Aquatic macrophyte growth season in Central and Northern European Union and the implications for aquatic macrophyte risk assessments for herbicides
Abstract
Under current European Union regulation, the risks to aquatic organisms must be assessed for uses of plant protection products (PPPs) that may result in exposure to the environment. For herbicidal PPPs, aquatic macrophytes are often the most sensitive taxa. For some herbicidal modes of action, macrophytes may be affected only while they are actively growing. For the risk assessment, it is therefore useful to know whether application timings would result in surface water exposure during periods when aquatic macrophytes are actively growing (therefore potentially resulting in effects). Toxicity endpoints, which are based on studies with active growth, may be overconservative in cases where exposure of PPPs will not co-occur with active macrophyte growth. A comprehensive literature search was performed, using systematic and manual approaches, with the aim of identifying the main active growth period for macrophytes in natural freshwater bodies in climates relevant to the Central and Northern zones of the European Union. The results of the searches were screened initially to identify all potentially relevant references, for which a full evaluation was then performed. Reliability was assessed using the principles of the Klimisch scoring system. As part of the full evaluation, growth periods were identified for each macrophyte species studied. Finally, the extracted growth periods were considered together to determine an overall active growth period for aquatic macrophytes representative of the Central and Northern EU zones. Based on this literature review, the active growth period identified for most aquatic macrophyte species representative of the Central and Northern EU zones is April to September. Relating to the regulatory implication of these results, it may be possible to conclude a low risk for aquatic macrophytes if the predicted surface water exposure period for certain PPPs is demonstrated to be outside the periods of active growth. Integr Environ Assess Manag 2024;20:1125–1139. © 2023 The Authors. Integrated Environmental Assessment and Management published by Wiley Periodicals LLC on behalf of Society of Environmental Toxicology & Chemistry (SETAC).
INTRODUCTION
Plant protection products (PPPs) and their metabolites can enter surface waters through surface runoff, soil drain-flow, and spray drift following application to crops, and can affect aquatic organisms, including aquatic macrophytes. In the European Union (EU), the risk to aquatic macrophytes from exposure to PPPs must be assessed under Regulation (EC) 1107/2009 (EC, 2009) before a product can be authorized. Often, aquatic macrophytes are the most sensitive aquatic organism group for PPPs with herbicidal modes of action (MoAs) due to the targeting of processes in primary producers. This is reflected in the data requirement to test aquatic macrophytes for herbicide submissions in the European Union (Arie Vonk & Kraak, 2020). However, for herbicides with certain MoAs, the target is often proteins involved directly in plant growth. For example, acetolactate synthase (ALS) inhibitors (e.g., sulfonylureas) target the protein ALS, disrupting how it functions. This is a key enzyme involved in the biosynthesis of branched-chain amino acids in plants (Zhou et al., 2007). Therefore, when exposed to these chemicals, effects on aquatic macrophytes are only observed when macrophytes are actively growing (Li et al., 2022).
The standard toxicity studies of macrophytes for risk assessment (e.g., Organisation for Economic Co-operation and Development [OECD], 2006, 2014) are performed under conditions where macrophytes are actively growing to maximize the detection of effects. This is the case for laboratory studies (where conditions are artificially maintained) and is also most often the case for higher tier, outdoor mesocosm studies, which are performed in spring and/or summer. Therefore, the current testing and risk assessment approach may be overly conservative for substances with reversible effects that only affect actively growing macrophytes and where exposure to aquatic macrophytes occurs mainly outside the growing period. For the purpose of a risk assessment, this will always require support of exposure modeling or data to determine when exposure of macrophytes is likely to occur. Professional experience of exposure modeling performed for regulatory submissions of herbicides has demonstrated that, depending on the sorption and degradation behavior, the critical surface water exposure events of some herbicidal substances may occur outside the growth season even if the herbicide is applied during the growth season, for example, due to drainage events in winter (FOCUS, 2001, 2015). Supporting Information: Appendix D provides an illustrative example.
