Survival of malignant mesothelioma and other rare thoracic cancers in Germany and the United States: A population-based study
Abstract
Evidence on survival of malignant mesothelioma (MM) and other rare thoracic cancers is limited due to the rarity of these cancer sites. Here, we provide a comprehensive overview of MM incidence and survival after MM and other rare thoracic cancers in Germany and the United States (US). Incidence was estimated from a German National Cancer Database and from the Surveillance, Epidemiology and End Results (SEER) 18 database for 2000–2014. Patients diagnosed in 1997–2013 with malignant epithelial tumors of the trachea (Etra), epithelial tumors of the thymus (Ethy) and MM were extracted from a German cancer survival database and from the SEER 13 database. Period analysis was employed to compute 5-year relative survival (RS). During 2000–2014, an annual average of 0.9 and 0.6 MM cases per 100,000 person-years was diagnosed in Germany and the US. Rates decreased in Germany and in the US. Patients with Ethy had highest 5-year RS with US patients surviving longer (69.1% compared to 63.7%, p = 0.02). Survival after Etra was comparable in both countries (Germany 33.6%, US 34.4%, p = 0.07). Survival in MM patients was poor overall (Germany 11.8%, US 12.1%, p < 0.01). Survival improvements were only observed in MM patients in Germany (10.8% [2002–2007] vs. 13.0% [2008–2013], p < 0.01). The lack of progress in survival for Etra and Ethy patients underlines the need of novel preventive, therapeutic and diagnostic approaches. MM incidence significantly decreased in Germany and in the US. Further monitoring of MM incidence is warranted given that a peak in incidence is expected in 2020–2030 in Western countries.
Abstract
What's new?
Certain cancer types that originate from the epithelial or mesothelial tissues of the thoracic cavity are exceedingly rare. As a consequence, little is known about their incidence and survival. Here, utilizing national databases, the authors estimated incidence and survival of malignant mesothelioma (MM) and other rare thoracic cancers in Germany and the United States. Analyses show that between 2000 and 2014, MM incidence declined in both countries, although only MM survival improved in Germany. No improvements were observed in either country for survival of epithelial tumors of the trachea and thymus, highlighting a need for preventive and therapeutic advances.
Abbreviations
-
- APC
-
- annual percentage change
-
- DCO
-
- death certificate only
-
- Ethy
-
- epithelial tumors of the thymus
-
- Etra
-
- epithelial tumors of the trachea
-
- MM
-
- malignant mesothelioma
-
- MM-PP
-
- MM of the pleura and pericardium
-
- MM-PT
-
- MM of the retroperitoneum and peritoneum and tunica vaginalis
-
- MT
-
- malignant thymoma
-
- ORTCs
-
- other rare thoracic cancers
-
- RS
-
- relative survival
-
- SCC
-
- squamous cell carcinoma
-
- SEER
-
- Surveillance, Epidemiology and End Results
-
- US
-
- United States
Introduction
Malignant mesothelioma (MM) and other rare thoracic cancers (ORTCs) include tumors originating from the mesothelium, epithelial tumors of the trachea (Etra) and epithelial tumors of the thymus (Ethy). Apart from MM (especially pleura mesothelioma) little information is available on the patterns of incidence and survival for these rare malignancies, as in the routine statistics and publications, these tumors are often grouped together with other tumors, although they have different etiologies. For example, tumors of the trachea are grouped with lung and bronchus tumors, tumors of the thymus are often grouped together with those of the heart and tumors of the mediastinum and pleura are grouped as other “other thoracic organs.”1
Similar to lung cancer, Etra are associated with active and passive smoking, occupational exposure to arsenic, asbestos, chromium, welding fumes and environmental exposure, for example, air pollution from traffic and industrial emissions.2 Survival of patients with Etra is low, comparable to those with lung cancer.3
The etiology of Ethy on the other hand is largely unknown with a complex biology. The most frequently diagnosed Ethy are thymomas. Compared to patients with tumors of the trachea and mesothelium, survival of patients with tumors of the thymus is favorable and mainly determined by tumor stage at diagnosis, morphology type and completeness of resection.4-6
Occupational asbestos exposure has been an established risk factor for MM for more than 50 years. However, the disease can also arise due to exposures to erionite, noncommercial amphiboles, or ionizing radiation, and from genetic predisposition or spontaneous occurrence.7 The most recent data available on mesothelioma epidemiology show that in many countries the incidence rate of the tumor does not present signs of attenuation,8 even though asbestos use had been banned in many industrialized countries for decades. The latency time for MM is known to be 30 or more years. The prognosis for patients with MM has been reported to be very low.3
We aimed to describe the incidence trends for MM in Germany and the United States (US) in 2000–2014. Furthermore, we provide detailed relative survival (RS) estimates for patients diagnosed with MM and ORTCs by sex, age, morphology and topography in Germany and the US for the period 2002–2013.
Materials and Methods
Incidence
The Center for Cancer Registry Data at the Robert Koch Institute in Berlin, Germany collects data for cancer cases diagnosed from 1999 onward. In this database, incidence for the entire population of Germany is estimated based on the incidence in regions with high completeness and completeness-corrected incidence in regions with insufficient completeness.9 From this database, overall and age group-specific (0–64, 65–74, 75+ years) annual incidence rates for MM (International Classification of Diseases 10th Revision (ICD-10) C45) per 100,000 person-years were computed by sex for the period 2000–2014. For the US, incidence estimates were extracted from the Surveillance, Epidemiology, and End Results (SEER)-18 Program for the same subgroups and period using SEER*Stat.10 The SEER-18 database includes data for cancer patients diagnosed from 2000 onward, collected from 18 regional cancer registries throughout the US that are chosen for their high-quality and epidemiologically significant population. It covers 28% of the total US population.11
The overall and sex-specific annual incidence rates for both countries were age-standardized to the world standard population (Segi 1960) by the direct method. Trends in age-standardized and age group-specific incidence rates in both countries were assessed using joinpoint regression.12 We employed the log-linear regression model to compute annual percentage change (APC). The t test was used to test whether the APC was statistically different from zero.
