Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City
Abstract
N-Nitroso compounds (NOC) are potent animal carcinogens and potential human carcinogens. The primary source of exposure for most individuals may be endogenous formation, a process that can be inhibited by dietary polyphenols. To estimate the risk of gastric cancer (GC) in relation to the individual and combined consumption of polyphenols and NOC precursors (nitrate and nitrite), a population-based case–control study was carried out in Mexico City from 2004 to 2005 including 257 histologically confirmed GC cases and 478 controls. Intake of polyphenols, nitrate and nitrite were estimated using a food frequency questionnaire. High intakes of cinnamic acids, secoisolariciresinol and coumestrol were associated with an ∼50% reduction in GC risk. A high intake of total nitrite as well as nitrate and nitrite from animal sources doubled the GC risk. Odds ratios around 2-fold were observed among individuals with both low intake of cinnamic acids, secoisolariciresinol or coumestrol and high intake of animal-derived nitrate or nitrite, compared to high intake of the polyphenols and low animal nitrate or nitrite intake, respectively. Results were similar for both the intestinal and diffuse types of GC. Our results show, for the first time, a protective effect for GC because of higher intake of cinnamic acids, secoisolariciresinol and coumestrol, and suggest that these polyphenols reduce GC risk through inhibition of endogenous nitrosation. The main sources of these polyphenols were pears, mangos and beans for cinnamic acids; beans, carrots and squash for secoisolariciresinol and legumes for coumestrol. © 2009 UICC
In Mexico, gastric cancer (GC) rates show an increasing trend with time,1 in contrast to other countries, and remains the 2nd leading cause of cancer mortality.2 Nitrate and nitrite are precursors for the endogenous formation of N-nitroso compounds (NOC), which are carcinogenic in animals and, potentially, in humans.3 Ingested nitrate is absorbed in the small intestine and ∼25% is excreted in the mouth, where oral bacteria reduce about 20% to nitrite (about 5% of ingested nitrate).In the acidic stomach, nitrite forms nitrous acid, which decomposes into various reactive nitrogen species (RNS). Nitrite and RNS react with nitrosatable compounds, mainly amines and amides, to form NOC.4 The formation of NOC is inhibited by some antioxidants, such as polyphenols5, 6 and vitamins C and E.4, 7 For this reason, a low consumption of these inhibitors of NOC formation, (INC) together with a high consumption of nitrate and/or nitrite, results in an increase in the endogenous formation of NOC.3
Contrasting with consistent results showing an increase in the risk of GC because of nitrite consumption,8 the association with nitrate intake is less certain. Some investigators observed no association with nitrate consumption9-16; whereas, others have observed a decrease in GC risk with increasing nitrate consumption.17, 18 These apparent contradictory results reflect the different sources of dietary nitrite and nitrate. When drinking water nitrate levels are not elevated, the major sources of dietary nitrate are vegetables3 that contain INC, thus inhibiting endogenous nitrosation. In contrast, the main source of dietary nitrite is usually preserved meats, which also contain amines and amides, precursors necessary for endogenous nitrosation.3
Polyphenols may play a role in preventing or inhibiting carcinogenesis by several mechanisms, including the reduction of cell proliferation, antiestrogenic/estrogenic activity, induction of cancer cell apoptosis, prevention of oxidation, induction of detoxification enzymes and regulation of immune responses.19 Moreover, under acidic conditions in the stomach, some polyphenols, such as flavanols and phenolic acids, act as INC by scavenging nitrite and RNS.5, 6 The epidemiological evidence for an inverse association between polyphenols consumption and risk of GC is limited,20-22 with some evidence for a protective effect for consumption of 2 subclasses of flavonoids, the flavonols20 and the flavonones.21 To date, the relationship of the consumption of other polyphenols such as phenolic acids, lignans, coumestrol with GC has not been evaluated, although these classes of polyphenols have been inversely associated with risk of other cancers.22-26
Four studies have evaluated the joint effects of nitrate or nitrite consumption and INC on GC risk.9, 13, 15, 27 In 3 of those studies,9, 15, 27 the highest risks were observed among people with high consumption of nitrate and/or nitrite and low consumption of vitamins C and/or E, subgroups of the population which would be expected to have the highest rates of endogenous nitrosation. No studies have evaluated other dietary INC, such as polyphenols. Therefore, the objective of our study was to evaluate the individual and joint effects of consumption of polyphenols, nitrate and nitriteon GC risk.