The aim of this work was to review the available literature to identify the main active growth period of aquatic macrophytes in natural freshwater bodies, with particular focus on determining the start of the growth period, being most relevant to spring applications of herbicides. In the context of the aquatic risk assessment framework for PPPs, the present review focused on freshwater bodies in climates relevant to the Central zone (CZ) and the Northern zone (NZ) of the European Union (CZ and NZ EU), as defined in Annex I of Regulation (EC) 1107/2009 (EC, 2009).
METHODS
Available literature data were used to answer the research question. No tests were performed as part of this work. To achieve this, a definition of “aquatic macrophytes” was chosen and a list of search terms was compiled; a systematic literature review was performed using text mining to identify potentially relevant literature sources; the literature search results were screened using set criteria for relevance; for those articles considered relevant, full reviews were performed and a reliability score was assigned; and finally, for those studies considered relevant and reliable, the data were evaluated to determine the main growth period for freshwater macrophytes in climate zones representative of CZ and NZ EU. Full details of the methods are provided as follows.
Systematic literature search
- (a)
Emergent (i.e., rooted to substrate with at least some plant structures emergent from the water);
- (b)
Floating leaved (i.e., rooted to the substrate with leaves floating on water surface);
- (c)
Submerged (i.e., no emergent plant structures; excludes reproductive organs that may emerge from the water, e.g., floating);
- (d)
Free-floating (i.e., not rooted to the substrate).
They were also identified taxonomically as being either monocotyledonous or dicotyledonous, with the exception of Ceratophyllum demersum of the primitive order Ceratophyllales. All four life-form groupings and three taxonomic groups were considered relevant to the scope of this review.
“Aquatic plant” OR macrophyte OR Pondweed OR “Pond Weed” OR “Aquatic Vascular Plant” OR “Freshwater plant” OR “Fresh water plant” OR “aquatic vegetation” OR “aquatic weed” OR “waterweed” OR “floating plant” OR “submerged plant” OR “submersed plant” OR “emerged plant” OR “Potamogeton” OR “Nymphaea” OR “Nuphar” OR “Myriophyllum” OR “Ceratophyllum” OR “Lemna” OR “Ranunculus” OR “Chara” OR “Hottonia” OR “Hydrocharis” OR “Alisma”
Excluding gray literature (n = 704), the search strategy highlighted 122 553 documents of potential interest to this literature review.
After this search, a text mining exercise was performed as follows: customized gazetteer (lookup) lists were created, which contained both the generic terms such as “aquatic plant” as well as common and scientific names at the species level, for example, pondweed and Potamogeton crispus. In total, the gazetteer list of terms describing aquatic macrophytes contained 356 entities (see Supporting Information: Appendix A). To identify which of the 122 553 records mentioned the aspects of interest to this particular project, a purpose-built text mining application was applied to each document in turn. The software used to build and execute the pipeline was General Architecture for Text Engineering (GATE), an open-source software toolkit written in Java (Cunningham et al., 2013). Only those documents that specifically mentioned a habitat, a growth term, and a temporal term, together with a mention of an aquatic macrophyte, were identified as a positive result.
The text mining application (which was built for the exercise) comprises several different steps, each performing a different function in the application. Generally, the approach taken was: (1) to tokenize (identify individual words and features) and sentence split the documents; (2) to use the gazetteer lists to identify any important keywords and phrases such as the habitat in question; to identify the document's title and abstract; (3) to search in the title and abstract for patterns matching the natural language expressions describing the time of growth of aquatic macrophytes in a specified habitat; and (4) to index the results.
As a result of applying the text mining pipeline to the document corpus, 169 peer-reviewed journal articles were identified as being of potential relevance to the project, and these were summarized in an Excel spreadsheet. For the gray literature review, 704 documents were downloaded and processed from Google Scholar, and 41 of those were identified as being potentially relevant using the text mining pipeline (after the duplicates were removed). Therefore, 210 (i.e., 169 + 41) references were identified as being potentially relevant.
