 72.7% of patients in Group 1 experienced SNHL ≥ MS2b compared to 33.9% in Group 2 (P < .01). A time-to-onset analysis was performed for 74 patients from Group 1 and 45 patients from Group 2. Median time to hearing loss (HL) ≥ MS2b was 1.2 years in Group 1 and 4.4 years in Group 2 (P < .01). Thus, audiological outcomes were better for patients who received CRT after PCth than before. This finding should be further evaluated and considered within clinical practice in order to minimize hearing loss in children and adolescents with CNS and HN tumors.
Graphical Abstract
What's new?
Treatment for pediatric central nervous system (CNS) and head and neck (HN) cancers includes a combination of surgery, radiation therapy, and chemotherapy. However, sensorineural hearing loss (SNHL) can arise from the combined action of radiation and chemotherapy. Here, the authors examined whether the sequence of therapies affected the incidence of SNHL. In a study of 119 patients, they found that 73% of patients who received radiation before chemotherapy experienced SNHL compared with 34% of those who received chemotherapy first. However, no studies have yet compared treatment outcomes based on sequence of therapy.
Abbreviations
-
- CI
-
- confidence interval
-
- CNS
-
- central nervous system
-
- CRT
-
- cranial radiotherapy
-
- EQD
-
- equivalent doses at fractionation
-
- HL
-
- hearing loss
-
- HN
-
- head-and-neck
-
- IMRT
-
- intensity-modulated radiation therapy
-
- MANOVA
-
- multivariate analysis of variance
-
- PCL
-
- PanCareLIFE
-
- PCth
-
- platin-based chemotherapy
-
- QoL
-
- quality of life
-
- SNHL
-
- sensorineural hearing loss
1 INTRODUCTION
The current multimodal treatment method used for most malignant pediatric central nervous system (CNS) and head-and-neck (HN) tumors include surgery of the primary tumor, cranial radiotherapy (CRT) and platin-based chemotherapy (PCth). One possible side-effect is sensorineural hearing loss (SNHL) resulting from the synergistic ototoxic effect of CRT and PCth.1-3 Ototoxicity-induced hearing loss (HL) typically begins in the high-frequency range of hearing, potentially progressing in severity and into lower frequencies over time.4
Several studies have found correlations between higher cochlear radiation dose and increased incidence, severity, irreversibility and shorter time-to-onset of SNHL.5-7 Much research has demonstrated the considerable ototoxic risk of cisplatin treatment.8-11
The combined use of CRT and cisplatin has a greater ototoxic effect than either of those treatments alone.2, 6, 12-15 Their temporal sequence appears to influence the development of posttreatment HL, with literature demonstrating no additional deterioration in hearing thresholds where cisplatin was given before CRT, and more severe ototoxicity when the order was reversed.9, 11, 14-17
The evidence concerning the ototoxic effect of carboplatin varies and can be related to patient or treatment-associated factors.18-21 Administration of carboplatin before or after radiation is expected to cause less HL compared with cisplatin. Whereas a threshold dose for carboplatin or the expected time-to-onset of SNHL when carboplatin and CRT are combined were not defined in previous studies, Keilty et al found an additive effect with a carboplatin dose greater than 1000 mg/m2 being associated with an increasing grade of HL in children and adolescents.22
The present multicountry study reports retrospective analyses of SNHL following different temporal sequences of CRT and PCth administration in a large European cohort of children and adolescents with malignant brain and HN tumors assembled within the framework of the PanCareLIFE (PCL) project.23, 24 The degree, progression and time-to-onset of hearing impairment were compared among patients treated with CRT either prior to or subsequent to PCth.
2 MATERIALS AND METHODS
2.1 Patients
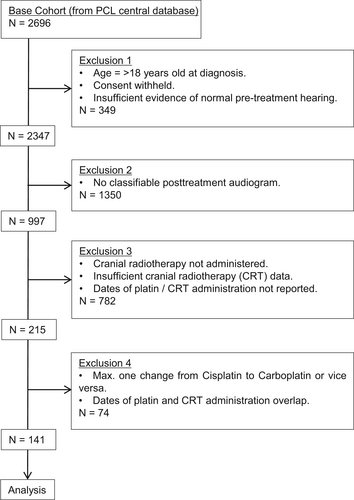
This retrospective study included data of pediatric and adolescent cancer patients who participated in Work Package 5 of the PCL consortium (http://www.pancarelife.eu).23, 24 This data was gathered from 12 data providers (mostly pediatric oncology clinics) in 7 European countries between January 2016 and April 2018 (Supplementary Data, Table 1). The inclusion criteria specified age at diagnosis younger than 18 years old, treatment with cisplatin and/or carboplatin as well as cranial radiotherapy and no evidence of HL prior to the start of their treatment (Figure 1). Key exclusion criteria were no posttreatment audiogram, HL pretreatment as evidenced by pretreatment audiograms (these patients were excluded to ensure a focus on therapy-, not tumor-related HL or HL due to other causes), low cochlear radiation dose (mean cochlear dose <35 Gy to both cochlea; patients with a mean cochlear dose >35 Gy to either cochlea were included), and treatment with otoprotective drugs.6, 25 Full exclusion criteria are described in detail in the Supplementary Data. The 141 individuals of this cohort meeting these criteria were included in the present analysis. Following the methods of survival analyses, the follow-up period ends when participants either died or were lost to follow-up. We have no information on tumor recurrence, or second malignancies.
