A perivascular niche in the bone marrow hosts quiescent and proliferating tumorigenic colorectal cancer cells
Abstract
Disseminated tumor cells (dTCs) can frequently be detected in the bone marrow (BM) of colorectal cancer (CRC) patients, raising the possibility that the BM serves as a reservoir for metastatic tumor cells. Identification of dTCs in BM aspirates harbors the potential of assessing therapeutic outcome and directing therapy intensity with limited risk and effort. Still, the functional and prognostic relevance of dTCs is not fully established. We have previously shown that CRC cell clones can be traced to the BM of mice carrying patient-derived xenografts. However, cellular interactions, proliferative state and tumorigenicity of dTCs remain largely unknown. Here, we applied a coculture system modeling the microvascular niche and used immunofluorescence imaging of the murine BM to show that primary CRC cells migrate toward endothelial tubes. dTCs in the BM were rare, but detectable in mice with xenografts from most patient samples (8/10) predominantly at perivascular sites. Comparable to primary tumors, a substantial fraction of proliferating dTCs was detected in the BM. However, most dTCs were found as isolated cells, indicating that dividing dTCs rather separate than aggregate to metastatic clones—a phenomenon frequently observed in the microvascular niche model. Clonal tracking identified subsets of self-renewing tumor-initiating cells in the BM that formed tumors out of BM transplants, including one subset that did not drive primary tumor growth. Our results indicate an important role of the perivascular BM niche for CRC cell dissemination and show that dTCs can be a potential source for tumor relapse and tumor heterogeneity.
Abstract
What's new?
Prior studies indicate that perivascular bone marrow niche can induce quiescence of disseminated tumor cells (dTCs), making bone marrow a suspected dTC reservoir. In this study, quiescent and proliferating colorectal cancer (CRC) cells were detected in the perivascular bone marrow niche of mice harboring patient-derived xenografts. Xenotransplantation and clonal tracking of dTCs in the bone marrow revealed distinct tumor-initiating cell subsets and demonstrated the ability of dTCs to serve as a cell source for tumor relapse and tumor heterogeneity. These functional data should prompt further studies to better define the prognostic value of dTCs in the clinical setting.
List of Abbreviations
-
- BM
-
- bone marrow
-
- CD
-
- cell diameter
-
- CMV
-
- cytomegalovirus
-
- CRC
-
- colorectal cancer
-
- dTC
-
- disseminated tumor cell
-
- ECGM2
-
- endothelial cell growth medium
-
- EDTA
-
- ethylenediaminetetraacetic acid
-
- EGFP
-
- enhanced green fluorescent protein
-
- EPCAM
-
- epithelialcell adhesion molecule
-
- ET
-
- endothelial tube
-
- HSC
-
- hematopoietic stem cell
-
- HUVEC
-
- human umbilical vein endothelial cell
-
- IF
-
- immunofluorescence
-
- IS
-
- insertion site
-
- LAM-PCR
-
- linear amplification-mediated polymerase chain reaction
-
- MANOVA
-
- multivariateanalysis of variance
-
- MEM
-
- minimal essential medium
-
- MSC
-
- mesenchymal stroma cell
-
- NSG
-
- NOD.Cg-Prkdc scidIl2rg tm1Wjl/SzJ
-
- OPN
-
- osteopontin
-
- PBS
-
- phosphate-buffered saline
-
- PDX
-
- patient-derived xenograft
-
- SNP
-
- single nucleotide
-
- TIC
-
- tumor-initiating cell
-
- TSC
-
- tumor spheroid culture
Introduction
Worldwide, colorectal cancer (CRC) is the second leading cause of cancer-related death.1 Depending on stage and localization, local CRC is treated with surgery, radiotherapy and/or chemotherapy. Improvement of resection and radiation techniques has limited local relapse rates to less than 10%.2 In a substantial proportion of CRC patients, however, relapse occurs with distant metastases, adding to an overall relapse rate of up to approximately 50%.2 In these patients, individual tumor cells must have disseminated early, not detectable by imaging at the time of diagnosis, and thereby escaped local treatment approaches.
In a subset of CRC patients with local tumors, disseminated tumor cells (dTCs) can be found in peripheral blood and bone marrow (BM) already at the time of surgery, and the detection of rare dTCs in the BM was associated with poor prognosis in several clinical studies.3 Still, the prognostic value of CRC dTCs is not completely understood, as some clinical studies failed to show a significant impact of dTC numbers on patient outcome, possibly due to heterogeneous study designs.3
Liver and lungs are much more frequently affected by distant CRC metastasis compared to the BM. Yet, BM aspirates can be obtained from patients with very limited effort and risk of complications and might allow for identifying patients at risk for relapse before overt metastases occur.