Manual search
An ad hoc manual search was also done to identify any further useful articles which may have been overlooked by the systematic search. Various relevant terms were entered manually into Google Scholar, for example, “aquatic plant growth season” or “macrophyte growth period.” Also, some sources were referenced in the body of another study, and thus included in the search. In total, six additional references were identified.
Screening
-
Is the study of freshwater aquatic macrophytes?
Only freshwater species were considered relevant. If the study had been performed in a natural freshwater system, then it was assumed that the macrophyte species found therein were freshwater aquatic macrophytes.
-
Could the study contain data on seasonal growth?
Only references presenting data on macrophyte growth (e.g., biomass, percentage cover) over time were considered relevant. Studies only reporting consolidated seasonal or annual growth were not considered relevant.
-
Did sampling occur over a full year to allow determination of the start and end of the growth period?
References containing data on macrophyte growth across the whole year, or at least the start of the year, were preferred to determine the start of the growth period. However, exceptions were made where references presented data that allowed inference of the start of the growth period, even if data had not been collected during this time, for example, if growth data collected until November indicated zero biomass, and sampling began again in April and also indicated zero biomass, it would suggest that no growth occurred between November and April. Where such articles were fully evaluated, they were allocated a reliability score of 3 to indicate the higher level of uncertainty in such extrapolations (see the following for discussion regarding reliability assessment).
-
Was the study undertaken in a region climatically relevant to CZ and NZ EU?
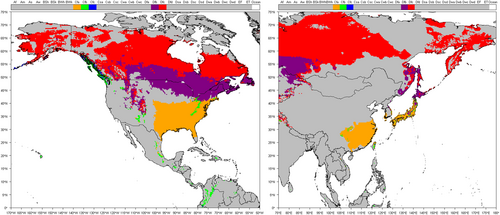
Here, the CZ and NZ are defined according to the zonal classification used in the evaluation of pesticides in the EU (Regulation [EU] 1107/2009). Any studies performed in these zones were considered relevant to the purpose of the review with no further consideration. Studies performed outside these zones compared the location's climate with NZ and CZ climates. For this purpose, the Köppen–Geiger classification was chosen (Köppen & Geiger, 1936). The Köppen–Geiger classification system is available in a digital format with the classifications updated using temperature and precipitation data for 1951–2000 (Climate and Infectious Diseases Group, 2019). The Köppen–Geiger classification is considered suitable because of the level of granularity it uses and because it was used as the partial basis for the European and Mediterranean Plant Protection Organization (EPPO) climate classifications that are used to identify comparable regions for efficacy studies on a European (European and Mediterranean Plant Protection Organization [EPPO], 2014) and global (EPPO, 2010) scale. Both are part of EPPO Standards PP1 Efficacy evaluation of plant protection products of which standards 1/181 and 1/152 are used to define “good experimental practice” under Regulation EC 1107/2009. Köppen–Geiger is therefore considered a suitably harmonized system.
The relevant Köppen–Geiger system classification for CZ and NZ (as illustrated in Figure 1) is detailed in Table 1. Figure 2 presents a map showing the regions relevant to the assessment of aquatic macrophyte growth seasons whose climates are comparable with CZ and NZ conditions. Some areas of the southern hemisphere have a climate comparable with CZ or NZ, such as New Zealand (Cfb), southeast Australia (Cfa, Cfb), and the Pampas region of South America (Cfa, Cfb). However, the southern hemisphere latitudes were excluded, primarily because of differences in the timing of the seasons and thus when aquatic macrophytes will be actively growing. These regions could also be excluded because of differences in the species present.
This approach aimed to identify the broad range of climates that can be considered comparable with CZ and NZ conditions. It should be noted that the herbicidal PPPs of concern (i.e., those with a MoA affecting actively growing macrophytes) may not be used in all areas of these climatic zones. However, the main purpose of the rapid screening phase was the identification of aquatic macrophyte growth data based on literature studies conducted in areas with a climate comparable with CZ and NZ.
-
Reference available in English?
References that were not presented in English (n = 3) were not evaluated. These exclusions are not considered to significantly affect the conclusions.