| Diagnosis | Total number of patients/% (male/female) | Age at diagnosis, years, median/range | Age at cranial radiotherapy, years, median/range | Indicated radiation dose, Gy, median/range | Mean cochlear dose, left, Gy, median/range | Mean cochlear dose, right, Gy, median/range | Cisplatin cumulative dose, mg/m2, median/range | Carboplatin cumulative dose, mg/m2, median/range |
|---|---|---|---|---|---|---|---|---|
| Medulloblastoma | 93/41.8 (55/38) | 7.7/2-18 | 7.9/2.6-18.4 | 59/46-72 | 59/33-71 | 60/30-71 | 350/140-630 | 1800/1200-3000 |
| Ependymoma | 48/21.6 (29/19) | 6.3/2-16 | 6.4/2.1-16.2 | 56/44-74 | 47/25-64 | 49/28-65 | 210/140-280 | 6600/1200-10 200 |
| Astrocytoma/glioblastoma | 17/7.6 (6/11) | 7.1/4-16 | 7.1/4-16 | 57/52-61 | 44/18-53 | 39/21-48 | 160/80-320 | 2000/600-2700 |
| Rhabdomyosarcoma | 11/5.0 (6/5) | 7.5/4-16 | 7.7/4.3-16.4 | 59/48-68 | 43/25-51 | 45/27-53 | – | 1400/1100-2100 |
| Germ cell tumor/germinoma | 12/5.4 (4/8) | 9.6/2-16 | 9.7/2.4-16 | 52/40-54 | 41/24-53 | 37/21-50 | 150/65-260 | 9450/2100-14 700 |
| Pinealoblastoma | 10/4.5 (7/3) | 8.5/3-15 | 8.7/3.2-15.4 | 54/45-60 | 33/18-45 | 31/23-39 | 350/140-350 | 1500/600-2400 |
| Optic glioma | 9/4.0 (6/3) | 5.5/2-12 | 5.5/2-12.2 | 52/50-54 | – | – | 270/135-360 | 4800/1800-8100 |
| Oligodendroglioma | 4/1.8 (1/3) | 5.2/3-11 | 5.2/3.1-11 | 54/46-59 | – | – | 250/90-315 | – |
| Retinoblastoma | 4/1.8 (4/0) | 2.7/0.4-5 | 2.8/0.5-5 | 50/46-54 | – | – | – | 1200/600-1800 |
| Rhabdoid tumor | 4/1.8 (3/1) | 3.1/2-4.4 | 3.6/2.2-4.6 | 52/45-57 | 39/20-43 | 41/21-47 | – | 1150/900-1800 |
| Nasopharyngeal carcinoma | 3/1.3 (2/1) | 14.7/8-16 | 14.9/9-18 | 57/54-59 | 38/28-49 | 37/25-42 | 300/225-450 | 1000/500-1450 |
| Total | 215 (123/92) | 7.6/2.9-14.3 | 7.7/3.4-14.5 | 55/47-61 | 43/24-55 | 42/24-52 | 246/120-357 | 3255/1010-5135 |
2.2 Audiological methodology
The audiological results were grouped as clinically-relevant HL (≥2b Muenster classification, or >40 dB HL at 4 kHz or above), or clinically-nonrelevant/normal hearing (Muenster <2b).26 The analysis of posttreatment HL was based on each patient's worst classified audiogram after the end of platin/CRT treatment. Time-to-onset of SNHL was defined as the time between the start of treatment (CRT/PCth) and the time of the first audiogram showing a hearing loss Muenster ≥2b in at least one ear (measured up to a maximum of 5.5 years). Audiological test methodology (test protocols, equipment and calibration standards) is well-established by international standards and measurements were performed in European University clinics. Specifics of analysis methodology were discussed between centers, and validation of results was performed centrally by trained audiologists at the Audiological Reference Center in Muenster, Germany. A detailed description of the audiological methodology is given in the Supplementary Data.
