Human glioblastoma-initiating cells invade specifically the subventricular zones and olfactory bulbs of mice after striatal injection
Tel.: +32-4-366-59-50, Fax: +32-4-366-59-12
Abstract
In patients with glioblastoma multiforme, recurrence is the rule despite continuous advances in surgery, radiotherapy and chemotherapy. Within these malignant gliomas, glioblastoma stem cells or initiating cells have been recently described, and they were shown to be specifically involved in experimental tumorigenesis. In this study, we show that some human glioblastoma cells injected into the striatum of immunodeficient nude mice exhibit a tropism for the subventricular zones. There and similarily to neurogenic stem cells, these subventricular glioblastoma cells were then able to migrate toward the olfactory bulbs. Finally, the glioblastoma cells isolated from the adult mouse subventricular zones and olfactory bulbs display high tumorigenicity when secondary injected in a new mouse brain. Together, these data suggest that neurogenic zones could be a reservoir for particular cancer-initiating cells.
Malignant gliomas are the commonest primary brain tumors in adult and their most aggressive form, glioblastoma multiform (GBM, WHO grade IV), is also the most prevalent subtype.1 Despite aggressive treatments combining surgery, chemotherapy and radiotherapy, the median survival hardly reaches 12 to 15 months, and virtually, all tumors relapse within 5 years from diagnostic.2, 3 A better understanding of the mechanisms leading to tumor recurrence is thus mandatory.
Several years ago, the discovery of a highly tumorigenic cell population, cancer stem cells4 or cancer-initiating cells5 in malignant gliomas shed some light on the mechanisms of tumor recurrence. Although their origin remains debated, these cancer-initiating cells exhibit self-renewal capacities and express multiple neural stem cell (NSC) markers.6 They may find shelter in perivascular niches and escape conventional treatments7, 8 and can, when xenografted in animal models, initiate the formation of complete glial tumors. The existence of such niches could help explain why gliomas recur within a short distance from the initial tumor margins. Distant recurrences, however, also exist and are increasingly seen as patient survival is prolonged with the use of new treatment regimen. The recurrent tumor then frequently grows in the vicinity of ventricles, even if the tumor did not initially grow close to this structure.9, 10 Two explanations to this phenomenon may be suggested. First, adult NSC could be involved in the pathogenesis of brain tumors, with glioblastoma-initiating cells (GIC) emerging from genetic mutations in adult NSC leading to cancer.11-15 After treatment, some GIC might subsist and spread in the neurogenic zones and could give rise to new tumors. Since the discovery of the persistence of neurogenesis in the adult mammalian16, 17 and human brain,18 different studies have demonstrated that the subventricular zone (SVZ) of the forebrain is the largest neurogenic niche, whereas dentate gyrus in the hippocampus is a more restrictive zone, with a lower neuronal production.19, 20 The precise physiological environment present in the SVZ, consisting of specialized cell organization and vascularization, supports the NSC maintenance and their asymmetric division.21 The second, non exclusive explanation, is that GIC, which show characteristics that are similar to those of NSC, could present a tropism for the neurogenic zones and migrate toward the subventricular area where they would physically escape surgery and radiation therapy, become quiescent and thus, resist to chemotherapy, and later resume growth.
In this article, using orthotopic xenografts of human malignant glioma cell line and primary GBM cultures in adult immunodeficient mice, we showed that some tumor cells migrate specifically toward neurogenic zones (i.e., the SVZ). In the SVZ, these cells then express markers of NSC and mimic their behavior by migrating to olfactory bulbs (OB). When isolated and reinjected in other animals, these distant tumor cells are highly tumorigenic. Together, these results suggest that the migration of GIC into the adult neurogenic regions may hypothetically contribute to the relapse of GBM and maybe to the late periventricular pattern of recurrence observed in GBM.
Abbreviations
AO: anterior olfactory nucleus; ATCC: American type culture collection; CPu: caudate putamen (striatum); Dcx: doublecortin; DG: dentate gyrus; DMEM: Dulbecco's modified Eagle medium; EGF: epidermal growth factor; E/OV: ependymal and subependymal layer/olfactory ventricule; FGF-basic: fibroblast growth factor; FBS: foetal bovine serum; GB138: primary glioblastoma initiating cells; GBM: glioblastoma multiforme; GIC: glioblastoma-initiating cells; GlA: glomerular layer of the accessory olfactory bulb; GrO: granular cell layer of the olfactory bulb; NSC: neural stem cell; PBS: phosphate buffer saline; PFA: paraformaldehyde; RMS: rostral migratory stream; RT: room temperature; SVZ: subventricular zone; TM: tumor mass; OB: olfactory bulb; WHO: world health organization
Material and Methods
Animals
We used adult P40 female immunodeficient athymic nude mice, Crl:NU-Foxn1nu, obtained from Charles River® animal facilities (Charles River Laboratories®, Wilmington, UK). All animals were taken care of in accordance with the declaration of Helsinki and following the guidelines of the Belgium ministry of agriculture in agreement with EC laboratory animal care and use regulation (86/609/CEE, CE of J n°L358, 18 December 1986). The athymic nude mice were housed in sterilized filter-topped cages.