Survival
A pooled national data set from the German Cancer Survival Project13 was used for survival analyses for Germany. The pooled data set includes cancer registry data for patients diagnosed in 1997–2013 from 12 of the 16 federal states in Germany, covering 35% of the total German population (28.3 million inhabitants in 2013). Regions were included in the pooled data set based on data quality as described in detail elsewhere.13 The SEER-13 data set,14 which contains data for patients diagnosed from 1975 onward and covers 13.4% of the US population (42.7 million inhabitants in 2013), was used for survival analyses for the US. The larger SEER-18 data set was not taken into account for survival computation because it would only allow estimation of 5-year period survival estimates, the main survival outcome, from the period 2005 onward. Patients of 15 years or older, diagnosed with a first malignant tumor of Etra, Ethy and MM in 1997–2013 and passive mortality follow-up until December 2013 were included. Death certificate only (DCO) cases were excluded from the analysis.
Patients were selected according to a list provided by the surveillance of rare cancers in Europe (RARECARE).15 In the RARECARE study, cancer is classified according to ICD-O-3 anatomic sites (Etra: C33, Ethy: C37, MM subdivided in MM of the pleura and pericardium [MM-PP]: C38 and MM of the retroperitoneum and peritoneum and tunica vaginalis testis [MM-PT]: C48, C63.7). Analysis for patients with Etra was restricted to Tier 1 of the RARECARE listings because of small case numbers. For Ethy and MM, the Tier 2 subgroups of malignant thymoma (MT), MM-PP and MM-PT had sufficient case numbers to be analyzed separately; morphology codes included in the computations were selected according to ICD-O-3 and as provided by the RARECARE study.15
RS was estimated by period analysis.16 Period analysis has been shown to provide more up-to-date survival estimates than traditional cohort analysis. In period analysis, the survival analysis is restricted to some recent calendar period by left truncation of survival times at the beginning of the period of interest in addition to right censoring at its end. It has been shown that period analysis closely predicts the survival later observed for patients diagnosed in the period of interest.16 RS was calculated as the ratio of the observed survival in the group of cancer patients divided by the expected survival of a comparable group in the source population.17 The expected survival was derived from life tables according to the Ederer II method,18 stratified by age, sex, and calendar year, as obtained from the German Federal Statistical Office and the Center for Disease Control and Prevention19 in the US.
Five-year age-standardized RS was calculated for the period 2002–2013, for which the most up-to-date and stable survival estimates could be derived from the given data sets. We assessed RS by cancer subgroup, sex and age group in both countries. RS differences between patients in Germany and the US and sex differences within countries were tested for statistical significance using model-based period analysis.20 In the model-based analysis, the numbers of deaths were modeled as a function of the year of follow-up, age (in age-standardized analysis) and country (in country comparisons) or sex (in sex comparisons) by Poisson regression with the logarithm of the person-years at risk as offset. Furthermore, trends in 5-year RS for the periods 2002–2007 and 2008–2013 within countries were investigated by model-based period analysis. Survival estimates were age-standardized according to the International Cancer Survival Standard using weights for five age groups (15–44, 45–54, 55–64, 65–74, 75+) for cancers whose incidence rate increase with age.21
All survival calculations were performed with SAS statistical software package (version 9.2, SAS Institute Inc, Cary, North Carolina), using macros developed for standard and model-based period analysis.20, 22 Statistical tests were two-sided with α = 0.05.
Data availability
The data that support the findings of our study are available from the included cancer registries. Restrictions apply to the availability of these data, which were used under license for our study. Data are available from the authors with the permission of the cancer registries.
Results
MM incidence
In Germany in 2000–2014, 24,510 cases of MM were diagnosed including 19,313 (79%) men and 5,197 (21%) women (Table 1). The age-standardized incidence rate in 2000–2014 for men and women combined was 0.9 per 100,000 person-years. Men had a five times higher rate compared to women (1.5 vs. 0.3). A very strong age gradient in incidence rate was observed with an average annual rate of 1.0, 12.6 and 18.2 in the age groups 0–64, 65–74 and 75+ years, respectively, in men and 0.3, 2.4 and 3.1 in women.