2.3 Radiotherapy
Radiotherapy data were gathered from the PCL database and additionally from the database of the Department of Phoniatrics and Pedaudiology of University Hospital Münster, Germany. Participants were included in the present analysis if mean cochlear dose was available for both cochleae and exceeded 35 Gy in at least one ear. Notably, dose did not have to exceed 35 Gy in both ears for inclusion. A detailed description of the radiotherapy regimes and techniques is presented in the Supplementary Data.
2.4 Chemotherapy
The dose schedules, route and duration of administration, and hematologic criteria for chemotherapy are described in detail in the relevant treatment protocols corresponding to the specific CNS and HN malignancies (Supplementary Data, Table 2).
| Therapy sequence | CRT → PCth | PCth → CRT | P-values | ||
|---|---|---|---|---|---|
| Sex (male/female) | 59/29 | 36/17 | .53 | ||
| Age at diagnosis, years, median/range | 9.5/2-15 | 7.2/0.4-18 | .09 | ||
| Age at diagnosis, years | <9 years (No./%) | 43 (48.9) | 33 (62.3) | .12 | |
| ≥9 years (No./%) | 45 (51.1) | 20 (37.7) | |||
| Age at CRT, years, median/range | 9.7/3-16 | 7.9/2-18.4 | .13 | ||
| Mean cochlear dose, Gy ± SD | Right | 43 ± 28 | 39 ± 32 | .23 | |
| Left | 40 ± 33 | 41 ± 27 | .40 | ||
| Mean cochlear dose, Gy | Right | ≤45 Gy (No./%) | 67 (76.1) | 36 (67.9) | .29 |
| >45 Gy (No./%) | 21 (23.9) | 17 (32.1) | |||
| Left | ≤45 Gy (No./%) | 69 (78.4) | 39 (73.6) | .51 | |
| >45 Gy (No./%) | 19 (21.6) | 14 (26.4) | |||
| Total cisplatin dose, mg/m2, median range | 270/175-630 | 240/140-450 | .08 | ||
| Total cisplatin dose, mg/m2 | ≤200 mg/m2 (No./%) | 26 (39.4) | 9 (40.9) | .9 | |
| >200 mg/m2 (No./%) | 40 (60.6) | 13 (59.1) | |||
| Total carboplatin dose, mg/m2, median range | 3900/1200-8100 | 4500/900-10 200 | .52 | ||
| Patients with HL (No./%) 82/58.2 (all groups) | 64/72.7 | 18/33.9 | <.01 | ||
| Year of treatment | Before 2010 (No./%) | 54/61.4 | 23/43.4 | .04 | |
| After 2010 (No./%) | 34/38.6 | 30/56.6 | |||
| Diagnosis | Medulloblastoma, No./% | 43/48.8 | 21/39.6 | .17 | |
| Ependymoma, No./% | 21/23.8 | 12/22.6 | .21 | ||
| Degree of HL, mean threshold, dB ± SD, right/left | 0.125 kHz | 18.4 ± 6.5/16.3 ± 6.5 | 15.0 ± 5.0/13.6 ± 8.7 | .35 | |
| 0.25 kHz | 14.2 ± 5.5/14.0 ± 6.1 | 13.6 ± 8.7/11.4 ± 4.8 | .09 | ||
| 0.5 kHz | 14.5 ± 10.2/13.0 ± 9.7 | 16.2 ± 10.4/15.4 ± 9.2 | .10 | ||
| 1 kHz | 15.2 ± 13.2/13.2 ± 11.9 | 15.8 ± 13.1/13.9 ± 10.0 | .63 | ||
| 2 kHz | 16.4 ± 17.0/16.5 ± 16.7 | 15.7 ± 15.2/14.4 ± 11.1 | .48 | ||
| 3 kHz | 24.5 ± 18.0/23.4 ± 21.5 | 14.7 ± 12.5/15.9 ± 19.3 | .15 | ||
| 4 kHz | 30.5 ± 22.0/29.4 ± 23.7 | 16.5 ± 16.5/18.9 ± 18.0 | <.01 | ||
| 6 kHz | 45.5 ± 24.0/41.6 ± 24.4 | 24.0 ± 20.6/26.7 ± 23.3 | <.01 | ||
| 8 kHz | 51.4 ± 26.1/50.2 ± 26.2 | 31.0 ± 22.2/32.6 ± 27.5 | <.01 | ||
- Note: Values meeting the level of significance (p < 0.05) were marked in bold.
- Abbreviations: CRT, cranial radiotherapy; HL, hearing loss; PCth, platin-based chemotherapy.
2.5 Statistical analyses
The statistical analysis of the research questions was performed on the basis of a two-tailed test and 5% significance level, using SPSS software (version 26—IBM Corp. Released 2019).
Descriptive statistics were used to describe patient characteristics and differences between groups. Continuous treatment variables were also dichotomized to include group-based testing. These included the one-way Welch-ANOVA-Test for continuous variables as well as the chi square test for categorical variables.