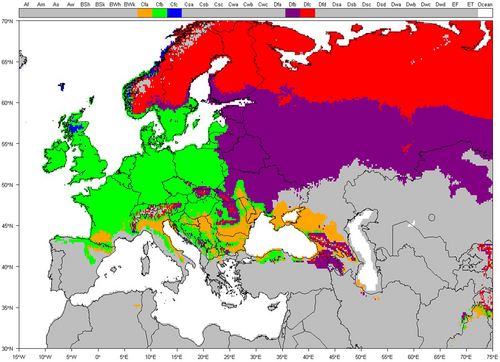
| Relevant to: | ||
|---|---|---|
| Climate zone (explanation of three-character code) | CZ | NZ |
| Cfa (warm temperate, fully humid, hot summer) | Southern and eastern parts | None |
| Cfb (warm temperate, fully humid, warm summer) | Majority | Southern part |
| Cfc (warm temperate, fully humid, cool summer) | Limited: upland | None |
| Dfb (snow, fully humid, warm summer) | Limited: upland | Eastern and Central parts |
| Dfc (snow, fully humid, cool summer) | Limited: upland | Northern part |
Full evaluations
A full evaluation summary was produced for each reference that was considered relevant to the scope of this review after the screening step. Full evaluation summaries included information on the source of the study, the methods used, data collected, and overall conclusion regarding the growth period for the specific species studied. For the purpose of these evaluations, growth was classified as either “minor” or “major” growth. There was no standard definition of what constitutes “minor” and “major” growth; these were chosen using expert judgment and considering all growth data from a single study as a whole. This approach was chosen because studies used different methods to measure growth, and it was not possible to standardize these. For example, several studies used dry weight (biomass) as a measurement of growth, but the sampling of plant matter depended on the species chosen and the aim of the study. Other studies used different measurements of growth; for example, Farmer & Spence (1987) measured leaf production of Lobelia dortmanna L. Full details on how growth periods were chosen for each study are given in the Supporting Information: Appendix C (Summaries of fully evaluated references), but an example is also provided here.
In the study by Best and Visser (1987), seasonal growth measurements of the submerged macrophyte C. demersum L. were taken in a mesotrophic lake (in the Netherlands). Plant growth information was collected over three years (1978, 1979, and 1981). Different sampling methods were used: biomass as ash-free dry weight was measured by harvesting a plot bimonthly over the three years (reported as AFDW m−2); biomass for individual plants was reported by harvesting individual plants only in 1978 and 1981 (reported as AFDW plant−1); finally, plant density was measured by dividing the total weight of the standing crop by individual plant weights (reported as number of plants m−2). The results for each of these methods were presented numerically as well as graphically in the study report of Best and Visser (1987, p. 235).
For the review, biomass and density data were both considered in relation to where growth was occurring, and data from all available years were considered. For example, based on a standing crop, growth began between late March and end April—initially at a slow rate of growth (a shallow incline, i.e., minor growth) and increasing to major growth (a steeper incline) until a peak around September. After September, the biomass declined (assumed to be no growth of measured biomass). The other measurements were considered in a similar way. The available biomass per plant data suggested that the growth occurred between mid-August and mid-October and was slowest from January to mid-June. The available plant density data suggested that growth began from late March, declining from end-June/early July onward, with a second growth period from end October. These results were then combined to determine a single growth period for the species C. demersum: Based on this study, the start of the growth season for C. demersum is late March, with the end of the growth season being mid-October, although noting that some minor growth may occur into winter. This was then presented in a tabulated format to make the results easier to interpret (Table 2).
| Data source | Location | Northern (N)/Central (C) Zone EU | Species | Macrophyte type | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Best and Visser (1987) | Lake Vechten, The Netherlands | C & N | Ceratophyllum demersum | Submerged dicot | ||||||||||||||||||||||||
It is acknowledged that this method of classification is subjective. However, it is considered useful to distinguish between months where there was a low level of “minor” growth, compared with months where most rapid “major” growth occurred.