| Country | Variable | Number of cases (%) | Incidence rate 2000–2014 | Trend 1 Years (APC) | Trend 2 Years (APC) | Trend 3 Years (APC) |
|---|---|---|---|---|---|---|
| Germany | Overall1 | 24,510 | 0.9 | 2000–2003 (−8.0, p < 0.01) | 2003–2009 (1.4, p = 0.1) | 2009–2014 (−4.7, p < 0.01) |
| Men1 | 19,313 (100%) | 1.5 | 2000–2003 (−9.9, p < 0.01) | 2003–2006 (3.5, p = 0.6) | 2006–2014 (−2.3, p < 0.01) | |
| 0–64 years | 4,786 (24.8%) | 1.0 | 2000–2003 (−12.5, p < 0.01) | 2003–2014 (−3.8, p < 0.01) | ||
| 65–74 years | 7,810 (40.4%) | 12.6 | 2000–2014 (0.2, p = 0.6) | |||
| 75+ years | 6,717 (34.8%) | 18.2 | 2000–2002 (−18.2, p < 0.01) | 2002–2014 (2.6, p < 0.01) | ||
| Women1 | 5,197 (100%) | 0.3 | 2000–2006 (−6.6, p < 0.01) | 2006–2010 (8.9, p = 0.3) | 2010–2014 (−12.9, p < 0.01) | |
| 0–64 years | 1,351 (26.0%) | 0.3 | 2000–2014 (−1.5, p = 0.1) | |||
| 65–74 years | 1,716 (33.0%) | 2.4 | 2000–2014 (−0.5, p = 0.5) | |||
| 75+ years | 2,130 (41.0%) | 3.1 | 2000–2005 (−8.1, p < 0.01) | 2005–2008 (10.3, p = 0.3) | 2008–2014 (−1.3, p = 0.3) | |
| US | Overall1 | 12,480 | 0.6 | 2000–2008 (−0.6, p = 0.2) | 2008–2014 (−3.1, p < 0.01) | |
| Men1 | 9,581 (100%) | 1.0 | 2000–2014 (−2.6, p < 0.01) | |||
| 0–64 years | 2,120 (22.1%) | 0.4 | 2000–2008 (−0.1, p = 0.9) | 2008–2014 (−5.3, p < 0.01) | ||
| 65–74 years | 2,793 (29.2%) | 7.7 | 2000–2014 (−3.0, p < 0.001) | |||
| 75+ years | 4,668 (48.7%) | 17.4 | 2000–2014 (−0.7, p = 0.1) | |||
| Women1 | 2,899 (100%) | 0.2 | 2000–2014 (−2.2, p < 0.01) | |||
| 0–64 years | 996 (34.4%) | 0.2 | 2000–2014 (0.00, p = 1.00) | |||
| 65–74 years | 668 (23.0) | 1.6 | 2000–2008 (4.8, p < 0.01) | 2008–2014 (−6.7, p < 0.01) | ||
| 75+ years | 1,235 (42.6%) | 2.9 | 2000–2014 (0.0, p = 0.9) |
- APCs significantly different from zero (p ≤ 0.05) are printed in italics.
- 1 Age standardized (world standard population).
In the US, a total of 12,480 MM patients were diagnosed in 2000–2014 within the SEER-18 database including 9,581(76.8%) men and 2,899 (23.2%) women (Table 1). The age-standardized incidence rate in 2,000–20,014 was 0.6 per 100,000 person-years, with men showing approximately a five times higher incidence rate compared to women (1.0 vs. 0.2, Table 1). As in Germany, a strong age gradient was observed in the US with an average annual rate of 0.4, 7.7 and 17.4 in the age groups 0–64, 65–74 and 75+ years in men and 0.2, 1.6 and 2.9 in women.
MM incidence trends by sex and age group in Germany and the US in 2000–2014 are illustrated in Figure 1 and results from joinpoint regression models are shown in Table 1. Between 2000–2003 and 2009–2014, age-standardized MM incidence combined for men and women significantly decreased (APC = −8.0, p < 0.01 and APC = −4.7, p < 0.01, respectively) in Germany. Sex-specific incidence trends showed significant annual decreases in the incidence rate of MM in men during 2000–2003 (APC = −9.9, p < 0.01) and 2006–2014 (APC = −2.3, p < 0.01). However, trends strongly varied by age. Whereas incidence in more recent years declined among men below 75 years of age, it increased in the age group 75+ years in 2002–2014. Among women, a statistically significant decrease was observed in 2000–2006 (APC = −6.6, p < 0.01) and 2010–2014 (APC = −12.9, p < 0.01).
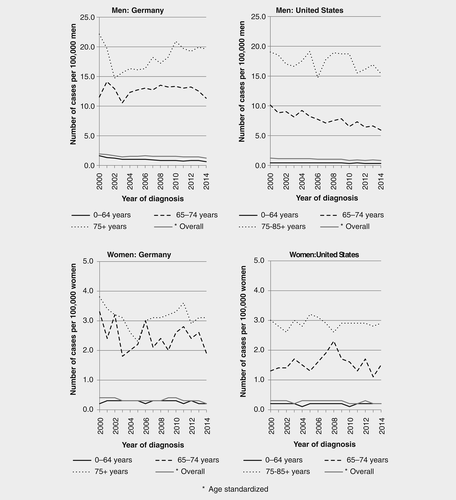
In the US, age-standardized MM incidence for men and women combined slightly and continuously decreased in 2000–2014, with a significant decrease in 2008–2014 (APC = −3.1, p < 0.01; Fig. 1, Table 1). This decrease was mainly attributed to a significant decrease in men observed in 2000–2014 (APC = −2.6, p < 0.01), particularly in the age groups 0–64 years (APC in 2008–2014 = −5.3, p < 0.01) and 65–75 years (APC in 2000–2014 = −3.0 p < 0.01). In women, a significant decrease was observed in 2000–2014 (APC = −2.2, p < 0.01), mainly contributed by women in the age group 65–74 years in 2008–2014 (APC = −6.7, p < 0.01).