Outcome analyses included time-to-event analyses in n = 119 patients and incidence analyses in n = 141 patients. Outcome was clinically relevant hearing, as defined as a Muenster classification ≥2b score.
We used cox regressions for time-to-event analyses and logistic regressions for incidence analyses. We first tested a priori defined variables known or hypothesized to be associated with posttherapeutic hearing loss, including dose of cisplatin, mean cochlear dose, age, sex, year of treatment (to reflect improvements in treatment algorithms), and therapy sequence. We included any variables in the multivariable model that showed a trending association (P < .1) with outcomes in univariable analyses.
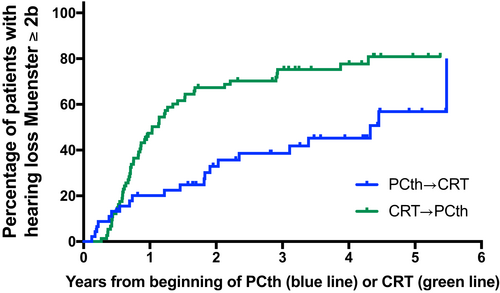
A Kaplan-Meier analysis was performed in order to visualize the time-to-event rate of hearing function of the 119 patients in the two treatment groups who had sufficient audiological data. It shows the probability of developing a hearing loss of ≥2b (Muenster classification) over time following the start of treatment.
3 RESULTS
Patient demographics and therapy variables are presented in Table 1. Detailed patient characteristics are also provided in the Supplementary Data (Results/Addition). 58.1% of participants were male (123/215). The median age at diagnosis was 7.6 years. The median cumulative RT dose applied to individuals ranged from 47 to 61 Gy, and the cochlear Dmean varied from 24 to 52 Gy (right)/24 to 55 Gy (left). The cumulative cisplatin dose ranged from 120 to 357 mg/m2 and varied according to the tumor entity. The cumulative carboplatin dose administered ranged from 1010 to 5135 mg/m2. More than 90% of patients were treated between 2000 and 2016, the remainder was treated between 1992 and 2000. Patients were treated in centers across Europe with no more than 31.9% of patients treated at the same center (Supplementary Data, Table 1). The diagnoses of this cohort are also provided (Supplementary Data, Table 3).
| Univariable logistic regression, n = 141 | Multivariable logistic regression, n = 141 | Univariable time-to-event analysis, n = 119 | Multivariable time-to-event analysis, n = 119 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (CI) | P | OR (CI) | P | HR (CI) | P | HR (CI) | P | |
| Treatment groups | ||||||||
| PCth → RT | Reference 1.00 | Reference 1.00 | Reference 1.00 | |||||
| RT → PCth | 5.19 (2.48-10.84) | < .001 | 3.77 (1.71-8.29) | .001 | 2.31 (1.38-3.88) | .001 | 1.88 (1.08-3.30) | .026 |
| Sex | ||||||||
| Male | Reference 1.00 | Reference 1.00 | ||||||
| Female | 0.90 (0.44-1.85) | .78 | 0.83 (0.51-1.36) | .46 | ||||
| Cumulative dose of cisplatin, mg/m2 | 1.004 (1.002-1.006) | .001 | 1.003 (1.001-1.006) | .013 | 1.002 (1.000-1.003) | .022 | 1.001 (1.000-1.003) | .11 |
| Age at diagnosis, years | 1.07 (0.99-1.16) | .088 | 1.03 (0.94-1.12) | .56 | 1.07 (1.02-1.13) | .011 | 1.04 (0.98-1.10) | .20 |
| Age at diagnosis | ||||||||
| <9 years | Reference 1.00 | Reference 1.00 | ||||||
| ≥9 years | 1.46 (0.74-2.87) | .27 | 1.51 (0.96-2.36) | .075 | ||||
| Dmean cochlear, Gy | 0.96 (0.90-1.02) | .21 | 0.97 (0.93-1.01) | .19 | ||||
| Dmean cochlear | ||||||||
| 35-45 Gy | Reference 1.00 | Reference 1.00 | ||||||
| >45 Gy | 0.69 (0.35-1.36) | .29 | 0.73 (0.40-1.32) | .29 | ||||
| Year of treatment | ||||||||
| Before 2010 | Reference 1.00 | Reference 1.00 | Reference 1.00 | Reference 1.00 | ||||
| After 2010 | 0.54 (0.27-1.06) | .075 | 0.63 (0.30-1.35) | .24 | 0.95 (0.61-1.50) | .84 | 1.01 (0.63-1.59) | .98 |
- Note: Values meeting the level of significance (p < 0.05) were marked in bold.