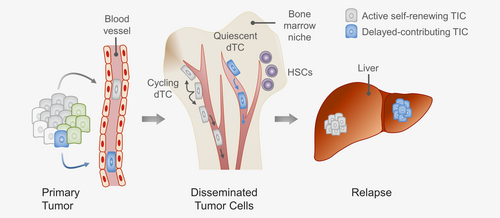
The BM constitutes a complex microenvironment that maintains and controls hematopoietic stem cell (HSC) activity. HSCs frequently localize near blood vessels, and the perivascular niche has been identified as an important regulator of HSCs.4, 5 Moreover, dTCs can be detected in the BM of breast cancer patients, and the perivascular BM niche can induce dTC quiescence.6
We and others have previously shown that only a small fraction of stem cell-like tumor-initiating cells (TICs) within individual CRC tumors drives tumor and metastasis formation.7, 8 Using genetic barcoding and a clonal tracking approach in a xenotransplantation model, we demonstrated that TIC clones disseminate from the primary tumor to the BM.8 Thus, it is conceivable that the BM might serve as a reservoir for metastatic CRC TICs.
Most existing studies on CRC dTCs focus on their detection and quantification in BM aspirates.9 This strategy precludes the analysis of dTCs in their cellular context in the BM and limits their functional investigation.
Here, we used an organotypic microvascular niche model to examine whether patient-derived CRC cells interact with endothelial cells in vitro. In a patient-derived xenograft (PDX) model, we analyzed the localization and proliferative activity of dTCs in the BM niche in vivo using confocal immunofluorescence (IF) imaging. To assess tumorigenicity and self-renewal capacity of individual dTCs, we performed serial xenotransplantations of the BM from PDX-carrying mice and used genetic barcoding to track individual TIC clones in primary tumors, BM and serial dTC-derived tumors. Thereby, we provide novel insights into the metastatic cascade of CRC and functional evidence for the potential prognostic relevance of dTCs for CRC patients.
Materials and Methods
Purification and culture of primary human CRC cells
Human tissue samples were obtained in compliance with the declaration of Helsinki and informed consent was received from every patient, as approved by the institutional Ethics Review Board. CRC samples from 10 patient samples (Supporting Information Table S1) were received from the University Hospital Heidelberg. Tumors were processed and tumor cells cultivated as tumor spheroid cultures (TSCs) as previously described.10 All TSCs were authenticated using single nucleotide polymorphism (SNP) profiling within the last 3 years (Multiplexion, Heidelberg, Germany). All experiments were performed with mycoplasma-free cells.
Generation and imaging of microvascular niche cultures
BM-derived mesenchymal stroma cells (MSCs) were received from the Paracelsus University Hospital Salzburg and expanded as previously described,11 modified by supplementation of the minimal essential medium (MEM) alpha (#22561; Gibco, Carlsbad, CA) with 2.2 mM dipeptamin (Fresenius Kabi, Bad Homburg vor der Höhe, Germany). Microvascular niche cultures were generated as described.6, 12 Per well of a 96-well plate, 7 × 103 mCherry- or enhanced green fluorescent protein (EGFP)-expressing primary human umbilical vein endothelial cells (#FC-0003; HUVECs, CellSystems, Troisdorf, Germany) were cocultivated with 5 × 104 MSCs in endothelial cell growth medium (#C-22011; ECGM2, PromoCell, Heidelberg, Germany) to obtain an ideal density of endothelial tubes (ETs) as determined by prior testing of different cell ratios and cell media. Tube formation was monitored by fluorescence microscopy (Zeiss, Oberkochen, Germany; Axiovert 200, AxioCam MRm and AxioVision).
For tumor cell tracking, 100 singularized EGFP+ TSC cells were added 5 days after seeding of mCherry+ HUVECs and nonlabeled MSCs. Next, either pure ECGM2 or a 1:1 mixture of ECGM2 and matrigel (#354248; Corning, Corning, NY) were added to the wells. For matrigel solidification, the plate was incubated for 30 min at 37°C. Afterward, first images were taken (t0).
Time-lapse imaging of whole wells was performed in seven 5-hr intervals after addition of tumor cells and after 85 hr using confocal laser-scanning fluorescence microscopy (Zeiss, Oberkochen, Germany; LSM 710 ConfoCor 3 and ZEN2010). Five images per well and time point covering a z-stack of 24 μm were merged and analyzed (Fiji, see also Supporting Information Methods S1 and S2).
Xenotransplantation of primary CRC cells
Approved by the institution's animal care and ethical committee, NOD.Cg-Prkdc scidIl2rg tm1Wjl/SzJ (NSG) mice were obtained from the Jackson Laboratory and housed according to all applicable laws and regulations. Xenografts were generated as described before.10 For BM transplantations, 1 × 106 to 2 × 107 whole BM cells were resuspended in 50 μl of a 1:1 matrigel-medium mixture and injected under the kidney capsule of 31 NSG mice. Mice were sacrificed 8–16 weeks after transplantation. Xenografts were assessed by pathologists as described.10
IF staining of femur bone sections
Whole murine femur bones were fixed and decalcified as described13 and sectioned using the CryoJane Tape Transfer System (#39475206; Leica Biosystems,Wetzlar, Germany). Femur slides were permeabilized using a 0.1% Tween-20 solution (#P9416; Sigma-Aldrich, St. Louis, MO), incubated with primary antibodies, followed by incubation with fluorescence-labeled secondary antibodies. DNA was stained using Hoechst 33342 (#H1399; Thermo Fisher Scientific, Waltham, MA). Analysis was performed by conventional (Zeiss, Axio Scan.Z1 and ZEN2) and confocal fluorescence microscopy (Leica Microsystems, Wetzlar, Germany; SP5 and LAS AF). Antibodies are listed in Supporting Information Table S2.