MM and ORTC survival
In Germany and the US, 8,745 and 8,054 MM/ORTC patients with a diagnosis in 1997–2013 were extracted from survival data sets (Table 2). After exclusion of patients notified by DCO (Germany: 9.5%, US: 1.0%), 7,917 and 7,970 patients remained for analysis. The majority of cases had MM (Germany 86.5%, US 73.0%), with the most frequent being MM-PT (Germany 91.9% vs. US 89.5%). Among patients with Etra, squamous cell carcinoma (SCC) was the most frequent subsite (Germany 70.9% vs. US 66.8%). MT was the most common cancer diagnosed among patients with Ethy (Germany 85.4% vs.US: 91.2%). The median ages (years) at diagnosis for both sexes combined in Germany vs. US were 63 vs. 65 for Etra, 63 vs. 60 for Ethy and 71 vs. 73 for MM. Over 90% of all cancers were histologically verified in Germany (93.6%) and in the US (96.7%).
| Tier | Cancer sites/Morphology subtypes (ICD-0-3) | Germany | United States | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) diagnosed1 | N without DCO | Median age2 | HV%3 | N (%) diagnosed | N without DCO | Median age2 | HV%3 | ||
| Germany | |||||||||
| 1 | Epithelial tumor of trachea (C33) ICD-10 C33 | 299 (100%) | 297 | 63 | 96.3 | 310 (100%) | 308 | 65 | 99.7 |
| 2 | Squamous cell carcinoma with variants of trachea | 212 (70.9%) | 212 | 64.5 | 96.2 | 207 (66.8%) | 206 | 68 | 99.5 |
| 2 | Salivary gland type tumors of trachea | 57 (19.1%) | 56 | 56 | 96.4 | 84 (27.1%) | 84 | 53.5 | 100.0 |
| 2 | Adenocarcinoma with variants of trachea | 30 (10.0%) | 29 | 66 | 96.6 | 19 (6.1%) | 18 | 70 | 100.0 |
| 1 | Epithelial tumors of thymus (C37) ICD-10 C37 | 789 (100%) | 774 | 63 | 94.8 | 1,851 (100%) | 1,847 | 60 | 98.7 |
| 2 | Malignant thymoma | 674 (85.4%) | 661 | 62 | 94.4 | 1,689 (91.2%) | 1,686 | 60 | 98.6 |
| 2 | Squamous cell carcinoma of thymus | 93 (11.8%) | 92 | 65.5 | 98.9 | 25 (1.4%) | 25 | 59 | 100.0 |
| 2 | Adenocarcinoma with variants of thymus | 10 (1.3%) | 10 | 62 | 80.0 | 118 (6.4%) | 117 | 65 | 100.0 |
| 2 | Lymphoepithelial carcinoma of thymus | 4 (0.5%) | 4 | 54 | 100.0 | 11 (0.5%) | 11 | 48 | 100.0 |
| 2 | Undifferentiated carcinoma of thymus | 8 (1.0%) | 7 | 64 | 100.0 | 8 (0.4%) | 8 | 50 | 100.0 |
| 1 | Malignant mesothelioma ICD-10 C45 | 7,657 (100%) | 6,846 | 71 | 93.3 | 5,893 (100%) | 5,815 | 73 | 95.9 |
| 2 | Mesothelioma of pleura and pericardium (C38) | 7,036 (91.9%) | 6,255 | 71 | 93.3 | 5,277 (89.5%) | 5,205 | 74 | 95.5 |
| 2 | Mesothelioma of retroperitoneum and peritoneum and tunica vaginalis testis (C48, C63.7) | 621 (8.1%) | 591 | 67 | 93.4 | 616 (10.5) | 610 | 65 | 99.5 |
| Overall | 8,745 | 7,917 | 70 | 93.6 | 8,054 | 7,970 | 71 | 96.7 | |
- Abbreviations: DCO, death certificate only cases; HV, histologically verified; N, number of cases.
- The proportion of DCO cases was 9.5% in Germany and in the US 1.0% for malignant mesothelioma and other rare thoracic cancers combined during the period 2002–2014. Tier 1 values are hightlighted in bold.
- 1 Percentage of cancer subtype within a cancer entity.
- 2 Median age at diagnosis.
- 3 Percentage of histologically verified cancer diagnosis.
Survival of Etra
The overall 5-year RS of patients with Etra was comparable in both countries (Germany 33.6% vs. US 34.4%, p = 0.07, Table 3). Five-year RS for men was lower than the overall estimate in both countries and significantly higher in the US (31.5%) than in Germany (28.4%, p = 0.01). Five-year RS for women and by age could not be estimated reliably due to small case numbers.