Cell cultures
One primary GIC primary culture (GB138) was established from acutely resected human GBM originated from a woman and validated as previously described.22, 23 Briefly, tumor tissue was washed twice in phosphate buffer saline (PBS, Gibco®), minced and placed in T75 flask (Corning®, Corning, NY, USA) in DMEM containing 10% of foetal bovine serum (FBS, Invitrogen®, Merelbeek, Belgium). After 2–3 weeks, cells were collected and resuspended at the density of 25,000 cells/mL in serum-free defined medium consisting of DMEM/F12 containing B27 (Invitrogen®) and supplemented daily with recombinant human epidermal growth factor and recombinant human fibroblast growth factor 2 (EGF, 20 ng/mL and FGF-2, 10 ng/mL, respectively, Preprotech®, Rocky Hill, NJ, USA). Under these culture conditions, cells were maintained as floating spheres. When spheres became visible under microscope, we tested their ability to self-renew using secondary spheres culture assays. Briefly, spheres were incubated 15 min at 37°C in papaïn (Wothington®, Lakewood, NJ, USA), the reaction was stopped with ovomucoïde (Wothington®) and then, cells were mechanically dissociated. Finally, the cell suspension was cultured as the primary culture sphere assays.
Glioma cell line U87MG was obtained from the American Type Culture Collection (ATCC) and cultivated in serum-containing medium. U87MG/DsRed cells expressing red fluorescent protein were also used. To stimulate the formation of floating spheres, cells were cultivated in B27-supplemented DMEM/F12 medium as described above. Cultures were maintained at 37°C under a humidified atmosphere containing 5% carbon dioxide.
Intracranial transplantation of tumor cells into nude mice
Mice were anaesthetized with an intraperitoneal injection of a Rompun® (Sedativum 2%, Bayer®, Bruxelles, Belgium) and Ketalar® (Ketamin 50 mg/mL, Pfizer®, Bruxelles, Belgium) solution (V/V) prepared just before injection. A suspension of 50,000 or 100 GBM cells in 2 μL of PBS solution was injected into the right striatum of the athymic nude mice. The mouse head was maintained within a stereotactic frame, allowing a precise and reproducible injection site, using the intersection of the coronal and sagittal sutures (bregma) as reference. The coordinates used were 0.5 mm anterior and 2.5 mm lateral from the bregma and at a depth of 3 mm. This injection site is 1.2 mm distant from the SVZ. Mice were sacrificed at 24 hr, 1, 2, 3 or 4 weeks after injection for U87MG cells and after 4, 6, 8, 10 weeks for GB138 GIC. For secondary injections, the OB, the SVZ and the tumor mass (TM) were isolated from 400 μm thickness sections obtained with a Leica vibratome (Leica VT1000S, Groot Bijgaarden, Belgium). Thereafter, tissues were incubated in papaïn (Wothington®, Lakewood, NJ, USA) for 30 min at 37°C, and ovomucoïde (Wothington®) was added to stop the dissociation. Then, U87MG cells were plated in six-well dishes in DMEM containing 10% of FBS and GB138 cells in serum-free defined medium at the density of 25,000 cells/mL. Human cells were selected with one passage allowing the selection of more than 99% of human cells. GBM cells were then passed and grafted as described above in a secondary mouse brain or cultured as floating spheres in specific medium.
Processing of tissue sections and cell cultures for immunolabelling
Mice were anaesthetised with a Nembutal® injection (Pentobarbital 60 mg/mL, Ceva Sante Animal®, Bruxelles, Belgium) before an intracardiac perfusion with a NaCl 0.9% solution (VWR International®, Prolabo, USA), followed by paraformaldehyde (P.F.A.) 4% at 4°C (4,3g/L NaOH, 40g/L paraformaldehyde, 18.8 g/L NaH2PO4). Brains were removed and postfixed in P.F.A. 4% for 2 hr, then cryoprotected for 48 hr in PBS containing 20% sucrose before being frozen at −20°C in a 2-methylbutane solution (Sigma®, Bornem, Belgium). Fourteen micrometer thick coronal and sagittal sections were cut on a cryostat and stored at −20°C.