Purification of BM cells
Femur bones were harvested and BM was flushed out using phosphate-buffered saline (PBS) (#14190; Gibco,Carlsbad, CA) supplemented with 1% penicillin/streptomycin (#15140; Gibco). After centrifugation, the pellet was resuspended in erythrocyte lysis buffer (150 mM ammonium chloride [#09718; Sigma-Aldrich], 10 mM potassium hydrogen carbonate [#P748; Roth, Karlsruhe, Germany], 100 μM disodium ethylenediaminetetraacetic acid [EDTA] [#E6635; Sigma-Aldrich]), centrifuged and resuspended in PBS.
Spheroid-forming assays
A total of 1 × 106 to 2 × 107 purified BM cells from PDX mice potentially comprising dTCs were cultivated in TSC conditions. Medium was changed twice a week for 4–16 weeks. Cultures were monitored weekly for EGFP+ tumor spheroid formation.
Lentivirus production and clonal tracking
Lentiviral vectors encoding EGFP or mCherry under the control of the human cytomegalovirus (CMV) promoter were generated as described.8 Transductions were performed at multiplicities of infection from 1 to 100. For clonal tracking linear amplification-mediated polymerase chain reaction (LAM-PCR) analysis was performed on 50–500 ng of DNA isolated from xenografts and BM cells using the restriction enzyme MluCI (#R0538; New England Biolabs, Ipswich, MA) as described.14, 15 LAM-PCR amplicon sequences were separated according to the introduced barcode, trimmed and aligned to human genome assembly (HG19, GRCh37).
Statistical analyses
dTCs were categorized (direct attachment, distance up to and distance more than two cell diameters to endothelial cells), and were analyzed as compositional data. To describe the relationship between the relative frequency of attached TSC cells and time in the microvascular niche cocultures a linear mixed model with random effect for replicate was used.
For analysis of the dTC distribution in whole femur slides a random distribution of events was simulated. For this purpose, the EGFP signal of EGFP-tagged tumor cells in the BM was arbitrarily shifted along the longitudinal BM axis. The localization of shifted tumor cells in the BM was compared to the detected distribution of dTCs globally and pairwise using multivariate analysis of variance (MANOVA).
Proliferation events and proportions of Ki67-stained cells were compared using t-tests as specified. p-Values below 0.05 were considered significant. Calculations were performed with R version 3.4.3 and R package compositions.
Data availability statement
The data that support the findings of this study will be made available from the corresponding authors upon reasonable request.
Results
CRC cell localization in a microvascular niche model
To analyze a potential interaction between primary human CRC cells and primary human endothelial cells in vitro, we adapted a previously described organotypic microvascular niche model using defined culture conditions that favor cultivation of both HUVECs and TSCs.6, 12 mCherry-expressing HUVECs were cocultivated with primary human MSCs, which stopped HUVECs from proliferating and induced the formation of an organized capillary-like endothelial network after approximately 5–11 days (Fig. 1 a). In control experiments without MSCs, endothelial network formation did not appear (Fig. 1 a).
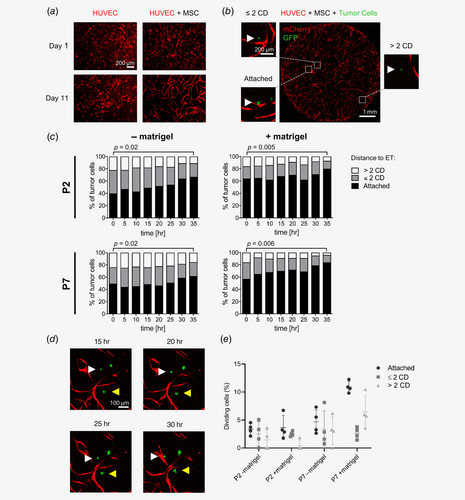
To study interactions of primary TSC cells with HUVECs in this model, two patient-derived CRC TSCs with a metastatic phenotype in previous xenotransplantation experiments were chosen (P2 and P7). Formation of PDX from P2 and P7 under the kidney capsule of NSG mice were frequently accompanied by distant metastases to the spleen (P2) or multiple organs (P7; Supporting Information Fig. S1). Singularized EGFP+ TSC cells from P2 and P7 were added to the coculture 5 days after seeding of MSCs and HUVECs, which allowed for assessing interactions of EGFP+ TSC cells with the mCherry+ ET network by fluorescence microscopy. In parallel, matrigel was added to the coculture to assess a potential effect of extracellular matrix components on localization and migration of tumor cells and to exclude diffusion-mediated effects.
Using automated time-lapse imaging and tumor cell tracking four cocultures per condition (P2 and P7, with and without matrigel) were analyzed for 35 hr and 16–86 (median 48) TSC cells were monitored per coculture (Supporting Information Method S1). Three different categories were used to describe distances between TSC cells and ETs: direct attachment, close colocalization (distance up to two tumor cell diameters [CDs]) and no colocalization (distance more than two CDs; Fig. 1 b, Supporting Information Table S3).