- a Logistic regression was used to assess any posttherapeutic occurrence of hearing loss classified ≥2b according to the Muenster classification26 in n = 141 patients. Cox regression time-to-event analyses was used to evaluate onset of hearing loss classified ≥2b according to the Muenster classification in n = 119 patients. Treatment sequence, age and cumulative dose of cisplatin that showed a trending association (P < .01) with outcomes in univariable analyses were included in the multivariable model.
3.1 Severity, progression and incidence of posttreatment HL
The degree and progression of posttreatment hearing impairment was analyzed for 141 patients, of whom 88 received CRT prior to PCth (Group 1) and 53 vice versa (Group 2) (Table 2).
We found a significant main effect for the treatment order variable (CRT before PCth vs PCth before CRT; P = .01) (Table 2). The test power was high (.915 observed acuity) and the effect size was good (partial Eta-squared .39). This factor explained 39% of the total variance with all other included factors controlled. The incidence of clinically-relevant posttreatment HL (≥2b Muenster classification) was found to be higher in patients who received CRT prior to PCth than vice versa (72.7% vs 33.9%, respectively; P < .01; odds ratio 3.32 [95% CI: 1.83-6.22]) (Table 2).
The severity of SNHL was significantly different (P < .01) between Groups 1 and 2 at the frequencies 4, 6 and 8 kHz. Hearing thresholds did not differ significantly between the groups (P > .05) in the low-mid frequency range (Table 2). Posttreatment progression of HL did not differ between the two groups (P > .05).
No significant difference between Group 1 and Group 2 patients was found for the variables sex, diagnosis, age at diagnosis and age at time of CRT, mean cochlear radiation dose or cumulative cisplatin and carboplatin doses (Table 2).
Univariable logistic regression suggested that therapy sequence and cumulative dose of cisplatin significantly influenced therapy outcome. Additionally, year of treatment (before or after 2010) and age at diagnosis tended to be associated with HL. No associations with HL were seen for sex and mean cochlear dose (Table 3).
Multivariable logistic regression including the variables that showed trending associations with HL (therapy sequence, cumulative dose of cisplatin, year of treatment, and age at diagnosis) confirmed therapy sequence and cumulative dose of cisplatin as independent variables affecting posttreatment HL (Table 3).
3.2 Time-to-onset of HL
One hundred and nineteen patients had a sufficient number of audiograms (≥3) to be included in this evaluation, of whom 74 patients received CRT prior to PCth (Group 1) and 45 patients received CRT after PCth (Group 2) (Table 4). Detailed patient characteristics are also provided (Supplementary Data, Results). While audiogram acquisition times were not standardized, there was no significant difference in the timing of the first audiogram after therapy completion between groups (P = .28).
| Therapy sequence | CRT → PCth | PCth → CRT | P-valuesa | |
|---|---|---|---|---|
| Sex (male/female) | 43/31 | 30/15 | .41 | |
| Age at diagnosis, years, median/range | 9.8/2-16 | 8/0.4-15.3 | .13 | |
| Age at CRT, years, median/range | 9.9/2-16.7 | 9.1/1.2-16 | .11 | |
| Mean cochlear dose, Gy ± SD | Right | 42 ± 18 | 41 ± 22 | .27 |
| Left | 44 ± 25 | 42 ± 24 | .36 | |
| Total cisplatin dose, mg/m2, median range | 245/150-530 | 230/140-420 | .15 | |
| Total carboplatin dose, mg/m2, median range | 4100/1200-8800 | 4500/1300-10 200 | .44 | |
| Median time-to-onset of HLb, years ± SD | 1.2 ± 0.4 | 4.4 ± 0.7 | <.01 | |
- Note: Values meeting the level of significance (p < 0.05) were marked in bold.
- Abbreviations: CRT, cranial radiotherapy; HL, hearing loss; PCth, platin-based chemotherapy.
- a 5% level of significance in difference of time-to-onset of HL between various treatment groups analyzed with Kaplan-Meier test.
- b Time-to-onset of HL defined as at least 2b (≥2b) according to Muenster classification scale.26
A time-to-event analysis for the treatment group factor (CRT before PCth vs PCth before CRT) and for hearing impairment ≥2b was performed for all observations from the start of therapy up to 5.5 years later (median follow-up 1.6 years). Median time to onset of HL ≥2b were 1.2 years (confidence interval [CI] 0.9 to 1.5) in Group 1 and 4.4 years (CI 2.0 to not reached) in Group 2 (P < .01) (Table 4, Figure 2). Univariable time-to-event analyses demonstrated that dose of cisplatin, age at diagnosis, and therapy sequence were associated with time-to-hearing loss. In multivariable modeling, only therapy sequence remained independently associated with the outcome (Table 3).
4 DISCUSSION
The present study analyzes hearing impairment in patients treated with CRT prior to or after PCth by comparing two groups of patients with a wide spectrum of malignant CNS and HN neoplasms.