| Germany | United States | Country | Sex (p value1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer sites/subtype | Factor | N | RS (SE) | N | RS (SE) | Diff | p value2 | Germany | US |
| Epithelial tumors of trachea | Overall3 | 297 | 33.6 (3.6) | 308 | 34.4 (3.3) | −0.8 | 0.0685 | ||
| Men3 | 204 | 28.4 (4.5) | 170 | 31.5 (3.7) | −3.1 | 0.0121 | |||
| Epithelial tumors of thymus | Overall3 | 774 | 63.7 (2.8) | 1,847 | 69.1 (1.9) | −5.4 | 0.0249 | 0.10 | 0.62 |
| Men3 | 428 | 60.6 (4.2) | 960 | 68.3 (2.7) | −7.7 | 0.0205 | |||
| Women3 | 346 | 67.3 (3.9) | 887 | 69.7 (206) | −2.4 | 0.5277 | |||
| Age 15–64 | 432 | 67.6 (2.7) | 1,126 | 75.4 (1.6) | −7.8 | 0.0067 | |||
| Age 65+ | 342 | 64.7 (3.9) | 721 | 66.0 (2.8) | −1.3 | 0.9919 | |||
| Malignant thymoma | Overall3 | 661 | 68.0 (3.1) | 1,686 | 71.7 (2.0) | −3.7 | 0.1179 | ||
| Men3 | 353 | 64.7 (4.7) | 872 | 70.0 (2.9) | −5.3 | 0.1536 | |||
| Women3 | 308 | 71.5(4.1) | 814 | 73.2 (2.7) | −1.7 | 0.5374 | |||
| Age 15–64 | 375 | 72.1 (2.8) | 1,039 | 77.4 (1.6) | −5.3 | 0.0728 | |||
| Age 65+ | 286 | 68.4 (4.2) | 647 | 69.4 (3.0) | −1.0 | 0.9633 | |||
| Malignant mesothelioma | Overall3 | 6,846 | 11.8 (0.6) | 5,815 | 12.1 (0.6) | −0.3 | <0.0001 | ||
| Malignant mesothelioma of PP | Overall3 | 6,255 | 9.9 (0.7) | 5,205 | 9.5 (0.7) | 0.4 | <0.0001 | <0.0001 | 0.0002 |
| Men3 | 5,159 | 8.3 (0.7) | 4,137 | 8.6 (0.8) | −0.3 | <0.0001 | |||
| Women3 | 1,096 | 15.7 (1.6) | 1,068 | 12.5 (1.5) | 3.2 | 0.0126 | |||
| Age 15–64 | 1,566 | 11.8 (1.0) | 1,191 | 11.5 (1.2) | 0.3 | 0.0132 | |||
| Age 65–74 | 2,580 | 8.8 (0.7) | 1,491 | 8.0 (0.9) | 0.8 | <0.0001 | |||
| Age 75+ | 2,109 | 6.1 (0.8) | 2,523 | 6.0 (0.7) | 0.1 | 0.0038 | |||
| Malignant mesothelioma of PT | Overall3 | 591 | 21.5 (2.1) | 610 | 24.9 (2.3) | −3.4 | 0.7172 | 0.06 | 0.0035 |
| Men3 | 342 | 20.6 (2.8) | 340 | 20.2 (3.0) | 0.4 | 0.8878 | |||
| Women3 | 249 | 22.9 (3.4) | 270 | 29.0 (3.8) | −6.1 | 0.6171 | |||
| Age 15–64 | 235 | 30.5 (3.6) | 294 | 32.5 (3.4) | −2.0 | 0.7238 | |||
| Age 65–74 | 219 | 19.9 (3.5) | 162 | 24.4 (4.7) | −4.5 | 0.5747 | |||
| Age 75+ | 137 | 12.5 (4.2) | 154 | 18.1 (4.6) | −5.6 | 0.8544 | |||
- Abbreviations: Diff, difference in 5-year RS for Germany versus the US; N, number of cases; PP, pleura and pericardium; PT, retroperitoneum and peritoneum and tunica vaginalis testis; RS, relative survival, SE, standard error. Significant p values (<0.05) are printed in bold.
- 1 p value for sex comparison within country from model-based analyses.
- 2 p value for country comparison from model-based analyses.
- 3 Age-standardized using five age-groups (15-44, 45-54, 55-64, 65-74, 75+ years).
Survival of Ethy
Patients with Ethy showed the highest age-standardized 5-year RS in both countries. Five-year RS was lower in Germany (63.7%) than in the US (69.1%, p = 0.02, Table 3), which was mainly based on the strong country difference among men (60.6 vs. 68.3%, p = 0.02) and among patients aged 15–64 (67.6 vs. 75.4%, p < 0.01). Women showed higher survival estimates than men in Germany (67.3 vs. 60.6%), but differences were not significant (p = 0.10). Age differences were mainly observed in the US with 5-year RS of 75.4 and 66.0% in patients aged 15–64 and 65+, respectively.
Five-year RS for patients with the most common morphology subtype of Ethy, MT, was slightly better than survival for all Ethy patients with 68.0% in Germany and 71.7% in the US. Subgroup differences for MT patients were generally comparable to patterns for all Ethy patients.
Survival of MM
Age-standardized 5-year RS for MM patients was poor in both countries with a slightly better survival in the US (Germany: 11.8%, US 12.1%, p < 0.01; Table 3). MM-PP patients showed an even poorer prognosis with an age-standardized 5-year RS of 9.9% in Germany and 9.5% in the US. In this subgroup, men showed significantly lower 5-year RS compared to women in Germany (8.3 vs. 15.7%, p < 0.01) and the US (8.6 vs. 12.5%, p < 0.01). Survival decreased by age in both countries from approximately 12% for patients aged 15–64 to 6% in patients aged 75+ years. In all subgroups except for men, survival was slightly but significantly higher in Germany.
Age-standardized 5-year RS for MM-PT patients was generally higher (Germany: 21.5%, US: 24.9%), and no significant country differences were observed. Sex differences were only observed in the US with significantly higher 5-year RS in women (29.0 vs. 20.2%, p < 0.01). Five-year RS decreased with age from 30.5% and 32.5% in Germany and the US, respectively, for patients aged 15–64 years to 12.5% and 18.1% for patients aged 75+ years.