Reliability assessment
-
Test procedure in accordance with generally accepted scientific standards and described in sufficient detail—Reliability 1;
-
Study well documented, meets generally accepted scientific principles, acceptable for assessment—Reliability 2;
-
Significant methodological deficiencies or unsuitable test system—Reliability 3;
-
Secondary literature or documentation insufficient for assessment—Reliability 4.
RESULTS
Rapid screening
Of the 216 references that were screened for relevance, 25 (11.6%) were considered relevant, 188 (87%) were not considered relevant, and 3 (1.4%) were not included because they were not presented in English. Studies were mostly rejected at this stage either because the data were not suitable for determining seasonal growth patterns or because the location of the study was not climatically relevant to CZ or NZ EU. The reasons for rejecting each study were recorded, and this information is provided in Supporting Information: Appendix B.
Full evaluations
Of the 25 studies that were considered relevant and were fully evaluated, no study was given a reliability score of 1, 18 references were given a reliability score of 2, and seven were given a reliability score of 3. However, growth periods for those studies with a score of 3 were in general agreement with those with a score of 2 (see Table 3); therefore, all 25 references were used to draw overall conclusions. Summaries were prepared for these 25 studies using a standardized format. These summaries provide rationales for decisions on the relevance and reliability, as well as summarizing the methods and results, and finally presenting proposals for growth periods for the species in question. The summaries for all 25 studies are provided in Supporting Information: Appendix C.
| Data source | Reliability | Location | Waterbody type | Climatic classification | Relevant zone | Species | Species occurs in CZ/NZ?a | Life-form type |
|---|---|---|---|---|---|---|---|---|
| Wetzel and Howe (1999) | 2 | Natural wetland. Alabama, USA | Wetland | Cfa | C | Juncus effusus | Yes | Emergent monocot |
| Dawson (1976) | 2 | Bere Stream, UK | Stream | Cfb | C & N | Ranunculus penicillatus var. calcareus | Yes | Submerged dicot |
| Shamsudin and Sleigh (1995) | 2 | River Itchen, UK | River | Cfb | C & N | Ranunculus penicillatus var. calcareus | Yes | Submerged dicot |
| Brux et al. (1987) | 2 | Fen ditch, Brandgraben, Oldenburg, Germany | Ditch | Cfb | C & N | Potamogeton alpinus | Yes | Submerged monocot |
| Kunii and Maeda (1982) | 2 | Ojaga-ike pond, Japan | Pond | Cfa | C | Trapa natans | Yes | Floating leaved dicot |
| Kunii and Maeda (1982) | 2 | Ojaga-ike pond, Japan | Pond | Cfa | C | Hydrilla verticillata | Yes | Submerged monocot |
| Kelly et al. (1983) | 2 | Gryde River, NW Jutland, Denmark | River | Cfb | C & N | Natural community | Yes | Various |
| Kunii and Maeda (1982) | 2 | Ojaga-ike pond, Japan | Pond | Cfa | C | Potamogeton crispus | Yes | Floating leaved monocot |
| Haury and Aïdara (1999) | 2 | River Scorff, France | River | Cfb | C & N | Oenanthe crocata | Yes | Emergent dicot |
| Kunii and Maeda (1982) | 2 | Ojaga-ike pond, Japan | Pond | Cfa | C | Elodea nuttallii | Yes | Submerged monocot |