4.1 Therapy sequence
Our study found that the temporal sequence of CRT and PCth had a significant effect on the presence of clinically-relevant HL after treatment. This effect was more severe in patients treated with CRT before platin than vice versa (72.7% vs 33.9%, respectively), as shown by the higher incidence of clinically-significant HL and more severe hearing thresholds. Numerous studies have shown an increased ototoxic effect from the combined use of CRT and cisplatin in pediatric CNS and HN cancer patients in general.1-3, 11-15 Some group of authors demonstrated that the extent, time-to-onset and clinical course of HL may reduce or at least not deteriorate if cisplatin is given before CRT.15, 16
Kortmann et al found a more prevalent ototoxic effect in medulloblastoma patients with postirradiation PCth than vice versa (34% and 10%, respectively).17 Unfortunately, the cumulative cisplatin dose applied in this prospective study differed considerably between the two groups (560 vs 80 mg/m2, respectively), meaning that cumulative platin dose could not be ruled out as a significant factor. This issue does not affect the current study, as platin doses were similar between the groups.
One possible mechanism to explain an increase in HL after prior irradiation is the development of hyperemia after irradiation, which may increase the permeability of the inner ear and/or CNS barriers, thereby decreasing the normal tolerance of inner ear tissue to cisplatin.27, 28 Schell et al speculated that cisplatin-based ototoxicity correlates with the destruction of cochlear outer hair cells but preservation of inner hair cells, which typically leads to a bilateral HL in the higher frequencies.11 However, the administration of CRT prior to cisplatin may reduce the resistance of the inner hair cells to platinum drugs, leading to increased HL on the side that was exposed to a cochlear radiation dose.2, 9, 11 Histopathological changes in inner ear structures after CRT and cisplatin treatment are described in detail in the Supplementary Data.
4.2 Age, sex and other variables
The literature referring to the role of age and sex on hearing loss in CRT/PCth treatment is inconsistent.1, 5, 9, 11-13, 16, 25, 29 Interestingly, our findings indicate that advanced age may be associated with increased risk of hearing loss, but only in univariable analysis. The association is lost in the multivariable model. Older childhood age5, 13, 16, 25 and younger age11, 29, 30 have both been shown to predispose to increased ototoxicity. No systematic effect of sex on audiological outcome was found in our study.
The impact on hearing outcome of other pertinent patient-related factors, such as otitis media, cerebrospinal fluid shunt and localization of primary brain tumors was not evaluated in this study because of an unfavorable ratio of the number of variables to the number of participants which precluded valid statistical analysis.5, 9, 11, 12, 15, 16, 30, 31
The treatment-related factor cochlear Dmean was found to have a nonsignificant effect on hearing outcome in this study. However, cochlear Dmean has been shown in other studies to be a statistically-significant factor in determining the incidence and degree of SNHL.1, 3, 7, 12, 32 The evaluation of the pure ototoxic effect of cochlear Dmean in this study was complicated by the additional detrimental effect of cisplatin and/or carboplatin on hearing thresholds. Many studies have led to the suggestion that the cochlear Dmean dose may supersede cisplatin in affecting long-term sensorineural sequelae (see Supplementary Data).3, 5, 9, 29, 30 Current radiation dose constraints for the cochlea (Dmean ≤45 Gy) do not consider the additional ototoxic effect of platin drugs.6, 25 Some authors recommended limiting the cochlear Dmean to ≤35 Gy when platin-based Cth is additionally used.25, 32
Increasing use of cisplatin showed associations with incidence and time-to-onset of HL in univariable and multivariable modeling (Table 3). Thus, as expected, cisplatin is one key factor for HL in this patient group. However, many studies have shown cumulative or median cochlear radiation dose and not cumulative cisplatin dose to be a predicting factor for ototoxicity associated with late onset HL.5, 6, 9, 12, 29, 30 These results lead to the suggestion that the radiation dose may supersede cisplatin in affecting long-term sensorineural sequelae after more than 12 months posttreatment.
Data regarding other treatment-related factors which potentially affect the risk of ototoxicity, such as radiation dose per fraction or the effect of different radiation techniques were too sparse for analysis.5, 9, 11, 12, 15, 16, 30, 31 However, to take into account novel treatment techniques, we have decided to include the treatment year (dichotomized to before or after 2010) as a surrogate parameter. We find that patients treated later than 2010 tend to do slightly better than those treated before in terms of HL in univariable analyses, but association is lost in a multivariable setting (Table 3). This result is unsurprising because the increased use of IMRT techniques in the last decade is likely to reduce cochlea radiotherapy dose. The effect of RT fraction dose on HL is described in detail in the Supplementary Data.
Given the intergroup similarity in the factors age, sex, platin and radiotherapy dosages, and Dmean for cochlea in our study (Table 2), we assume that the therapy sequence is the critical factor in determining the greater posttreatment audiological impairment found in patients who received CRT prior to PCth.