In cocultures without matrigel, 33–50% (P2) and 40–65% of TSC cells (P7) directly attached to ETs at the first time point (t0), 36–40% (P2) and 22–31% of TSC cells (P7) were localized within two CDs, and 13–27% (P2) and 13–29% of TSC cells (P7) more than two CDs distant (Fig. 1 c, Supporting Information Table S3). In cocultures with addition of matrigel, 47–86% (P2) and 47–66% of TSC cells (P7) were attached to ETs (t0), 5–27% (P2) and 25–29% of TSC cells (P7) were located within two CDs and 7–32% (P2) as well as 5–28% of TSC cells (P7) more than two CDs distant (Fig. 1 c, Supporting Information Table S3). Importantly, the proportion of cells directly attached to ETs consistently increased over 35 hr across both patient samples and coculture conditions, which was significant when comparing t0 and t35 (Fig. 1 c, Supporting Information Table S3), was afterward stable in cocultures without matrigel and even more pronounced in cocultures with matrigel (t85, Supporting Information Table S3).
To assess whether this observation could be explained by migration of TSC cells toward ETs or selective survival, individual TSC cells were followed over time, demonstrating multiple TSC cells, initially more than two CDs distant from HUVECs, approaching and finally attaching to HUVECs (Fig. 1 d). Notably, the equally increasing proportion of TSC cells attached to ETs over time after addition of matrigel to the coculture argues against a diffusion-mediated effect.
As absolute numbers of TSC cells attached to ETs increased over time proliferation events in each category were compared (Supporting Information Method S2). This comparison demonstrated a trend toward higher proliferation activity of TSC cells attached to ETs versus cells up to or more than two CDs distant from ETs, which was only significant for P7 after addition of matrigel (p = 0.006; two-sided, paired t-test; Fig. 1 e, Supporting Information Table S4). Thus, increase of TSC cells attached to ETs over time resulted from migration toward ETs and a by trend higher proliferative activity of TSC cells at ETs. In summary, these findings suggest that TSC cells migrate toward, preferentially localize and proliferate close to ETs in this coculture model in vitro.
Detection of colorectal dTCs in the BM
As the in vitro model does not recapitulate blood flow and migration of tumor cells toward ETs that occur from outside of the ET lumen, we aimed to analyze the localization of dTCs in the BM in their cellular context in vivo. Thus, immunohistological BM examination was performed using a xenotransplantation model. We have previously demonstrated that CRC TIC clones marked with individual barcodes can be traced to the BM after xenotransplantation of primary TSC cells under the kidney capsule of NSG mice.8 This injection site has been shown to be more sensitive for the detection of tumorigenic cells than conventional xenotransplantation sites.16 Thus, TSCs derived from 10 different CRC patient samples were transduced with an EGFP-encoding lentiviral vector and 104 to 106 marked TSC cells were injected under the kidney capsule of a total of 46 NSG mice (Fig. 2 a, Supporting Information Table S1). Transduction efficiencies varied from 80 to 98% (mean 91%) depending on the individual TSC. After 8–16 weeks, tumor formation occurred in all 46 transplanted mice.
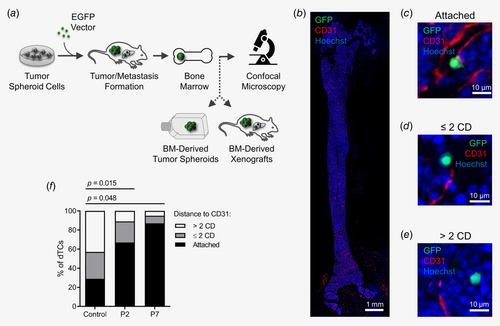
To identify dTCs in the BM, cryosections of murine femur bones were generated, whole femur slides were stained using antibodies against EGFP and analyzed by confocal IF microscopy (Fig. 2 b). Complementarily, antibodies against the epithelial cell adhesion molecule (EPCAM) were applied to detect rare EGFP−EPCAM+ dTCs. Per patient sample, 1–11 mice (mean 4.6) were transplanted and at least four whole femur slides were analyzed (Supporting Information Table S1).
Remarkably, dTCs were identified in PDX mice from 8 out of 10 patient samples. A total of 1018 single dTCs with up to 38 dTCs per whole femur slide (median per mouse 3.5 dTCs/slide) were detected in the BM of PDX mice from these patient samples (Supporting Information Table S1). As expected, the highest numbers of single dTCs per slide were identified in mice with PDXs from P2 (median per mouse 8.6 dTCs/slide, range 3.1–10.8) and P7 (median per mouse 4.3 dTCs/slide, range 3.2–7.8), which reflected the high metastatic activity of these TSCs in the xenograft model (Supporting Information Fig. S1 and Table S1). Collectively, these observations illustrate that dTCs are rare, but can be identified by IF in xenografts from most CRC patients.
Localization of colorectal dTCs in the BM
Next, the localization of detected dTCs in the BM was assessed. dTCs were distributed across the trabecular region of the BM and the diaphysis. Strikingly, 1018 identified dTCs were found as solitary cells. Only three mice—all transplanted with TSC cells from P7—harbored in total one spot in the BM with a cluster of seven dTCs and four spots with clonal metastatic outgrowth each consisting of more than 100 tumor cells (Supporting Information Fig. S2). Otherwise, neither further dTC clusters nor any other spot with a metastatic clone were identified in the BM of PDX mice.