For immunocytofluorescence, cells were plated on coverslips coated with polyornithine for 1 hr at room temperature (0.1 mg/mL, Sigma®). After a short washing in PBS, cells were fixed by incubation for 15 min at room temperature (RT) in P.F.A. 4% solution, then washed three times for 5 min in PBS.
Immunolabelling and histology
Permeabilization and blocking of unspecific binding sites were obtained by a 30 min incubation at RT in the blocking solution (5% donkey serum and 0.3% Triton X-100 in PBS). Primary antibodies were diluted in a carrier solution containing 0.2% donkey serum and 0.1% Triton X-100 in PBS. We used antibodies directed against Nestin (chicken polyclonal IgG, 1:250, Novus Biologicals®, Littleton, CO, USA), GFAP (mouse monoclonal IgG, 1:500, Sigma®, Bornem, Belgium), human mitochondria (mouse monoclonal IgG1, 1:250, Millipore®, Brussels, Belgium), human nuclei (mouse monoclonal IgG1, 1:250, Millipore®), Sox2 (rabbit polyclonal IgG, 1:250, Abcam®, Cambridge, UK and goat polyclonal IgG, 1:200, Santa-Cruz®), Ki67 (rabbit polyclonal IgG, 1:200, Abcam®), CD133 (rabbit polyclonal IgG, 1:250, Abcam®), CD31 (rabbit polyclonal IgG, 1:500, Abcam®), Doublecortin (goat polyclonal IgG, 1:250, Santa-Cruz®), β-III-tubulin (rabbit polyclonal IgG, 1:1000, Covance®, Belgium), NeuN (rabbit polyclonal IgG, 1:500, Chemicon®) and GABA (guinea pig polyclonal IgG, 1:750, Chemicon®). Brain sections or fixed cells were incubated with primary antibodies at RT for 2 hr or at 4°C for the night. Three 15 min washes were performed in PBS at RT. We used biotinylated (Vector®, Brussels, Belgium) or RRX-, FITC- and Cy5-conjugated (Jackson Immunoresearch Laboratories®, West Grove, PA, USA) secondary antibodies. All secondary antibodies were diluted at 1:500 in the carrier solution. Finally, tissue sections were washed three times with PBS and coverslipped in an assembly DAPI-containing Vectashield® solution (Hard Set Mounting Medium®, Vector laboratory, Burlingame, CA, USA) when appropriate. The slides and cultures were stored in the dark at 4°C. Omission of primary antibodies resulted in a complete loss of detectable signal. Moreover, some slides were stained with Hematoxylin/Eosin to observe the tumor mass.
Image acquisition and data analysis
Immunostained sections were imaged and examined using a laser-scanning confocal microscope equipped with a krypton/argon gas layer (Olympus® Fluoview 1000, Aartselaar, Belgium). We also used Zeiss Axiovert 10® microscope (Car Zeiss®, Zaventem, Belgium) coupled with Mercator® software (Explora Nova®, La Rochelle, France) for automatically cell counting within anatomical regions. Figures were composed using ImageJ® (public domain Java image processing program, author: Wayne Rasband, National Institute of Mental Health, Bethesda, Maryland, USA) and Gimp software® (GNOME foundation, Cambridge, MA, USA).
Statistical analysis
Brain xenografts and tumor sphere experiments were repeated at least three times and representative results are illustrated. Where applicable, chi-square test was used to compare tumorigenicity between groups. Quantitative data were presented as means ± SD and a p value of <0.05 was considered statistically significant.
Results
Validation of the intrastriatal xenograft of GBM cells as a model of cancer cell migration
To test whether human glioblastoma (GBM) cells are attracted to neurogenic zones, human U87MG cells were injected into the right striatum of immunodeficient mice using a stereotactic frame. This site is 1.2 mm distant from the ipsilateral subventricular zone (SVZ) but also 1.1 mm from the dentate gyrus in the hippocampus (Fig. 1a). We first checked that the injection is not responsible of expelling cells into the SVZ by sacrificing animals 24 hr after the surgery. We located human cells in the adult mouse brain using antibodies specifically directed against human mitochondria (Fig. 1b) or nuclei. The species-specificity of these antibodies was confirmed by the selective labeling of human astrocytes but not mouse astrocytes (data not shown) and by the injection of cells of the U87MG cell line previously stably transfected with a plasmid expressing a red fluorescent protein (Fig. 1d, DsRed). The small red tumor formed after 2 weeks was specifically labeled with the antibodies directed against human mitochondria (Fig. 1d, green) or nuclei (data not shown). In contrast, the mouse brain was completely clear of any signal in these conditions.