Trends in survival
For patients with Etra and Ethy and the Ethy subgroup MT, no significant changes in age-standardized 5-year RS between 2002–2007 and 2008–2013 were observed (Table 4). For MM, age-standardized 5-year RS increased significantly from 10.5% in 2002–2007 to 13.1% in 2008–2013 in Germany (p < 0.01), whereas no improvement was observed in the US (11.1 and 11.4%). The improvements in Germany were observed in both subtypes, MM-PP and MM-PT, but were more pronounced in the latter (+1.9% units vs. +8.4% units). Increases were observed in all sex and age subgroups of MM-PP and MM-PT in Germany, but they were only significant among men with MM-PP (+1.6% units, p = 0.02) and among women with MM-PT (+12.2% units; p = 0.02). In the US, no significant changes in 5-year RS between 2002–2007 and 2008–2013 were observed in any subgroup.
| Cancer sites/subtype | Germany 2002–2007 RS (SE) | 2008–2013 RS (SE) | Diff | p Value1 | United States 2002–2007 RS (SE) | 2008–2013 RS (SE) | Diff | p Value1 | |
|---|---|---|---|---|---|---|---|---|---|
| Epithelial tumors of trachea | Overall2 | 33.9 (4.3) | 31.2(4.0) | −2.7 | 0.63 | 31.6 (4.4) | 37.2 (4.5) | 5.6 | 0.34 |
| Epithelial tumors of thymus | Overall2 | 69.7 (3.6) | 60.9 (3.4) | −8.8 | 0.05 | 68.0 (2.6) | 69.0 (2.2) | 1.0 | 0.75 |
| Malignant thymoma | Overall2 | 71.8 (3.8) | 66.0 (3.8) | −5.8 | 0.23 | 70.0 (2.7) | 72.0 (2.2) | 2.0 | 0.51 |
| Malignant mesothelioma | Overall2 | 10.5 (0.7) | 13.1 (0.7) | 2.6 | 0.0004 | 11.1 (0.7) | 11.4 (0.7) | 0.3 | 0.62 |
| Malignant mesothelioma of PP | Overall2 | 9.1 (0.7) | 11.0 (0.7) | 1.9 | 0.0055 | 8.3 (0.7) | 8.6 (0.7) | 0.3 | 0.71 |
| Men2 | 7.8 (0.7) | 9.4 (0.7) | 1.6 | 0.0201 | 7.1 (0.8) | 7.7 (0.8) | 0.6 | 0.40 | |
| Women2 | 14.3 (1.9) | 17.5 (2.0) | 3.2 | 0.13 | 12.6 (1.8) | 10.9 (1.6) | −1.7 | 0.36 | |
| Age 15–64 | 10.9 (1.2) | 13.8 (1.4) | 2.9 | 0.09 | 10.6 (1.4) | 12.4 (1.6) | 1.8 | 0.35 | |
| Age 65–74 | 8.6 (0.9) | 10.0 (0.9) | 1.4 | 0.17 | 6.2 (1.1) | 6.9 (1.1) | 0.7 | 0.58 | |
| Age 75+ | 6.1 (1.0) | 8.0 (1.0) | 1.9 | 0.06 | 4.4 (0.7) | 4.2 (0.7) | −0.2 | 0.71 | |
| Malignant mesothelioma of PT | Overall2 | 17.2 (2.5) | 25.6 (2.8) | 8.4 | 0.0123 | 23.4 (2.9) | 23.8 (2.8) | 0.4 | 0.93 |
| Men2 | 17.2 (3.4) | 22.8 (3.5) | 5.6 | 0.20 | 21.4 (4.0) | 17.4 (3.3) | −4.0 | 0.41 | |
| Women2 | 17.6 (3.0) | 29.8 (4.5) | 12.2 | 0.0230 | 23.8 (4.3) | 32.4 (4.9) | 8.6 | 0.16 | |
| Age 15–64 | 26.4 (4.8) | 34.8 (5.0) | 8.4 | 0.20 | 31.2 (4.6) | 34.3 (4.7) | 3.1 | 0.63 | |
| Age 65–74 | 14.6 (4.3) | 24.1 (4.5) | 9.5 | 0.09 | 23.3 (6.2) | 26.6 (6.4) | 3.3 | 0.68 | |
| Age 75+ | 10.4 (4.9) | 17.2 (6.0) | 6.8 | 0.26 | 17.2 (5.8) | 13.1 (4.6) | −4.1 | 0.51 | |
- Abbreviations: Diff, difference in 5-year RS for the two periods, that is, 2008–2013 − 2002–2007; PP, pleura and pericardium; PT, retroperitoneum and peritoneum and tunica vaginalis testis; RS, relative survival; SE, standard error. Significant p values (<0.05) are printed in bold.
- 1 p Value for comparing the 5-year RS for 2002–2007 versus 2008–2013.
- 2 Age standardized using five age groups (15–44, 45–54, 55–64, 65–74, 75+ years).
Discussion
This is the first comprehensive population-based study on incidence and survival of patients with MM and ORTCs in Germany, including a comparison with the US. MM incidence decreased between 2000 and 2014 in Germany and the US, with overall 0.9 and 0.6 cases per 100.000 person-years, respectively. Five-year RS for MM was 12.1% in the US and 11.8% in Germany. Significant improvements in survival were only observed in Germany with 10.5% in 2000–2007 and 13.1% in 2008–2013. These improvements were strongest for the subtype MM-PT. Survival of Etra was comparable in both countries with 34% 5-year RS and did not improve over time. Survival of Ethy patients was higher in the US, with 69.1 versus 63.7%, and also showed no improvement over time.
Incidence of MM
As in previous studies, we found the highest numbers of cases diagnosed in men and in the older age groups in Germany and the US.3, 23 Occupational exposure of men to asbestos and a long latency period of over 30 years of this malignancy have been reported to be the probable explanations of these findings.3, 23 The age-standardized incidence rate of 1.5 and 0.3 per 100,000 for men and women, respectively, seen in Germany concurs with previous reports that have also reported similar rates in Germany and in some central European countries, where asbestos production and use has been banned for at least the last 20 years.8, 24 The much higher incidence rate of MM in men is in accordance with pervious studies3, 23 and is hypothesized to arise mostly from differential occupational exposures to asbestos and its products.