Material and methods
Study population
We conducted a population-based case–control study of GC in Mexico City between January, 2004 and December, 2005. Cases were patients with histologically confirmed gastric adenocarcinomas without a history of another type of cancer, who were at least 20 years old and resided in the study area. Patients were recruited in 9 of the main tertiary care hospitals in Mexico City, where 60% of the GC cases are diagnosed (Hospital de Oncología, Hospital de Especialidades del Centro Medico Siglo XXI, Hospital de Especialidades La Raza, Hospital 20 de Noviembre, Hospital Adolfo López Mateos, Hospital General, Instituto Nacional de Ciencias Medicas y Nutrición Salvador Zubirán, Hospital Juárez and Instituto Nacional de Cancerología). A total of 263 patients with a histopathological diagnosis of GC were identified and 257 agreed to participate (response rate of 97.7%). A board-certified gastropathologist reviewed each GC diagnosis and classified them as intestinal, diffuse or mixed, according to Lauren's criteria.28
For each case, up to 2 healthy controls without a history of cancer, who resided in the same geographic area as the cases, were selected and matched to the cases by age (±5 years) and gender. Eligible controls were identified from a sampling frame of households (a representative list of domiciliary addresses) used for the Mexican National Health Survey. If more than 1 member of a household fitted the eligibility criteria, 1 was chosen at random to be interviewed. When no one in the selected household fitted the eligibility criteria, interviewers sought participants in the house to the right of that which was originally selected. A total of 478 of 507 eligible controls agreed to participate (response rate of 94.3%). The study protocol was approved by the Committee of Research and Ethics of Mexico's National Institute of Public Health.
Interviews
After obtaining informed consent, interviewers administered structured questionnaires that collected information about the participant's sociodemographic characteristics, medical history, lifestyle factors and dietary patterns. Cases were interviewed at the hospital and controls in their homes. The dietary information was ascertained for the time period of 3 years before diagnosis for cases and 3 years before the interview for controls.
Helicobacter pylori CagA positivity
Serum samples were obtained at the time of the interview by nurse phlebotomists. The presence of immunoglobulin G antibodies against Helicobacter pylori (H. pylori) CagA+ antigen was determined by an ELISA based on the presence of serum IgG antibodies against orv220, a 65,000 Dalton recombinant cagA-encoded protein purified from Escherichia coli.29 CagA positivity (representing carriage of a cagA+ strain) was defined as an optical density of >0.35.29
Dietary information
We used a 127-item food frequency questionnaire developed for the Mexican population, which was recently updated and previously validated.30 Frequency of consumption of the total standard portion sizes per day was obtained in 10 categories ranging from never consumed to consumed 6 times a day. The intake of nutrients, polyphenols, nitrate and nitrite, was determined by multiplying their content in each food portion by the daily frequency of consumption, using the Microsoft Visual FoxPro 6.0 program.31 We adjusted the frequency of consumption of fruits and vegetables according to their availability in the market. For example, for plums, the total estimated frequency was divided by 4, because they are typically only available 3 months/year.