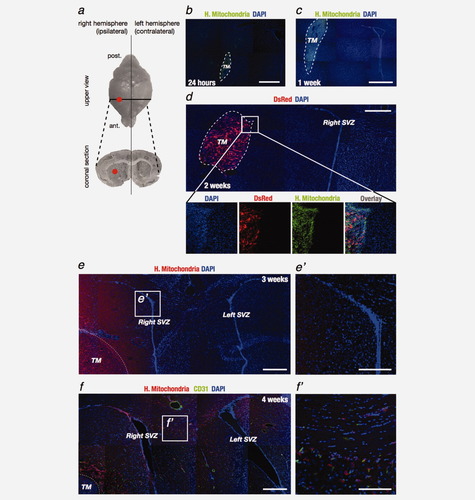
Immunodeficient mice injected with human U87MG cell suspension (50,000 cells/2 μL) develop tumor mass and human GBM cells fill the largest part of the right striatum at 3 weeks postinjection and invade the ipsi- and contra-lateral subventricular zones (SVZ) after 4 weeks. (a) Human cells are slowly inoculated in the right striatum (red point) following stereotactical coordinates. Human U87MG cells are recognized using an antibody specifically directed against human mitochondria, while mouse and human cell nuclei are visualized using a DAPI staining. (b) Mice are sacrificed 24 hr and (c) 1 week after the surgery and (d) the injection of red U87MG cells (transfected with a DsRed plasmid, red) for 2 weeks validated the anti-human mitochondria antibody (H. Mitochondria, green). (e) Three weeks after the xenograft, U87MG cells (H. Mitochondria, red) diffuse slightly in the striatum and (e′) are not present in the SVZ. (f) Interestingly, 4 weeks postinjection, tumor cells are found at the ipsilateral SVZ. Moreover, some human cells migrate to the contralateral SVZ. (f′) This migration occurs mainly along the corpus callosum and does not seem to be linked to blood vessels labelled with an antibody directed against CD31 (endothelial cells, green). Nuclei are counterstained with DAPI (blue, b-f). Scale bar = 150 μm for b and c, = 120 μm for d, = 130 μm for e, = 44.5 μm for e′, = 150 μm for f and = 51 μm for f′. TM: tumor mass; SVZ: subventricular zone.
GBM cells invade the adult mouse subventricular zones and migrate to the OB after their injection
Four weeks after the injection in the right striatum, as mice developed TM, we identified U87MG cells in the right SVZ and in the left (contralateral) SVZ (Fig. 1f). These cells appeared to have migrated and reached these regions during the 4th week postinjection, as they are not seen during the first 3 weeks postimplantation (Fig. 1e). Migrating human cells were observed in one the main fiber tract structure connecting the right and left SVZ (i.e., the corpus callosum, Fig. 1f′) but did not follow the blood vessels (Fig. 1f, CD31-staining of endothelial cells). No cells could be observed in the right or left dentate gyrus (data not shown). In the SVZ, U87MG cells were intermingled with normal NSC (Fig. 2a). As adult NSC in the SVZ can divide asymmetrically and give rise to neuroblasts that differentiate while migrating toward the OB through the rostral migratory stream (RMS),24 we looked for the presence of human cells both in the RMS and in the OB. Human U87MG cells were indeed found migrating to the granular layer of OB (Fig. 2b). To better analyze this migration, we performed sagittal sections of the left striatum, opposite to the tumor injection site, and demonstrated the presence of glioma cells in the more anterior part of the left SVZ, throughout the left RMS and into the glomerular layer of the left OB (Fig. 2c).