The majority of all detected dTCs was localized at extravascular sites. To quantitatively analyze the localization of dTCs we focused on BM of PDX mice from P2 and P7, as in the BM of these mice sufficient numbers of single dTCs were identified (P2: 652 dTCs; P7: 349 dTCs; Supporting Information Table S1). Previously defined categories were used to describe the distance between single dTCs and endothelial cells. Metastatic spots were excluded from quantitative analysis, as the BM niche was overgrown by tumor cells within these spots, which would have significantly biased the localization analysis of solitary dTCs.
In femur sections, 64–71% (P2) and 68–93% of dTCs (P7) localized directly attached to CD31+ endothelial cells, 20–27% (P2) and 5–16% of dTCs (P7) within two CDs, and 9–16% (P2) and 2–16% of dTCs (P7) more than two CDs distant (Figs. 2 b–2 f, Supporting Information Fig. S3 and Table S5). The distribution of dTCs in the BM according to the three categories was compared to a random distribution of events in the BM, which was significantly different (P2: p = 0.015; P7: p = 0.048; Figs. 2 b–2f, Supporting Information Table S5), strongly arguing against a stochastic distribution of dTCs at perivascular sites. These findings clearly demonstrate that primary CRC dTCs preferentially disseminate to perivascular sites in this xenograft model in vivo.
Colorectal dTCs and the HSC niche
As HSCs are frequently localized adjacent to blood vessels in the BM4, 5 and prostate cancer cells have been shown to competitively disseminate to HSC niches,17 we wondered whether perivascular regions occupied by dTCs were shared with HSCs.
HSCs have been shown to be enriched in the CD150+ and CD48− population and do not express lineage markers (CD150+CD48−lin−).18 In the BM of PDX mice, 5–7% (P2) and 5–7% of dTCs (P7) localized attached to CD150+CD48−lin− cells, 3–14% (P2) and 5–22% (P7) of dTCs within two CDs, and 79–92% (P2) and 71–89% of dTCs (P7) were detected more than two CDs distant (Figs. 3 a and 3 b, Supporting Information Table S5). Comparison of the distribution of dTCs in the BM to a random distribution of events according to the three categories was not significantly different (p = 0.19; Fig. 3 b). Thus, only a minor subfraction of dTCs shared a niche with HSCs, while most of the dTCs localized independently in the perivascular BM niche.
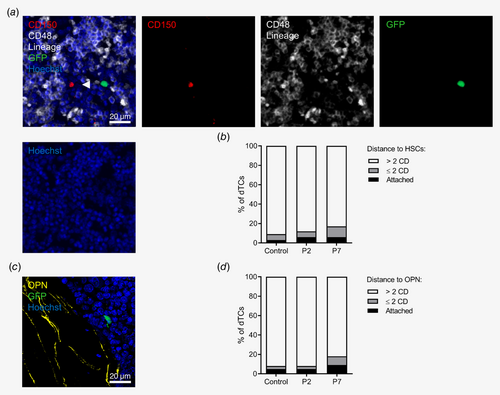
Colorectal dTCs and the subendosteal BM niche
Still matter of ongoing debate, the endosteal region is supposed to provide an additional and/or alternative niche for the maintenance of HSCs.5 In the BM of PDX mice, 3–9% (P2) and 4–14% of dTCs (P7) were attached to the osteopontin+ (OPN) endost, 0–16% (P2) and 6–18% of dTCs (P7) were localized within two CDs, and 81–93% (P2) and 68–91% of dTCs (P7) were more than two CDs distant (Figs. 3 c and 3 d, Supporting Information Table S5). The distribution of dTCs from both P2 and P7 in relation to the endost was compared to a random distribution of events in the BM according to the three categories and was not significantly different (p = 0.18). These results suggest that—despite sharing a preference for perivascular sites with HSCs—CRC dTCs do rarely occupy subendosteal regions in the BM.
Proliferative activity of colorectal dTCs in the BM
Dormancy of breast cancer cells in the perivascular BM niche has been shown to be induced by endothelium-derived signals.6 To analyze the proliferative activity of primary CRC dTCs in the BM, cryosections of femur bones were stained for Ki67 and analyzed by IF imaging (Figs. 4 a and 4 b). The frequency of cycling dTCs (Ki67+) in the BM of PDX mice from P2 ranged from 5 to 28% of dTCs (mean 15%; Fig. 4 c, Supporting Information Table S6). To assess BM-related changes in proliferative activity, Ki67+ tumor cells in primary xenografts were compared to Ki67+ dTCs from the respective patient sample in the BM. In primary PDXs from P2, Ki67+ tumor cells ranged from 13 to 30% of tumor cells (mean 21%), which was similar to the Ki67+ fraction of dTCs in the BM (Fig. 4 c, Supporting Information Table S6). Ki67+ dTCs in the BM of PDX mice from P7 ranged from 28 to 41% of dTCs (mean 34%; Fig. 4 d, Supporting Information Table S6) similar to the Ki67+ fraction of tumor cells between 30 and 39% (mean 34%) in primary xenografts from the same patient sample (Fig. 4 d). Ki67+ tumor cells in three spots with a metastatic clone in the BM ranged from 43 to 73% (mean 54%), which tended to be even higher than in primary tumors from the same patient sample (Fig. 4 d, Supporting Information Table S6). Interestingly, the proliferation activity of dTCs was similar in the different localization categories of dTCs related to CD31+ endothelial cells (Fig. 4 e, Supporting Information Table S6).