The macro- and micronutrient content of the food items was obtained using the Food Intake Analysis System computerized program version 3.0 (FIAS),32 which was developed for a large Mexican American population in Texas and contains nutritional information about many commonly eaten Mexican foods. The nutritional content of some local Mexican foods that were not in the database was added and adapted to the FIAS software, as we described previously.33 The primary food sources of polyphenols in this population are shown in Table I. The detailed methodology regarding the selection of polyphenol nutrient values used in our study was published elsewhere.34 The nitrate and nitrite content for foods in our study was obtained from several sources.35-37
| Polyphenols | Dietary sources1 |
|---|---|
| Flavonoids | |
| Flavonols | Pasta soup, onion, hot sauce, cooked tomato with garlic, potato. |
| Flavones | Hot sauce, pasta soup. |
| Flavanols | Broad beans, grapes, strawberry, pear, mango, yellow peach. |
| Phenolic acids | |
| Cinammic acids | Mango, pear, pinto beans, papaya, potato, pineapple, cooked tomato with garlic, orange juice. |
| Lignans | |
| Lariciresinol | Broccoli, pear, strawberry, yellow peach, cauliflower, orange, squash, mandarin, grapes, melon. |
| Pinoresinol | Mole, yellow peach, strawberry, broccoli, red plum, pear, cauliflower, squash, orange, carrot, melon, mandarin, tomato, pipian. |
| Secoisolariciresinol | Carrot, pinto beans, yellow peach, squash, melon, cooked tomato with garlic, potato. |
| Matairesinol | Red wine, mole, grapes, orange, mandarin. |
| Coumestan | |
| Coumestrol | Green peas, pinto beans, broad beans. |
- 1 That explain ≥10% to the daily intake in controls.
Statistical analysis
Nine subjects who reported a daily caloric intake greater than 4,500 kcal were excluded from the analysis, giving a final sample size of 248 cases and 478 controls. Selected dietary and other characteristics were compared between cases and controls, using the Mann-Whitney and the χ2 tests. Median intakes of individual foods and nutrients were adjusted for total energy intake using the residual method.38 We evaluated tertiles of distributions of polyphenols, nitrate and nitrite among controls in relation to GC risk. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using unconditional logistic regression analysis. We computed OR's adjusting for age (years), gender, energy (kcal/day), schooling (years), H. pylori CagA status (positive/negative), chili consumption (none, low, medium or high), salt consumption (never, rarely, frequently or with every meal) and alcohol intake (g/day). We also adjusted models for consumption of potential INC including vitamin C (mg/day) and vitamin E (ATE/day), fruits and vegetables (portions/day) and polyphenols (mg or mcg/day). In addition, we combined the intake data for nitrate, nitrite and polyphenols by computing the ratio of nitrate and nitrite consumption to the intake of each polyphenol of interest, and we evaluated tertiles of these ratios using those in the lowest tertile as the reference group. All models were also stratified by histological type (intestinal/diffuse) of GC. Trend tests were computed by including the categorical variables in the model as a continuous variable. All analyses were conducted using the Stata 9/SE statistical program.39
Results
Cases had significantly more years of schooling, higher prevalence of H. pylori CagA antibodies, higher total energy intake and greater consumption of alcohol, salt and chili than controls (Table II). Consumption of vegetables was significantly greater among controls (Table II). Controls had significantly higher consumption of cinnamic acids, total lignans, secoisolariciresinol and coumestrol (Table III). Consumption of flavanols and pinoresinol was also greater among controls, although differences were not statistically significant (Table III). Total nitrate, and nitrate and nitrite from fruits and vegetables, was significantly greater among controls; whereas, cases had significantly greater consumption of total nitrite and a greater consumption of nitrate and nitrite from animal sources; the latter was not statistically significant (Table III).