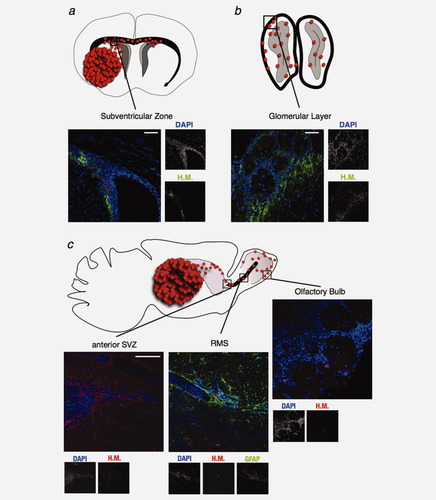
Some U87MG cells present in the neurogenic zone (subventricular zone or SVZ) migrate to the olfactory bulb (OB). (a) U87MG cells are observed both in the tumor mass (TM, represented by the numerous red circles) and in the ipsilateral subventricular zone on coronal sections processed on mice previously injected with 50,000 U87MG cells for four weeks (green). (b) Some human cells are found in the ipsilateral olfactory bulb (green). (c) As U87MG cells also migrate to the left SVZ, sagittal sections are obtained in the contralateral hemisphere. The migration of GBM cells from the SVZ to the OB occurs by the rostral migratory stream (RMS). Human U87MG cells (red) are present in the most anterior part of the left SVZ, in the left RMS characterised by an astrocytic barrier (GFAP positive cells, green) and in the glomerular layer of the left OB. (a–c) Nuclei are counterstained with DAPI (blue). H.M.: Human mitochondria signal. Scale bar = 40 μm for a and b and = 50 μm for c.
GB138 cells first established a GIC long term expanding culture (GB138 cells, see under “Cell Cultures”) and conclusively demonstrated its tumorigenic properties following serial orthotopic transplantations in immunodeficient mice. We next investigate if GB138 cells invade the SVZ niches in a similar fashion as their U87MG counterpart. Tumors formed by GB138 cells were infiltrative, do not form tumor mass and some of these cells were found in the ipsilateral SVZ 6 weeks after the injection (Supporting Information Table 1b). Two weeks later, GB138 cells migrate across the corpus callosum until the controlateral neurogenic niche (Supporting Information Table 1c). Moreover, some GB138 cells migrate through the RMS to the OB of both hemispheres within the next 2 weeks (Figs. 3a and 3b). Interestingly, we never observed GB138 cells in other anatomical structures such as the controlateral cortex or the dentate gyrus of the hippocampus, even 10 weeks after the implantation (Fig. 3a). These results suggest a tropism of GB138 invading cells for the SVZ and from there, for the OB.
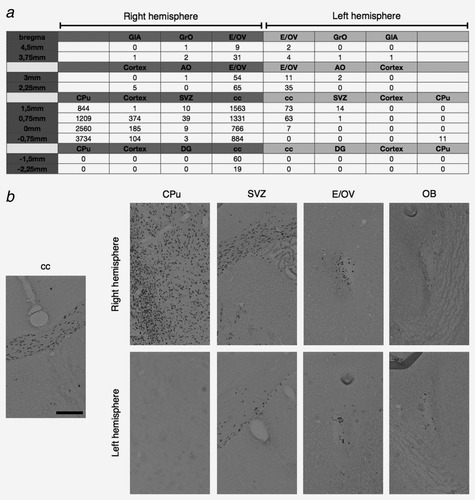
As U87MG cells, some GB138 cells invade the neurogenic niches when injected into the right striatum of immunodeficient mice. After 10 weeks, human cells labelled with an antibody directed against human nuclei are found in the ipsi- and contra- lateral SVZ. (a) Human cells quantification in the different anatomical regions with Mercator® software revealed that some GB138 cells specifically invaded the SVZ and are restricted to the left and right RMS in the anterior part of the mouse brain (b) as it is observed on coronal sections. The invasion of the contralateral hemisphere takes place through the corpus callosum (cc). Left part of the table corresponds to the right hemisphere (dark gray), and the right part of the table represents the left hemisphere (clear gray). GrO: granular cell layer of the olfactory bulb; GlA: glomerular layer of the accessory olfactory bulb; E/OV: ependymal and subependymal layer/olfactory ventricule; AO: anterior olfactory nucleus; SVZ: subventricular zone; CPu: caudate putamen (striatum); DG: dentate gyrus. Bregma column in a is the distance of the section from the bregma. Scale bar = 160 μm for b.