4.3 Potential effects of treatment-related HL
The synergistic ototoxic effect of cranial RT and PCth appears to most severely affect the high frequencies (4-8 kHz) (Table 2).1, 2, 7, 9, 11, 12, 14, 17 In younger children, untreated high-frequency SNHL can impede speech and language development, impair cognitive development and hinder the development of social skills.31, 33, 34 School-age children with HL may suffer reduced ability to understand speech in noise, leading to diminished attention span and worse academic performance.
Ototoxic effects can occur over a longer time-frame, with onset of HL after a median time of 3.6 years in children with brain tumors treated with RT alone, to SNHL continuing to worsen even 20-30 years after diagnosis among childhood cancer survivors.29, 31 Early detection and treatment of SNHL, as well as long-term posttherapeutic audiological monitoring, are therefore necessary in order to reduce considerable risks to the quality of life (QoL) of pediatric cancer patients. Bass et al recommend audiological follow-up every 6 months for the first 5 years post-RT and then annually for at least 5 additional years.31
4.4 Carboplatin
Carboplatin is less potent than cisplatin and higher doses are necessary to achieve a similar antitumor effect.35 As a platinum-based agent, carboplatin, like cisplatin, can potentially induce bilateral, irreversible, progressive, high-frequency SNHL in direct relation to the cumulative dose applied and depending upon patient-associated factors,8, 10, 18-20 and treatment-associated factors.36-40
Previous studies have been unable to determine a threshold dose for carboplatin or an expected time-to-onset of SNHL where carboplatin and CRT are combined. A valuable clue was provided by Keilty et al who found in children and adolescents an additive effect of radio- and chemotherapy on HL with a carboplatin dose of 1000 mg/m2 being associated with an increasing grade of HL.22 Pre- or postirradiation administration of carboplatin, however, could be expected to cause lower rates of HL than cisplatin, with ototoxicity rates of 0%-38% in the speech frequency range reported from carboplatin alone.8, 10, 18, 19, 35
4.5 Clinical perspectives
The clinical implications of our findings are challenging. The sequence of CRT and PCth and their respective doses are strictly regulated by the therapy protocol corresponding to the tumor entity and are, as a rule, based on large prospective studies evaluating therapy effectiveness. No clinical studies to date have compared the effects on treatment outcome of the sequence of CRT and PCth for pediatric CNS and HN tumors. This would be essential before any change to therapy protocol could be proposed.
Considering a change in the sequence of treatment may potentially be appropriate in palliative patients for whom hearing-related QoL has a stronger relevance. Other strategies, such as the use of cochlear-protective measures (eg, proton therapy or rotational intensity-modulated radiation therapy [IMRT]), would be of particular importance for patients receiving CRT before PCth, as would attempts to reduce the ototoxic effect of platinum compounds. In addition, the potential use of otoprotective pharmaceuticals during CRT and PCth, for example, sodium thiosulfate and amifostine, can be considered.41, 42 Such risks should be assessed on the basis of the oncological prognosis in each individual case.
In summary, our results are observational. Before any change is made to the sequence of CRT and PCth prescribed by therapeutic protocols with the aim of reducing the risk of HL in pediatric CNS and HN cancer patients, assessment of treatment effectiveness in large prospective trials with rigorous collection of both treatment data and outcome data is necessary.
4.6 Strengths and limitations
As a retrospective multicenter study, we were able to draw on a comparatively large sample but had less control over the quality and precision of some data recorded. There was a risk of potential selection bias due to the inclusion of patients with heterogeneous tumor entities and treatment protocols. Moreover, there were differences in radiation technique and radiation treatment plan implemented despite similar tumor localization. Data regarding such patient-related factors as post-RT otitis media, cerebrospinal fluid shunt and exact intracranial localization of primary brain tumors, as well as treatment-related factors such as radiation dose per fraction and applied radiation techniques were not sufficiently available in our dataset.
Posttreatment audiological monitoring could not be followed systematically in this retrospective dataset. Because audiological tests were conducted for surveillance, rather than in response to reported symptoms of HL, it would be more accurate to describe the time to onset analysis as time to confirmed identification of HL analysis.
4.7 Key findings
The key findings to emerge from the present study are as follows: (i) the incidence of clinically-relevant HL was greater in children treated with CRT before PCth than vice versa; (ii) children treated with CRT before PCth developed a significantly greater degree of SNHL in the high speech-frequency range (4-8 kHz) than those treated with CRT after PCth; (iii) the onset of clinically-significant SNHL (≥2b) was significantly earlier in children treated with CRT before PCth than vice-versa; (iv) the cumulative cisplatin dose has a bearing for the difference in audiological outcome between the groups; (v) age, sex, mean cochlear radiation dose and cumulative carboplatin dose had no significant effect on the audiological outcomes of either therapy group.