| Kunii and Maeda (1982) | 2 | Ojaga-ike pond, Japan | Pond | Cfa | C | Ceratophyllum demersum | Yes | Submerged Ceratophyllales |
| Kunii and Maeda (1982) | 2 | Ojaga-ike pond, Japan | Pond | Cfa | C | Myriophyllum spicatum | Yes | Submerged dicot |
| Haury and Aïdara (1999) | 2 | River Scorff, France | River | Cfb | C & N | Ranunculus penicillatus | Yes | Submerged dicot |
| Březinová and Vymazal (2015a) | 2 | Constructed wetlands in Číčenice and Mořina, Czech Republic | Wetland | Cfb | C & N | Phalaris arundinacea (reed canary grass) | Yes | Emergent monocot |
| Best and Visser (1987) | 2 | Lake Vechten, The Netherlands | Lake | Cfb | C & N | Ceratophyllum demersum | Yes | Submerged Ceratophyllales |
| Fair and Meeke (1983) | 2 | Ingle-Pingle Pool, UK | Pond | Cfb | C & N | Ceratophyllum demersum | Yes | Submerged Ceratophyllales |
| Kunii and Maeda (1982) | 2 | Ojaga-ike pond, Japan | Pond | Cfa | C | Vallisneria denseserrulata | No | Submerged monocot |
| Desmet et al. (2011) | 2 | River Aa, Belgium | River | Cfb | C & N | Various (natural community) | Yes | Various |
| Brock et al. (1989) | 2 | Wetland, Weerselo, The Netherlands | Wetland | Cfb | C & N | Hottonia palustris L. | Yes | Submerged dicot |
| Kunii and Maeda (1982) | 2 | Ojaga-ike pond, Japan | Pond | Cfa | C | Nelumbo nucifera | Yes | Floating leaved dicot |
| Madsen and Adams (1988) | 2 | Badfish Creek, Wisconsin, USA | Stream | Dfb | N | Potamogeton pectinatus | Yes | Submerged monocot |
| Rejmankova (1973) | 3 | Průhonice near Prague, Czech Republic | Pond | Cfb | C & N | Lemna minor | Yes | Unrooted floating monocot |
| Rejmankova (1973) | 3 | Průhonice near Prague, Czech Republic | Pond | Cfb | C & N | Lemna gibba | Yes | Unrooted floating monocot |
| Kunii (1988) | 3 | Ojaga-ike Pond, Chiba, Japan | Pond | Cfa | C | Trapa japonica | Unknown | Floating leaved dicot |
| Březinová and Vymazal (2015b) | 2 | Constructed wetlands in Číčenice, Czech Republic | Wetland | Cfb | C & N | Phalaris arundinacea (reed canary grass) | Yes | Emergent monocot |
| Sand-Jensen et al. (1989) | 2 | River Susa, Zealand, Denmark | River | Cfb | C & N | Potamogeton pectinatus | Yes | Submerged monocots |
| Sand-Jensen et al. (1989) | 2 | River Susa, Zealand, Denmark | River | Cfb | C & N | Sparganium emersum | Yes | Submerged monocots |
| Madsen and Adams (1988) | 2 | Badfish Creek, Wisconsin, USA | Stream | Dfb | N | Elodea canadensis | Yes | Submerged monocot |
| Van Dijk and Janse (1993) | 3 | Lake Veluwe, The Netherlands | Lake | Cfb | C & N | Potamogeton pectinatus L. | Yes | Submerged monocot |
| Liffen et al. (2011) | 3 | River Blackwater, Surry, UK | River | Cfb | C & N | Sparganium erectum | Yes | Emergent monocot |
| Smith et al. (1988) | 3 | Marshland in Wisconsin, USA | Marshland | Dfb | N | Typha latifolia L. | Yes | Emergent monocot |
| Farmer and Spence (1987) | 2 | Loch-na-Thuill, UK | Loch | Cfb | C & N | Lobelia dortmanna | Yes | Submerged dicot |
| Getsinger and Dillon (1984) | 2 | Lake Marion, USA | Lake | Cfa | C | Egeria densa | Yes | Submerged monocot |
| Jiang and Kadono (2001) | 2 | Kasai city, Hyogo prefecture, Japan | Pond | Cfa | C | Blyxa echinosperma | No | Submerged monocot |
| Hrivnák et al. (2009) | 3 | Streams in Danube basin, Slovakia | Stream | Cfb | C & N | Various amphiphytes | Yes | Various |