U87MG and GB138 human GBM cells express stem cells markers in vivo
To better characterize the phenotype of tumor cells found in the neurogenic zones, we studied the expression of Sox2, Nestin and CD133. Sox2 is a highly conserved HMG box transcription factor that plays an important role in the maintenance of NSC and is expressed in SVZ in the adult brain.25 Moreover, the inhibition of Sox2 in GIC leads these particular cells to stop their proliferation and to lose their tumorigenicity in immunodeficient mice.26 In our experiments, 2 weeks after stereotactic right striatal injection, Sox2-positive U87MG cells were present heterogeneously in the tumor mass (Fig. 4a). This observation is not surprising as it was recently shown that Sox2 is widely expressed in GBM.27 However, 4 weeks after surgery, Sox2-expressing U87MG cells were mainly observed at the edge of the tumor mass facing the neurogenic niche, along and into the SVZ (Figs. 4b and 4c). Unlike U87MG cells, GB138 cells form a more invasive tumor and are Sox2-positive wherever they are located in the brain, in the neurogenic zone, in the OB or during their migration through the corpus callosum and the RMS (Fig. 5a). We next examined the expression of Nestin. This intermediate filament-associated protein is expressed mostly in dividing cells during the early stages of development and the adult neurogenesis and now generally regarded as a classical marker for brain NSC.28 We demonstrated that Nestin is expressed by Sox2-positive U87MG invading cells (Fig. 4c) and by most of the GB138 cells (Fig. 5b). Finally, we used an antibody directed against CD133, a cell surface glycoprotein expressed in GBM.29 We did not observe any staining of U87MG cells both in vivo (and in vitro, data not shown). In contrast, GB138 cells largely expressed CD133 (Fig. 5c) and are labeled with an antibody directed against GFAP (Fig. 5d).
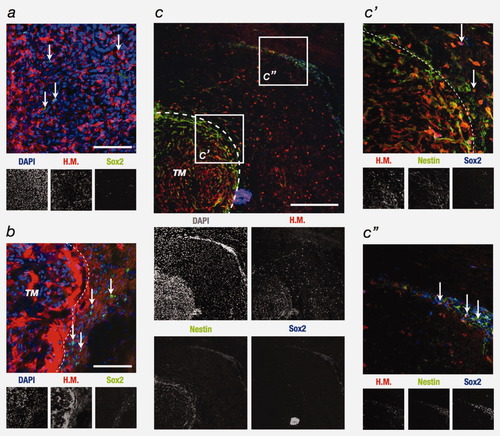
U87MG cells that have migrated into SVZ express Nestin and Sox2. (a) After two weeks, U87MG cells injected into the immunodeficient mice brain form a small tumor mass (red). At this stage, only several GBM cells are Sox2-positive and their localization is heterogeneous and scattered (red-green). (b) But after four weeks, U87MG cells have proliferated and a large tumor mass (TM) appears into the striatum. Now, U87MG Sox2-positive cells are observed in front of the right SVZ (red-green). (c′) In addition, these Sox2-positive cells expressed also largely Nestin (green), another immature cell marker. (c″) These Nestin- Sox2-positive U87MG cells are also observed within the ipsilateral SVZ (red-green-blue). (a and b) Arrows show human cells expressing Sox2. (c′ and c″) Arrows show human cells positive for Sox2 and Nestin antibodies. Nuclei are counterstained with DAPI. H.M.: Human Mitochondria signal (red). Scale bar = 40 μm for a and b and 60 μm for c.
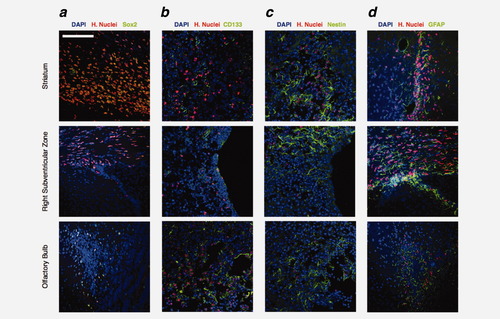
Human GB138 cells expressed (a) Sox2, (b) CD133, (c) Nestin and (d) GFAP (green) at 10 weeks after inoculation in immunodeficient mice brain. More precisely, GB138 cells expressed these markers whichever the localization is: striatum (upper panels), subventricular zone (middle panels) and olfactory bulb (lower panels). H.Nuclei: Human Nuclei (red). Nuclei were marked with DAPI (blue). Scale bar = 65 μm for a–d.
At the same time, we studied the expression of neuronal markers (NeuN, GABA and B-III-tubulin) to highlight the differentiation of U87MG cells migrating to the OB but did not observe any signals (data not shown). Indeed, true neuroblasts generated from NSC in SVZ differentiate into neurons while integrating the OB.30 However, some U87MG cells located in SVZ expressed Doublecortin (Dcx), a microtubule associated-protein (Supporting Information Fig. 1). Dcx plays a role in the neuroblasts migration to the OB both during development and in adulthood and is considered as an early marker of neuronal specification and differentiation.31 Interestingly, U87MG cells expressing Dcx are nestin-negative as it is observed within host neuroblast.