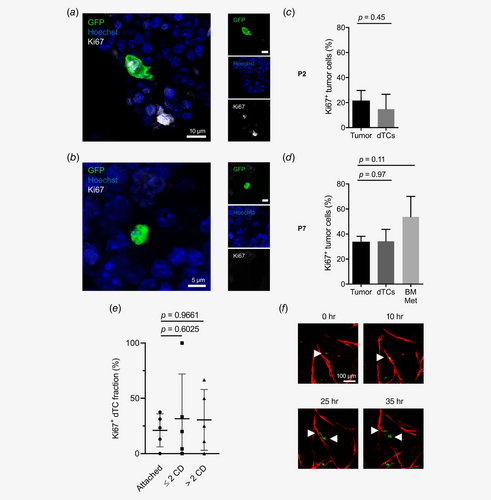
In summary, analysis of the proliferative activity of primary CRC dTCs in the BM revealed a majority of quiescent, but a substantial subfraction of actively cycling dTCs. The proportion of cycling and quiescent dTCs in the BM was not significantly different from the primary tumor and not dependent on the distance from the BM endothelium, arguing against induction of quiescence in these dTCs by signals exclusive for the perivascular BM niche.
Proliferation and separation of colorectal dTCs in the perivascular niche
Surprisingly, the cycling activity of a substantial subfraction of CRC dTCs in the BM was in stark contrast to the observation that—except for one spot with a dTC cluster and four spots with metastatic clones—1018 identified dTCs across eight different patient samples were found as isolated single cells.
Typically, tight junctions between individual tumor cells are established within an individual proliferating tumor cell clone in epithelial cancers,19 leading to the formation of compact tumor spheroids in vitro and solid tumor bulks in vivo. To explain the overt discrepancy of numerous cycling but solitary dTCs, we hypothesized that after proliferation of perivascular dTCs both daughter cells must have separated, and one daughter cell might have reentered the circulation. To obtain evidence for this hypothesis, we focused on proliferation events in our in vitro microvascular niche model. We indeed observed that the majority of proliferation events at the endothelium showed tumor cells dividing after approaching ETs, directly followed by separation of the daughter cells and migration into opposite directions along the endothelium (Fig. 4 f).
Self-renewal of disseminated CRC cells
To test whether dTCs in the BM of mice used for histological analysis included functional TICs that can be a potential source of secondary metastasis or only “bystanders” representing aggressive tumor growth, BM of these mice was harvested in parallel. Per BM sample, 1 × 106 to 2 × 107 purified BM cells were cultivated in serum-free medium supplemented with growth factors, which favors the proliferation and maintenance of TICs, and the formation of tumor spheroids. In total, BM from 56 PDX mice from 10 different patient samples was assessed. Corresponding to the highest frequency of dTCs in PDX mice from P2 and P7 as measured by IF, EGFP+ tumor spheroid formation occurred in BM cell cultures derived from P2 and P7 (Fig. 5 a and Supporting Information Table S1). In addition, 1 out of 11 BM cell cultures derived from P3 revealed tumor spheroid formation, while no dTCs were identified by IF imaging in these mice. No tumor spheroid formation was observed in BM samples from PDX mice derived from other patient samples.
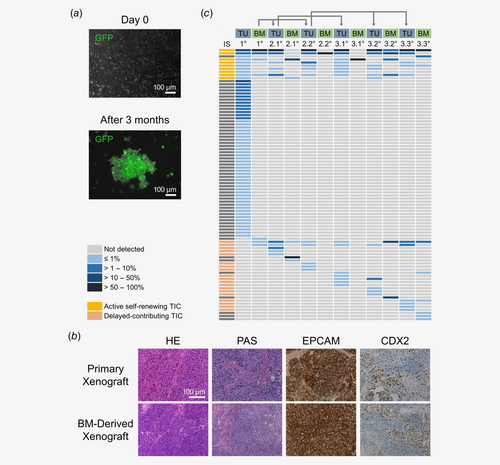
Even though tumor spheroid-forming capacity is supposed to be restricted to TICs, direct evidence that spheroid-forming cells and TICs represent identical tumor cell populations is lacking. To probe tumor-forming capacity of dTCs in the BM, 1 × 106 to 2 × 107 BM cells freshly purified from BM of PDX mice from P2, P3 and P7 were transplanted under the kidney capsule of NSG mice. Tumor formation occurred after 2–4 months in mice transplanted with BM cells of PDX mice from P2 and P7, but not from P3 (Supporting Information Table S1). Notably, histological and immunohistochemical analyses revealed a growth pattern and expression of colorectal adenocarcinoma markers in these secondary xenografts closely resembling the primary xenograft as well as the original patient tumor (Fig. 5 b).
To assess self-renewal potential of TICs in the BM, 3 × 105 to 1.3 × 107 tumor cells purified from secondary xenografts were transplanted into third generation NSG mice. Tumor engraftment was observed in 4 out of 4 transplanted tertiary xenografts from P2 and P7, respectively (Supporting Information Table S1). In summary, these observations indicate that, albeit rare, functional and self-renewing TICs are present in the BM of PDX mice.