| Variables | Cases (248) | Controls (478) | p1 |
|---|---|---|---|
| Age (years) | |||
| Median (P25–P75) | 59 (49–67) | 60 (49–70) | 0.29 |
| Gender (%) | |||
| Male | 54.0 | 54.0 | 0.99 |
| Schooling (years) | |||
| Median (P10–P90) | 6 (2–12) | 6 (0–12) | <0.01 |
| H. pylori CagA status (%) | |||
| Positive | 76.3 | 66.6 | <0.01 |
| Alcohol (%) | |||
| Consumption | 49.2 | 36.8 | <0.01 |
| Smoking (%) | |||
| Ever | 52.0 | 51.4 | 0.86 |
| Cigarettes (number/week) | |||
| Median (P25–P75) | 2.0 (0–35) | 0.2 (0–28) | 0.68 |
| Energy (kcal/day) | |||
| Median (P25–P75) | 2637.8 (2155.3–3046.9) | 2149.1 (1744.4–2595.8) | <0.01 |
| Vitamin C (mg/day)2 | |||
| Median (P25–P75) | 166.7 (131.3–217.5) | 170.7 (126.9–223.6) | 0.62 |
| Vitamin E (ATE/day)2 | |||
| Median (P25–P75) | 18.7 (16.1–20.8) | 18.5 (15.9–21) | 0.96 |
| Fruits (portions/day)2 | |||
| Median (P25–P75) | 1.7 (1.1–2.3) | 1.7 (1.1–2.5) | 0.41 |
| Vegetables (portions/day)2 | |||
| Median (P25–P75) | 3.5 (2.9–4.2) | 4.0 (3.4–4.9) | <0.01 |
| Added salt (%) | |||
| Rarely | 29 | 37.9 | |
| Frequently | 8.1 | 7.3 | |
| With every meal | 30.2 | 17.6 | <0.01 |
| Chili consumption (%) | |||
| A little | 18.6 | 21.8 | |
| Medium | 38.3 | 42.5 | |
| Much | 41.9 | 28.2 | <0.01 |
- P, percentile.
- 1 p value for Mann-Whitney (Median) or χ2 test (Percentages).
- 2 Energy adjusted by residual method.38
| Compounds | Cases (248) Median (P25–P75) | Controls (478) Median (P25–P75) | p2 |
|---|---|---|---|
| Polyphenols (mg/day) | |||
| Total | 52.60 (43.92–60.34) | 52.28 (44.28–60.85) | 0.72 |
| Flavonoids (mg/day) | |||
| Total | 50.54 (42.07–58.44) | 50.36 (42.29–59.14) | 0.86 |
| Flavonols | 35.84 (30.01–41.82) | 35.60 (29.99–41.67) | 0.78 |
| Flavones | 6.96 (4.83–8.89) | 6.55 (4.62–8.48) | 0.13 |
| Flavanols | 5.84 (3.44–9.48) | 6.91 (3.80–11.00) | 0.10 |
| Phenolic acids (mcg/day) | |||
| Cinnamic acids | 98.59 (76.87–123.28) | 108.22 (86.21–140.47) | <0.01 |
| Lignans (mcg/day) | |||
| Total | 321.98 (250.44–430.53) | 347.74 (268.99–473.50) | 0.01 |
| Lariciresinol | 179.79 (130.44–253.82) | 190.48 (135.46–277.24) | 0.11 |
| Pinoresinol | 85.31 (61.95–108.34) | 88.48 (65.53–129.49) | 0.10 |
| Secoisolariciresinol | 59.61 (45.18–71.63) | 67.68 (55.55–79.86) | <0.01 |
| Matairesinol | 0.72 (0.43–1.14) | 0.69 (0.40–1.17) | 0.37 |
| Coumestan (mg/day) | |||
| Coumestrol | 1.32 (0.72–1.85) | 1.62 (1.02–2.06) | <0.01 |
| Nitrate (mg/day) | |||
| Total | 101.90 (76.84–140.58) | 108.91 (81.95–157.05) | 0.02 |
| Animal | 2.52 (1.74–6.26) | 2.10 (1.53–6.27) | 0.07 |
| Fruits and vegetables | 93.05 (70.35–133.48) | 100.97 (74.17–149.52) | 0.02 |
| Nitrite (mg/day) | |||
| Total | 1.14 (0.96–1.37) | 1.10 (0.89–1.34) | 0.05 |
| Animal | 0.30 (0.18–0.58) | 0.23 (0.16–0.59) | 0.09 |
| Fruits and vegetables | 0.15 (0.12–0.22) | 0.17 (0.12–0.24) | 0.03 |
- 1 Energy adjusted by residual method.38
- 2 p value for Mann-Whitney test.
- P, percentile.