5 CONCLUSION
Children receiving CRT prior to PCth face a greater risk of ototoxicity than those receiving treatment in the reverse order (72.7% vs 33.9%, respectively). This finding should be further evaluated and considered within clinical practice in order to minimize hearing loss in children and adolescents with CNS and HN cancer.
AUTHOR CONTRIBUTIONS
The work reported in the paper has been performed by the authors, unless clearly specified in the text. Sergiu Scobioala: Term, Conceptualization, Methodology, Investigation, Writing—Original Draft, Data Curation. Ross Parfitt: Term, Conceptualization, Methodology, Investigation, Writing—Original Draft, Data Curation. Peter Matulat: Term, Conceptualization, Methodology, Formal analysis, Software, Data Curation. Julianne Byrne: Conceptualization, Methodology, Formal analysis, Writing—Review & Editing, Methodology, Formal analysis. Thorsten Langer: Writing—Review & Editing, Project administration, Formal analysis, Software. Fabian M. Troschel: Methodology, Writing—Review & Editing. Amélie Hesping: Writing—Review & Editing. Eva Clemens: Writing—Review & Editing. Peter Kaatsch: Project administration, Formal analysis, Writing—Review & Editing. Desiree Grabow: Data Curation. Melanie Kaiser: Data Curation. Claudia Spix: Data Curation. Leontien C. Kremer: Writing—Review & Editing. Gabriele Calaminus: Writing—Review & Editing. Katja Baust: Writing—Review & Editing. Claudia Kuehni: Methodology, Writing—Review & Editing. Annette Weiss: Methodology, Writing—Review & Editing. Sven Strebel: Writing—Review & Editing. Rahel Kuonen: Writing—Review & Editing. Susanne Elsner: Writing—Review & Editing. Riccardo Haupt: Writing—Review & Editing. Maria-Luisa Garré: Writing—Review & Editing. Bernd Gruhn: Writing—Review & Editing. Tomas Kepak: Writing—Review & Editing. Katerina Kepakova: Writing—Review & Editing. Jeanette Falck Winther: Writing—Review & Editing. Line Kenborg: Methodology, Writing—Review & Editing. Catherine Rechnitzer: Writing—Review & Editing. Henrik Hasle: Writing—Review & Editing. Jarmila Kruseova: Writing—Review & Editing. Ales Luks: Writing—Review & Editing. Herwig Lackner: Writing—Review & Editing. Stefan Bielack: Writing—Review & Editing. Jörn-Dirk Beck: Writing—Review & Editing. Heribert Jürgens: Writing—Review & Editing. Marry M. van den Heuvel-Eibrink: Methodology, Writing—Review & Editing. Oliver Zolk: Methodology, Formal analysis, Project administration, Writing—Review & Editing. Hans Theodor Eich: Methodology, Writing—Review & Editing. Antoinette am Zehnhoff-Dinnesen: Term, Conceptualization, Methodology, Investigation, Writing—Original Draft, Data curation, Project administration.
ACKNOWLEDGEMENTS
We want to acknowledge our special gratitude to Docent Dirk Deuster MD who has made crucial contributions to this work. Docent Deuster passed away in 2021 at age 50 and we will always remember his dedication to his patients and his research. His example continues to be an inspiration for our ongoing work. We want to thank the audiology assistants Monika Kleikamp and Gabriele Overmann for their untiring and valuable help to accomplish this study. We thank Mrs Kylie O'Brien for the activity in the PanCare network, providing administrative services for at least three different consortia. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This work was part of the PanCareLIFE project. PanCareLIFE is an EU FP7 project (Project No. 602030) and was funded by the FP7-HEALTH-2013-INNOVATION-1 HEALTH.2013.2.4.1-3 caLL: Investigator-driven supportive and palliative care clinical trials and observational studies HEALTH. In Switzerland, data collection and work for this publication was funded by the Swiss Cancer League and the Swiss Cancer Research Foundation (Grant Nos. KLS-3412-02-2014, HSR-4951-11-2019 and KLS/KFS-5711-01-2022).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
All local ethical committees have approved the use of the collected data from each institution for inclusion in this project, and written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
Individual PanCareLIFE patient data are not publicly available due to potential identification of individuals. PanCareLIFE data, aggregated for the purposes of the improvement of long-term care regarding fertility, ototoxicity and health-related QoL after cancer therapies, is available to approved researchers. Access to this and other anonymized, aggregated data may be granted under conditions agreed with the PanCareLIFE Publication Committee/Executive Board, and with appropriate data sharing agreements and permissions from data providers in place. All outputs are subject to the codes of practice for official statistics. Further information is available from the corresponding author upon request.