To conclude, we were unable to distinguish a specific profile of stem cells markers for GB138 cells invading the SVZ from those located in the bulk of cells in the striatum as we did for U87MG cell line. These results correlate with recent studies proving that self-renewing capacities of GIC seem to be not limited to a uniform population of cancer stem cells but can be linked with cell lineages expressing a range of markers.32
U87MG cells isolated from the TM, SVZ and the OB formed self-renewable spheres under specific in vitro conditions
To further investigate the biological properties of GBM cells found in the neurogenic zones, we decided to carry out their ability to initiate self-renewable “spheres” when cultured in specific conditions. Notably, GIC has to meet this criterion. Because of the very slow growth rate of GB138 cells, we decided to focus on U87MG cells. Thus, we isolated U87MG cells specifically from the TM, the ipsi- and contro- lateral SVZ and the OB of immunodeficient mice 4 weeks after their striatal injection (Fig. 6a). Cells obtained from these mouse brain regions were cultivated in a specific cell culture medium used for NSC and GIC. In these conditions, we could easily select U87MG cells that were able to form Nestin-positive spheres (Fig. 6b). Moreover, single cells obtained from the dissociation of these primary spheres have the ability to form secondary spheres that are also Nestin-positive (Fig. 6b). These experiments suggest the self-renewability of the U87MG cell populations derived from the four anatomical regions (TM, ipsi- or controlateral SVZ and OB).
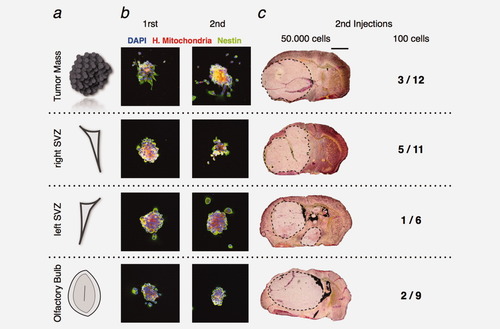
U87MG cells isolated from the TM, the SVZ and the OB form self-renewable spheres in vitro and preserve their tumorigenicity in vivo. (a) At four weeks postinjection, U87MG cells isolated from the tumor mass, the right SVZ, the left SVZ and the OB were seeded in the appropriate medium. (b) Within 5–6 days, we observed spheres containing Nestin-positive cells in the different cultures (green). New spheres containing Nestin-positive cells were obtained from single cell derived from primary spheres assessing their self-renewability. (c) Secondary injections of 50,000 U87MG cells isolated from the primary TM, the ipsilateral and contralateral SVZ and the OB rapidly give rise to the formation of new tumors within 2weeks (Hematoxylin/Eosin coloration). Colorations show the presence of tumors. In addition, several mice were injected with 100 U87MG cells isolated from the different regions and sacrificed 4 weeks later. The development of a tumor mass was reported for each population (number of mice with a tumor/total number of animal). Statistical analysis (chi-square test) was not significant. (b) Nuclei are counterstained with DAPI. Scale bar = 255 μm for b and = 1.8 mm for c.
Injection of distant invaders to new immunodeficient mice rapidly induced the formation of a tumor
The operational definition of GIC includes the ability to initiate tumor in xenograft models. To evaluate the experimental tumorigenicity of the migrating GBM cells, we performed injection of 50,000 human U87MG cells harvested from the primary tumor mass, the ipsilateral SVZ, the controlateral SVZ and the OB into immunosuppressed mice. Accordingly with former studies, mice grafted with these previously injected and then repurified U87MG cells had to be sacrificed 2 weeks after the grafting procedure because they were dying. Hematoxylin/Eosin (H/E) coloration showed the formation of a very large tumor in the right hemisphere (Fig. 6c). The large size of these secondary tumors observed 2 weeks after injection were never observed in the case of primary tumors 4 weeks after injection and prevents us to observe a new specific migration of human U87MG cells into neurogenic zones. It is now known that GIC responsible to experimental tumor represent a minor subset of the total cells within GBM, like in U87MG cell line.33 It is possible to estimate the amount of GIC using dilution assays through xenograft in immunodeficient mice brain.5 To do so, we then injected only 100 U87MG cells harvested from the primary tumor mass, the ipsilateral SVZ, the controlateral SVZ and the OB into the striatum of adult mice. As injection of 100 native U87MG cells never induce tumors, we observed the formation of three tumors out of 12 mice injected with 100 cells from the primary tumor mass, five tumors out of 11 mice injected with 100 cells from the controlateral SVZ, one tumor out of six mice injected with 100 cells from the ipsilateral SVZ and finally two tumors out of nine mice injected with 100 cells from the OB (Fig. 6c). Those tumors harbor the same infiltrative behavior as the naïve U87MG cells. Chi-square test analysis between the four conditions was not significant showing the same enrichment in GIC in the different populations of U87MG cells. In conclusion, we have demonstrated that a few GBM cells refugee in the neurogenic niches could initiate tumor.