Increasing consumption of cinnamic acids, secoisolariciresinol and coumestrol was associated with significant inverse trends in GC risk (Table IV) and remained after stratifying by histological type of GC (data not included in the table). The inverse associations for cinnamic acids, secoisolariciresinol and coumestrol also remained significant after further adjustment for vitamin C and E and for fruits and vegetables. Mutual adjustment of cinnamic acids, secoisolariciresionol and coumestrol intakes resulted in marginally significant inverse associations only for secoisolariciresinol and coumestrol. The intake of flavonoids (flavonols, flavones and flavanols) and lignans (lariciresinol, pinoresinol and matairesinol) did not show significant associations.
| Polyphenols | Model 1 OR (CI 95%) | Model 2 OR (CI 95%) | Model 3 OR (CI 95%) | Model 4 OR (CI 95%) |
|---|---|---|---|---|
| Phenolic acids | ||||
| Cinnamic acids (mcg/day) | ||||
| ≤93.8 | 1.00 – | 1.00 – | 1.00 – | 1.00 – |
| >93.8–127.0 | 0.83 (0.55–1.24) | 0.80 (0.53–1.20) | 0.88 (0.58–1.32) | 1.23 (0.79–1.92) |
| >127.0 | 0.52 (0.34–0.81) | 0.49 (0.31–0.78) | 0.61 (0.38–0.97) | 0.80 (0.49–1.31) |
| p for trend | 0.004 | 0.003 | 0.040 | 0.348 |
| Lignans | ||||
| Secoisolariciresinol (mcg/day) | ||||
| ≤60.0 | 1.00 – | 1.00 – | 1.00 – | 1.00 – |
| >60.0–75.5 | 0.45 (0.30–0.69) | 0.44 (0.29–0.68) | 0.47 (0.31–0.71) | 0.56 (0.35–0.90) |
| >75.5 | 0.42 (0.27–0.65) | 0.41 (0.26–0.64) | 0.47 (0.30–0.74) | 0.57 (0.32–0.99) |
| p for trend | <0.001 | <0.001 | <0.001 | 0.057 |
| Coumestan | ||||
| Coumestrol (mg/day) | ||||
| ≤1.3 | 1.00 – | 1.00 – | 1.00 – | 1.00 – |
| >1.3–1.9 | 0.45 (0.30–0.69) | 0.45 (0.30–0.69) | 0.45 (0.29–0.69) | 0.54 (0.34–0.86) |
| >1.9 | 0.45 (0.29–0.70) | 0.45 (0.29–0.71) | 0.42 (0.27–0.65) | 0.67 (0.39–1.16) |
| p for trend | <0.001 | <0.001 | <0.001 | 0.067 |
- Model 1. Adjusted by energy, age, gender, H. pylori CagA status, schooling and consumptions of salt, chili and alcohol.
- Model 2. Adjusted by variables in Model 1 plus vitamins C and E.
- Model 3. Adjusted by variables in Model 2 plus fruits and vegetables.
- Model 4. Adjusted by variables in Model 3 plus mutual adjustment by polyphenols (cinnamic acids, secoisolariciresinol and coumestrol).
- Based on 228 cases and 467 controls, because of missing values in one or more covariables.
High consumption of total nitrite, and nitrate and nitrite from animal sources was associated with an increased risk of GC (Table V). In contrast, high consumption of total nitrate or nitrate from fruits and vegetables (which accounted for >90% of nitrate intake) was associated with decreased risk. The sum of nitrite and nitrate from animal sources showed a similar positive association with GC risk as that for animal nitrate (OR: 1.87; CI 95%: 1.19–2.91, data not included in the table). The direction of the association was similar for both intestinal and diffuse types of GC; however, associations tended to be stronger for the diffuse type of GC.