Overall, the incidence in Germany decreased over the study period. Due to the long latency period, a peak in incidence is expected in 2020–2030 in Western countries including Germany.24 Therefore, further monitoring of MM incidence is warranted. MM incidence also decreased in 2000–2014 in the US and showed differences by sex and age. These findings are consistent with results of the study carried out by Henley et al., who assessed the incidence rate of MM in 50 states and the districts of Columbia.23 Differences in incidence rates between the US and Germany could reflect patterns of asbestos usage and or exposure, which began to decline earlier in the US23 compared to Germany.25
The overall incidence rate of 0.9 and 0.6 per 100,000 person-years in Germany and the US, respectively, most probably underestimated the incidence rate in regions with high asbestos exposure. The concept of differential occupational exposure to asbestos leading to a huge variation in the incidence rate and mortality of MM between regions inside a country is well known.8 For example, Henley et al. reported incidence rates by state ranging from 0.56 to 1.65 per 100,000 person-years. Similar regional variations with steeper gradients are observable in Germany with up to 10.0 cases per 100,000 person-years in some regions.26
Survival of patients with Etra
The overall 5-year age-standardized RS of approximately 34% observed in Germany and in the US in Etra patients is comparable to an overall crude 5-year RS of 33% reported by Siesling et al. for central European countries during the period 2000–2002.3 A strong regional gradient was observed in our study ranging from the lowest survival (1.7%) in the eastern bloc countries to the highest survival (33%) in central Europe. In addition, recent data published by the RARECARE project15 showed that there was an increase of 5-year RS from 15% in 2002–2004 to 22% in 2005–2007 when data for all the 94 European population-based cancer registries were included in the pooled database. Our 5-year RS estimate was already higher in both countries in 2002–2007 (Germany: 34%, US: 32%). However, we did not observe any progress in survival in 2000–2013. Nouraei et al. reported that most cases of Etra are diagnosed beyond the window of curative treatment,27 because patients with airway tumors typically present with symptoms which usually are mistaken for other respiratory pathology such as asthma, chronic obstructive pulmonary disease or pneumonia.28 Therefore, the lack of progress in the prognosis of this cancer observed in our study may be partly attributable to late stage diagnosis, which could not be assessed in our study due to the incompleteness of stage information (over 70% in the German data set; data not shown).
It has been described that over 80% of all Etra patients have a history of smoking.29 Therefore, the observation of more men than women (ratios: Germany 2.2 vs.1; US 1.2 vs. 1) being affected with Etra may in part reflect the smoking patterns in both countries.30 This observation is in line with some reports in the literature,3, 23 though it contradicts a previous study that reported that men and women were equally affected by this malignancy,27 especially those with SCC.31 We found a sex gradient in survival, with men showing lower survival than the overall group in Germany but not in the US. Subgroup analyses of Etra have been scarce but had reported tumor stage, morphology subtypes, age, complete resection of tumor and marital status to be important prognostic factors for survival.32 Therefore, differences in the distribution of the prognostic factors could in part explain the aforementioned observed differences in survival. Studies assessing these prognostics factors in detail would be needed to further explain the survival differences we have observed.
Survival of patients with Ethy
Overall, patients with Ethy showed the highest age-standardized 5-year RS in both countries. This finding is in line with results of the study conducted by Siesling et al.3 The majority of Ethy were MT. This subtype has been reported to be slowly growing with a generally good prognosis compared to thymic carcinoma, which have a poor prognosis despite aggressive treatment. Patients with Ethy in the US showed significantly better 5-year RS (69.1%) than those in Germany (63.7%), mainly due to significantly better survival in men and the younger age group. One explanation for this difference could be the higher proportion of patients with better prognosis (MT) in the US (91%) compared to Germany (85%). Tumor stage at diagnosis and morphology has been reported to be strong prognostic factors in patients with Ethy.4-6 Unfortunately, high proportions of missing stage information (about 70% in Germany) precluded analyses by tumor stage, which could have offered some explanations to the differences observed between Germany and the US. Another factor that might explain these differences could be differences in treatment. Multimodal treatment approaches have been reported to prolong life of Ethy patients.4 Especially surgery has been shown to be the mainstay of curative-intent treatment, with complete resection representing the most significant favorable prognostic factor on overall survival.6 Whether different patterns of treatment in Germany and the US could partly explain our finding could not be investigated, because detailed treatment data were not available in the assessed data sets.