Discussion
The concept of cancer-initiating cells34 has been extended to human glioblastoma multiforme (GBM) with the discovery in these tumors of multipotent cells capable of both self-renewal and malignant tumor formation.4 The concept was strengthened with the experimental demonstration that GBM formation in mice may arise from genomic instability in one or in several NSC (and not astrocytes).35, 36 Moreover, glial tumors originating from different brain regions are molecularly distinct and might arise from distinct populations of site-restricted progenitor cells.37, 38 Clinical practices also brought neurogenic niches and malignant glioma together. A recent study have showed that patients whose bilateral SVZ received greater than the median SVZ dose had a significant improvement in progression-free survival.39
Interestingly, cancer-initiating cells tend to hide in specific niches, such as the perivascular space, that influence their metabolism and maybe, their intrinsic treatment resistance.40 This is reminiscent of that of normal stem cells, which must remain located in neurogenic zones to maintain their stem cells characteristics.41 We have thus addressed the question whether the known neurogenic zone, the SVZ and the dentatus gyrus, could attract cancer-initiating cells of GBM or GIC.
To test this hypothesis, we performed stereotactic injections of U87MG cells in the striatum of immunodeficient mice. U87MG cells contain a very small population of multipotent and self-renewable cancer stem cells.33 We observed that, 3 to 4 weeks postinjection, U87MG cells that expressed the stem cell markers Nestin and Sox2 in the tumor mass were found mainly in the edge of the tumor facing the SVZ. Moreover, some of these Sox2/Nestin-positive U87MG cells migrated toward the ipsi- and contro-lateral SVZ of host mice, where all tumor cells expressed these markers. Interestingly, some GBM cells located in the neurogenic zones also migrated to the OB, but their potential of differentiation is limited to the expression of the immature neurons marker Dcx. However, we never observed U87MG cells into the dentatus gyrus of hippocampus, suggesting a specific tropism for SVZ from these Sox2/nestin-positive U87MG cells.
An important limitation of the use of U87MG cell line is that this brain tumor model did not resume typical characteristics of human GBM.42, 43 Therefore, we also used GIC derived from a primary GBM cell culture as they mimic more closely the infiltrative behavior of human GBM. When injected in immunodeficient mice brains, GIC derived from an acutely resected woman GBM (GB138 cells) show the same tropism for the neurogenic niches (SVZ) and then for the OB, but not for dentatus gyrus. However, by comparison with U87MG cells, all GB138 cells expressed classical stem cell markers wherever they are. This point is consistent with data demonstrating that cancer-initiating cells could be either CD133 positive or CD133 negative.44 According to this observation, numerous new studies describe GIC purification methods based on marker-independent strategies.45, 46
Indeed, when isolated and purified from the various invaded brain regions, U87MG cells maintained their capacity to form primary and secondary spheres expressing Nestin in vitro when (sub)cultured in stem cell conditions. Moreover, they induced rapidly the formation of very large tumors when reinjected in the striatum of immunodeficient mice. In fact, these tumors developed at least two times faster than the native tumors. This increased growth rate of secondary transplants (regarded as an aggressive behavior of the tumor) is largely described and is correlated with the enrichment of the fraction of GIC that yields faster growing tumors than other tumor cells.47 Therefore, we performed limiting dilution in vivo assays to determine variations in the frequency of cancer-initiating cells within U87MG cell populations. Interestingly, this approach allows us to highlight the GIC feature of GBM cells refugee in the SVZ and OB, like for those forming the tumor mass. Thus, although the distinct phenotype of cells present in the four anatomical regions is not clear, their biological behavior do appear similar or overlapping. Actually, GIC located in the neurogenic zones have competencies for self-renewal and experimental tumor initiation. This position distant from the tumor mass and their intrinsic characteristic of GIC may allow them to escape current treatments.48, 49
Taken together, our observations demonstrate that SVZ and OB can attract and harbor glioblastoma-initiating cells. Consequently, it is tempting to speculate that neurogenic zones may constitute a reservoir for tumor recurrence. Notwithstanding a possible “niche” influence of neurogenic zones on the intrinsic treatment resistance of tumor cells, the potential clinical implication of our findings may be important for the future definition of therapeutic target volumes.
Acknowledgements
The authors thank P. Ernst-Gengoux, O. Pierard and O. Hougrand for their expert technical assistance. P. Beukelaers, M. Bodson, D. Delvaux, R. Vandenbosch and J. Crèvecoeur are very appreciated for their helpful comments.