Long-term and delayed-contributing TIC types in the BM
Based on the semirandom integration pattern of lentiviral vectors into the host cell genome, lentiviral transduction can serve as genetic barcoding of individual cells. Highly sensitive insertion site (IS) analysis by LAM-PCR allows tracking of individual lentivirally marked cells and assessing their clonal output in vivo.14, 20 We have previously used genetic barcoding of primary CRC cells in serial xenotransplantation models to identify distinct types of TICs in colorectal tumors and metastases.8
To assess clonal dependencies of primary tumor and dTCs in the BM and to analyze which TIC types are present in the BM, DNA was isolated from a primary xenograft, the corresponding BM as well as from BM-derived secondary and tertiary tumors which formed under the kidney capsule in a transplantation series with xenografts from P2. Tumor cells that formed these tumors had been transduced with an EGFP-encoding lentiviral vector with an efficiency of approximately 80% and had been transplanted directly after transduction to avoid cell culture-mediated clonal selection.
In the primary xenograft 61 unique ISs were detected by IS analysis, corresponding to multiple TIC clones that have contributed to tumor formation (Fig. 5 c). Notably, only seven of these ISs were identified in the corresponding BM, confirming that only a subset of all TIC clones disseminated to the BM. Of those, six ISs were detected in secondary and/or tertiary tumors, indicating that indeed active self-renewing long-term TICs were present in the primary tumor and the BM and drove tumor formation in secondary and tertiary tumors.
Analysis of the IS repertoire in BM-derived secondary and tertiary tumors revealed 10–55 ISs representing multiple TIC clones forming these tumors. Most of these ISs (45–53%) were also detected in the original primary tumor. Still, across two secondary and three tertiary tumors, 5–7 ISs per tumor were identified that could not be detected in the original tumor, indicating that these clones had not substantially contributed to the primary tumor (Fig. 5 c). Thus, individual TICs must have disseminated from the primary tumor to the BM without quantitatively contributing to initial tumor growth, which prevents their detection by IS analysis. In secondary or tertiary BM-derived tumors these delayed-contributing TICs became active and substantially drove tumor growth. These observations suggest that dTCs in the BM comprise distinct TIC types with active self-renewing TICs maintaining primary and BM-derived tumor growth, whereas delayed-contributing TICs drive tumor formation substantially only in subsequent generations of BM-derived xenografts and not in the primary xenograft (Fig. 5 c).
Discussion
Understanding the relevance of dTCs for relapse of CRC patients might allow for more precisely assessing efficacy of adjuvant treatment strategies and guide treatment intensity based on dTC numbers.9 Monitoring of circulating and dTCs in patients can be achieved by “liquid biopsies,” which provide access to peripheral blood and BM.21-23 In contrast, solid organs like liver and lungs that represent the most frequent sites of distant metastasis in CRC patients do not allow a systematic analysis prior to overt metastatic lesions.
Solitary dTCs are present in the BM of a substantial number of CRC patients and were associated with poor outcome in several clinical studies.3 Still, their prognostic role is not fully defined. We here extend existing studies beyond correlation of dTC frequencies and prognosis and examine the functional relevance of CRC dTCs and their cellular context in more detail.
We found CRC dTCs in the BM of PDX mice from 8 out of 10 analyzed patient samples, and the frequency of dTCs reflected metastatic activity in our xenograft model. Importantly, the detection of dTCs in BM aspirations of CRC patients argues against a limitation of this observation to our xenograft model. Still, species-specific differences cannot be excluded, as they are inherent to xenograft models. Moreover, xenotransplantation of patient-derived cells into mice requires an efficiently depleted immune system, which precludes assessment of cells of the immune system interacting with dTCs in this model.
Our CRC patient cohort comprises primary tumors as well as metastatic lesions. Notably, we and others have previously shown that distant metastases are heterogeneous and also contain nonmetastatic cells.8 Thus, our findings cannot be assigned to either primary or metastatic tumors. No obvious parameter including tumor stage, tumor origin (Supporting Information Table S1), tumor grade, microsatellite status, mutation or gene expression profile (data not shown) fully explained why P2 and P7 TSCs metastasized more in our model than those derived from other patients. Still, intratumor heterogeneity as well as heterotopic tumor cell localization or species limitations might explain why tumor cells derived from metastatic lesions did not metastasize in our xenograft model. To which degree our findings can be generalized to less metastatic tumor cells remains to be determined.
Our collection of TSCs is derived from rather advanced tumors. However, injection of tumor cells under the kidney capsule offers the advantage of avoiding artificially high numbers of circulating tumor cells as a potential confounder. Here, tumor cells disseminate spontaneously from the primary tumor, which is in contrast to other models of metastasis formation comprising intravenous or intracardiac injections of tumor cells.6, 24 Although tumor cells could—in principle—disseminate during injection of tumor cells under the kidney capsule, we observed a patient-specific pattern of dTCs in the BM with very little variability between mice transplanted with cells from the same patient sample, arguing against injection-driven dissemination of tumor cells.