While survival for patients with Ethy is moderate in both countries, no significant progress has been made in the US. On the other hand, a borderline significant decrease was observed during the study period in Germany. These might be partly attributable to the rarity of the diseases leading to a major challenge in assessing innovative strategies, as there is lack of funding for clinical research for rare cancers. Their rarity also precludes the design of robust clinical trials that could lead to specific approval of drugs24 Therefore, the importance of patient-centered initiatives, such as establishing dedicated regional and international networks cannot be overemphasized.24
Survival of patients with MM
The poor prognosis of MM patients in both countries and in all subgroups is in accordance with previous reports.3, 33-35 One hypothesized reason for this poor prognosis is the long latency period, which leaves a majority of the patients to be diagnosed in advanced age, with no specific guidelines for their management. As a consequence, clinical uncertainty often results in suboptimal or excessively toxic treatment, which ultimately may lead to poorer outcomes as compared to younger patients36, 37 Older patients generally present with poorer health performance status with reduced tolerance to invasive diagnostic procedures which may lead to delay in diagnosis. Novel targets for screening (e.g., the pivotal role of BRCA-associated protein-1 in causing MM in both hereditary and sporadic settings) and therapy have only been recently identified.38 Elucidation of the precise mechanism of gene–environment interaction might lead to the prevention of at least some mesotheliomas. Furthermore, ongoing trials with high mobility box protein-1 and/or other biomarkers might provide a way to identifying patients exposed to asbestos and to identifying MM patients early in the course of the disease.38
Our observation of sex differences in survival by topography in both countries is in line with previous studies.39-41 Wolf et al.40 reported in their study on malignant pleural mesothelioma, including 702 patients who underwent extrapleural pneumonectomy, that women with epithelial morphology had a significantly longer survival. In addition, they reported an inherent survival advantage in female patients with epithelial disease that was not present in female patients with nonepithelial disease. A previous study evaluating the counts of asbestos bodies in lung tissues found that epithelial mesothelioma presented with a far lower burden in comparison to nonepithelial mesothelioma.42 Other studies have reported possible roles of hormone and sex steroids in the survival advantage observed in female MM patients compared to male patients.43
MM-PT patients had a survival advantage over MM-PP patients in both countries, which had been reported in previous studies.3 The reasons for this observed difference has not been fully elucidated. However, stage of disease, morphology and treatment with cisplatin-pemetrexed have been reported to be of prognostic importance in MM patients. Therefore, a detailed analysis by the aforementioned factors, which unfortunately were not available in the data sets for our study, might at least provide some explanations to the observed survival differences by topography.
Strengths and limitations
The major strength of our study was the large sample size contributed by 12 regional population-based cancer registries in Germany and 13 in the US. These large data sets offered the opportunity to estimate survival with sufficient precision for rare cancer types. The utilization of the period analysis approach made it possible to provide more up-to-date survival estimates by including the most recent calendar years. Furthermore, population-based data provide a more representative picture of prognosis than data provided by clinical series, which often refer to highly selective groups of patients of treatment patterns not representative of the general population.
A number of limitations should be considered when interpreting the results of our analysis. Firstly, in the absence of a national death index, most cancer registries in Germany rely on record linkage with vital statistics from the state that they cover and may miss deaths among patients who move out of the state. However, previous validation studies have suggested potential overestimation of survival due to deaths missed by migration to be very small.44 Secondly, no valid data on surgery, radiotherapy or chemotherapy is available in the German database and therefore we could not make definitive statements about how the use of specific treatment modalities affects survival. Thirdly, there is some evidence that survival estimates from the SEER-13 database may be higher than survival in the US population in general,45 therefore some caution is necessary when comparing survival in the two countries. Fourth, the proportion of DCO cases for MM and ORTC can be expected to be underestimated when relying on morphology codes, as DCO cases often do not have morphology coding. To get an approximation of the real DCO proportions, the ICD-10 codes for Etra, Ethy and MM were used showing an overall proportion of DCO cases of 9.5% in Germany and 1.0% in the US for the entire period of study. The higher DCO proportion in Germany can be partly explained by expected high DCO proportions in the initial years of newly established cancer registries, which are caused by dying cancer patients who were diagnosed before the establishment of the registry.46 Nonetheless, the exclusion of DCO cases in the analysis might lead to some overestimation of survival, as DCO cases can be expected to have shorter survival times.47, 48
Another complexity in the comparison of German and SEER survival estimates, which has to be considered, is the application of different multiple primary coding rules. In Germany, the rules of the International Agency for Research on Cancer are followed, which results in fewer cancer cases than the procedures followed in the SEER program.49 A further limitation is that 9 of the 12 German registries were not allowed to record and/or provide the exact day of diagnosis/birth of the patients. Therefore, only partial dates (month and year) could be used in survival analyses, which are expected to have introduced some imprecisions in survival estimates.50
Conclusions
The results of our study, which is the first detailed population-based incidence and survival analysis of MM and other rare thoracic cancers in Germany and in comparison with US data, could make an important contribution to the evidence of disease burden and survival of these rare cancer entities. They also point to lack of progress in survival for patients with Etra and Ethy. Novel preventive, therapeutic and diagnostic approaches are urgently needed to improve prognosis. In addition, further monitoring of MM incidence is warranted given that a peak in incidence is expected in 2020–2030 in western countries including Germany.
Acknowledgements
Members of the GEKID Cancer Survival Working Group: Karla Geiss, Martin Meyer (Cancer Registry of Bavaria), Andrea Eberle, Sabine Luttmann (Cancer Registry of Bremen), Roland Stabenow (Cancer Registry of Berlin and the New Federal States), Stefan Hentschel, Alice Nennecke (Hamburg Cancer Registry), Joachim Kieschke, Eunice Sirri (Cancer Registry of Lower Saxony), Bernd Holleczek (Saarland Cancer Registry), Katharina Emrich (Cancer Registry of Rhineland-Palatinate), Hiltraud Kajüter, Volkmar Mattauch (Cancer Registry of North Rhine-Westphalia), Alexander Katalinic, Nora Eisemann (Cancer Registry of Schleswig-Holstein), Klaus Kraywinkel (Robert Koch Institute, Berlin), Hermann Brenner, Lina Jansen (German Cancer Research Center). This work was supported by the German Cancer Aid (Deutsche Krebshilfe) [grant number 108257 and 110446].
Conflict of Interest
The authors declare that they have no conflict of interest.