| Nitrate and nitrite (mg/day) | All GC | Intestinal GC | Diffuse GC | |||
|---|---|---|---|---|---|---|
| OR (CI 95%) | p1 | OR (CI 95%) | p1 | OR (CI 95%) | p1 | |
| Nitrate | ||||||
| Total | ||||||
| ≤90.4 | 1.00 – | 1.00 – | 1.00 – | |||
| >90.4–141.7 | 0.93 (0.62–1.39) | 0.97 (0.54–1.75) | 0.97 (0.61–1.56) | |||
| >141.7 | 0.61 (0.39–0.96) | 0.035 | 0.76 (0.40–1.42) | 0.389 | 0.55 (0.32–0.93) | 0.032 |
| In animal | ||||||
| ≤1.7 | 1.00 – | 1.00 – | 1.00 – | |||
| >1.7–3.9 | 1.28 (0.82–2.00) | 1.53 (0.79–2.97) | 1.17 (0.69–1.98) | |||
| >3.9 | 1.92 (1.23–3.02) | 0.004 | 1.89 (0.97–3.67) | 0.063 | 1.99 (1.18–3.37) | 0.009 |
| In fruits and vegetables | ||||||
| ≤81.7 | 1.00 – | 1.00 – | 1.00 – | |||
| >81.7–134.9 | 0.93 (0.62–1.39) | 0.92 (0.51–1.66) | 1.02 (0.64–1.63) | |||
| >134.9 | 0.62 (0.40–0.97) | 0.038 | 0.73 (0.39–1.36) | 0.331 | 0.57 (0.33–0.97) | 0.047 |
| Nitrite | ||||||
| Total | ||||||
| ≤1.0 | 1.00 – | 1.00 – | 1.00 – | |||
| >1.0–1.2 | 1.07 (0.69–1.65) | 1.37 (0.72–2.64) | 0.88 (0.53–1.48) | |||
| >1.2 | 1.52 (0.99–2.34) | 0.052 | 1.76 (0.92–3.37) | 0.087 | 1.39 (0.84–2.29) | 0.186 |
| In animal | ||||||
| ≤0.2 | 1.00 – | 1.00 – | 1.00 – | |||
| >0.2–0.4 | 0.78 (0.50–1.21) | 0.65 (0.33–1.25) | 0.83 (0.49–1.42) | |||
| >0.4 | 1.56 (1.02–2.4) | 0.030 | 1.31 (0.71–2.39) | 0.334 | 1.74 (1.04–2.89) | 0.026 |
| In fruits and vegetables | ||||||
| ≤0.1 | 1.00 – | 1.00 – | 1.00 – | |||
| >0.1–0.2 | 0.81 (0.54–1.21) | 1.07 (0.59–1.95) | 0.7 (0.43–1.12) | |||
| >0.2 | 0.77 (0.50–1.18) | 0.216 | 1.06 (0.57–1.97) | 0.850 | 0.64 (0.39–1.06) | 0.069 |
- Based on 228 cases and 467 controls, because of missing values in one or more covariables and adjusted by energy, age, gender, H. pylori CagA status, schooling and consumptions of salt, chili and alcohol.
- 1 p value for trend.
We observed a significant increase in GC risk with increasing tertiles of the ratio of consumption of nitrate and nitrite from animal sources with those of cinnamic acids, secoisolariciresinol and coumestrol (Fig. 1). Results were similar for both histological types of GC, except for the ratio of nitrite from animal sources to secoisolariciresinol, for which the positive trend in risk was only marginally significant for the intestinal type of GC (data not shown).
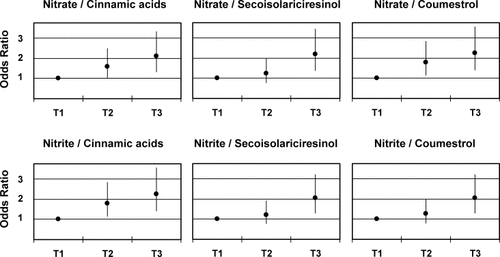
Ratios of nitrate and nitrite from animal sources with selected polyphenols intake and gastric cancer risk. Based on 228 cases and 467 controls and adjusted by energy, age, gender, H. pylori CagA status, schooling and consumption of salt, chili and alcohol. T1, tertile 1; T2, tertile 2; T3, tertile 3.
Discussion
Our results show, for the first time, a reduced risk for GC associated with higher consumption of several polyphenols including the cinnamic acids, secoisolariciresinol and coumestrol. We observed an increased risk of GC with higher consumption of nitrate and nitrite from animal sources, and risk was highest among those with high animal nitrate or nitrite intake and low polyphenol intake, and, similar patterns of risk for both the intestinal and diffuse types of GC. Our results also confirm the impact of known risk factors of GC40 such as salt intake and H. pylori seropositivity.