By far most of the dTCs detected in the BM of mice transplanted with TSC cells from eight different CRC patient samples were found at perivascular sites, a phenomenon described for normal and leukemic HSCs as well as a mouse model of breast cancer,4, 6, 25 but different from the preferential subendosteal localization of dTCs in prostate cancer.17 Remarkably, we did not find evidence for CRC dTCs occupying existing HSC niches, as colocalization of dTCs and HSCs was rare in our model. Even though a preference for perivascular localization of dTCs was observed in vitro and in vivo, we cannot exclude that few dTCs that localized independently are functionally relevant. Interestingly, a close physical contact between tumor cells and the abluminal surface of the blood vessel wall is essential for the survival of brain metastasis-forming cells.26
While the perivascular niche has been described as regulator of breast cancer dormancy,6 we find a substantial proliferative dTC subfraction equal in size to primary tumors in vivo, arguing against induction of quiescence in colorectal dTCs by the perivascular BM niche. Accordingly, by far most CRC patients do experience relapse within the first 2 years after surgery, which is in contrast to breast cancer patients that are at steady risk of recurrence for up to 20 years.27
To our surprise, despite harboring substantial subfractions of cycling cells, dTCs were almost exclusively found as solitary cells. As potential explanation, we observed individual tumor cells in vitro approaching ETs, followed by cell division and subsequent separation of both daughter cells into opposite directions along the abluminal endothelium. Since proliferating epithelial cells generally form tightly connected cell aggregates, these separation events suggest that some dTCs partially show characteristics of nonepithelial cells. Monitoring of such proliferation events by real-time imaging of the BM niche was hindered by the low dTC frequency, and thus ultimate proof of these events in vivo is currently missing. Still, localization of most dTCs directly at the abluminal endothelium and migration along the endothelium are reminiscent of “vessel cooption,” a nonangiogenic process through which tumor cells utilize preexisting tissue blood vessels to support tumor growth, survival and progression in a variety of tumors, but not yet described for dTCs in the BM.28
Xenotransplantations of BM samples from PDX mice confirmed the presence of TICs within dTCs, as formation of adenocarcinomas that histologically resemble the original patient tumor out of the BM could be observed repeatedly in secondary mice. These data further add to the hypothesis that the BM can serve as a reserve pool for dTCs which can trigger tumor relapse in patients after local therapy.29 Still, real-time imaging of the BM niche would be required to definitely proof that dTCs reenter the vasculature spontaneously.
In addition to the identification of active self-renewing TICs in the BM, clonal tracking revealed a substantial number of delayed-contributing TICs in the BM that did not quantitatively contribute to the primary tumor, but became activated and drove tumor growth after transplantation of BM into secondary mice, comparable to clonal kinetics in primary tumors and metastases.8 These delayed-contributing TIC clones might be derived from the fraction of quiescent tumor cells that could be detected in primary tumors and BM. Even though clonal tracking could only be performed for dTCs from one patient sample, delayed-contributing TICs were identified in multiple secondary and tertiary dTC-derived tumors. Notably, delayed-contributing TICs might explain the frequently observed genetic heterogeneity between the primary tumor and a metastatic tumor relapse,30 as they both can be driven by distinct TIC clones with different genetic aberrations.
In summary, our study demonstrates that patient-derived quiescent and actively cycling CRC dTCs disseminate to the perivascular niche in the BM and that distinct TIC types in the BM drive secondary and tertiary tumors (Fig. 6). A better mechanistic understanding of the interactions between dTCs and the perivascular niche might allow the design of therapeutic strategies that eliminate dTCs and might thus improve current treatment approaches. Moreover, our functional data provide a rationale for further systematic studies on the detection of dTCs in BM aspirates to better establish their role for patient outcome and their use for guiding treatment intensity.
Acknowledgements
This work was supported by grants from the German Research Foundation (Deutsche Forschungsgemeinschaft; Grant number: KFO 227/GL286/1-1; KFO 227/BA4806/1-2), NCT 3.0 Precision Oncology Program (NCT3.0_2015.4 TransOnco; NCT3.0_2015.54 DysregPT), the EU Framework Program Horizon 2020 (TRANSCAN-2 ERA-NET, TACTIC consortium), and the Deutsche Krebshilfe Priority program “Translational Oncology” (Colon-Resist-Net). L.M. was supported by scholarships of the German Research Foundation (Deutsche Forschungsgemeinschaft, KFO 227), the German Academic Scholarship Foundation (Studienstiftung des deutschen Volkes), and the Evangelisches Studienwerk e.V. Villigst. H.S. was supported by the Heinrich F.C. Behr scholarship sponsored by the DKFZ and the Medical Faculty Heidelberg. S.M.D. was supported by a scholarship from the Heidelberg School of Oncology. We thank Annika Heuser, Nina Hofmann, Tim Kindinger, Sylvia Martin, and Áine Prendergast for technical assistance. We thank the DKFZ Light Microscopy Facility, the High Throughput Sequencing Unit of the Genomics & Proteomics Core Facility and the Central Animal Laboratory Unit of the Center for Preclinical Research, DKFZ, for providing excellent services. We thank Andreas Trumpp for providing the Leica Biosystems CryoJane Tape Transfer System.
Conflict of Interest
The authors declare no potential conflicts of interest.