Our results for dietary nitrite are consistent with most previous studies, which observed increased risk of GC with higher total nitrite consumption.9, 17, 27, 41 In contrast to previous studies, we evaluated animal sources of nitrite and nitrate separately and clarified that animal sources of nitrite and nitrate were responsible for the increased GC risk in our study population. A separate evaluation of animal and plant sources of nitrate is particularly important because the majority of dietary nitrate intake comes from vegetables when drinking water levels are not substantially elevated.3 Most previous studies have not separated vegetable and animal sources of nitrate, which may have obscured associations with GC risk.17, 18
A recent review of the evidence for the carcinogenicity of ingested nitrate and nitrite by the International Agency for Research concluded that nitrate and nitrite ingestion under conditions that are likely to result in endogenous nitrosation is probably carcinogenic to humans.3 Support for this conclusion came partly from epidemiologic studies that found the highest GC risk among those with high consumption of dietary nitrite or nitrate and low consumption of vitamins C and/or E,9, 15, 27 a similar pattern to what we observed for nitrite and nitrate and specific polyphenols.
Cohort studies in Finland,42-44 Holland22 and the United States45 found no significant association between consumption of total or specific flavonoids and risk of GC. In contrast, case– control studies in Spain20 and Greece21 found significant inverse associations with intake of total flavonols and the flavone luteolin, and flavanones. We did not observe any significant associations with the flavonoid subgroups flavonols, flavones or flavanols. To our knowledge, no studies of GC have evaluated consumption of phenolic acids, lignans or coumestan. Thus, further studies are needed to clarify the associations between intake of specific polyphenols and GC risk.
On the basis of intake among controls, we found that the main sources of cinnamic acids were pinto beans, pear and mango; those of secoisolariciresinol were pinto beans, carrot and squash and those of coumestrol were some legumes (pinto beans, broad beans and green pea). A recent study46 demonstrated that pinto beans had a high total phenol content that was similar to that of cranberries and blueberries, one of the most important sources of polyphenols in fruits. The typical Mexican diet includes a high consumption of beans, which has been associated with a decreased risk of GC risk in a previous study in Mexico.47 Considering the potential importance that this food may offer for GC prevention, quantification of the polyphenol content in more than 200 varieties existing in the country48 deserves greater attention.
Our study has several limitations. Patients from private hospitals were not included and we were not able to determine if consumption patterns were different from those of our study population. Therefore, our results may not be generalizable to the entire Mexican population. Recall bias is a concern in case–control studies because cases may recall their dietary intakes more accurately than controls because of concerns about their disease. However, our study population was unlikely to have been aware of the specific dietary hypotheses we evaluated; therefore, differential reporting by case–control status would not be expected to be substantial. Nevertheless, our results may be underestimated because of nondifferential measurement error that is inherent in dietary assessments using questionnaires49 and food composition tables, for example, nitrate and nitrite levels are only our best estimation of the real levels that should vary during the seasons of the year.
Nitrite formed in vivo and that from animal sources are chemically indistinguishable in terms of its properties to react with NOC precursors. It might be possible that some in vivo formation of nitrite (from nitrate) took place, but no data from nitrate levels in the consumed vegetables by the study subjects are available to estimate its magnitude; however, we found that levels of nitrate in water in the study area are low (0.3–2.9 mg/L in drinking water),50 which limits the contribution of ingested nitrate from water in this urban population.
In conclusion, we found that a dietary pattern of high consumption of nitrate and nitrite from animal sources and low consumption of foods that are sources of specific polyphenols increased GC risk. These findings suggest that nitrate and nitrite may increase GC risk through the endogenous formation of carcinogenic NOC. Our results for dietary polyphenols and GC risk are novel and require confirmation in future studies.
Acknowledgements
The authors thank Ms. Veronica Lopez for the logistic field coordination and Dr. Perez-Perez for the laboratory support to determine the H. pylori status.