| Haury and Aïdara (1999) | 2 | River Scorff, France | River | Cfb | C & N | Apium nodiflorum | Yes | Emergent dicot |
| Haury and Aïdara (1999) | 2 | River Scorff, France | River | Cfb | C & N | Callitriche stagnalis | Yes | Floating leaved dicot |
| Jiang and Kadono (2001) | 2 | Kasai city, Hyogo prefecture, Japan | Pond | Cfa | C | Blyxa aubertii | No | Submerged monocot |
| Hrivnák et al. (2009) | 3 | Streams in Danube basin, Slovakia | Stream | Cfb | C & N | Various | Yes | Various |
| Hrivnák et al. (2009) | 3 | Streams in Danube basin, Slovakia | Stream | Cfb | C & N | Various hydrophytes | Yes | Various |
| Hrivnák et al. (2009) | 3 | Streams in Danube basin, Slovakia | Stream | Cfb | C & N | Various helophytes | Yes | Various |
| Hrivnák et al. (2009) | 3 | Streams in Danube basin, Slovakia | Stream | Cfb | C & N | Potamogeton nodosus | Yes | Floating leaved monocot |
| Hopson and Zimba (1993) | 3 | Lake Okeechobee Florida, USA | Lake | Cfa | C | Najas guadelupensis, Potamogeton illinoensis, Vallisneria Americana, Hydrilla verticillate | Naja. & Hyd.—Yes Pota. & Valli.—No |
Submerged monocots |
| Monthly growth periods for each data source | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data source | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | ||||||||||||
| Wetzel and Howe (1999) | ||||||||||||||||||||||||
| Dawson (1976) | ||||||||||||||||||||||||
| Shamsudin and Sleigh (1995) | ||||||||||||||||||||||||
| Brux et al. (1987) | ||||||||||||||||||||||||
| Kunii and Maeda (1982) | ||||||||||||||||||||||||
| Kunii and Maeda (1982) | ||||||||||||||||||||||||
| Kelly et al. (1983) | ||||||||||||||||||||||||
| Kunii and Maeda (1982) | ||||||||||||||||||||||||
| Haury and Aïdara (1999) | ||||||||||||||||||||||||
| Kunii and Maeda (1982) | ||||||||||||||||||||||||
| Kunii and Maeda (1982) | ||||||||||||||||||||||||
| Kunii and Maeda (1982) | ||||||||||||||||||||||||
| Haury and Aïdara (1999) | ||||||||||||||||||||||||
| Březinová and Vymazal (2015a) | ||||||||||||||||||||||||
| Best and Visser (1987) | ||||||||||||||||||||||||
| Fair and Meeke (1983) | ||||||||||||||||||||||||
| Kunii and Maeda (1982) | ||||||||||||||||||||||||
| Desmet et al. (2011) | ||||||||||||||||||||||||
| Brock et al. (1989) | ||||||||||||||||||||||||
| Kunii and Maeda (1982) | ||||||||||||||||||||||||
| Madsen and Adams (1988) | ||||||||||||||||||||||||
| Rejmankova (1973) | ||||||||||||||||||||||||
| Rejmankova (1973) | ||||||||||||||||||||||||
| Kunii (1988) | ||||||||||||||||||||||||
| Březinová and Vymazal (2015b) | ||||||||||||||||||||||||
| Sand-Jensen et al. (1989) | ||||||||||||||||||||||||
| Sand-Jensen et al. (1989) | ||||||||||||||||||||||||
| Madsen and Adams (1988) | ||||||||||||||||||||||||
| Van Dijk and Janse (1993) | ||||||||||||||||||||||||
| Liffen et al. (2011) | ||||||||||||||||||||||||
| Smith et al. (1988) | ||||||||||||||||||||||||
| Farmer and Spence (1987) | ||||||||||||||||||||||||
| Getsinger and Dillon (1984) | ||||||||||||||||||||||||
| Jiang and Kadono (2001) | ||||||||||||||||||||||||
| Hrivnák et al. (2009) | ||||||||||||||||||||||||
| Haury and Aïdara (1999) | ||||||||||||||||||||||||
| Haury and Aïdara (1999) | ||||||||||||||||||||||||
| Jiang and Kadono (2001) | ||||||||||||||||||||||||
| Hrivnák et al. (2009) | ||||||||||||||||||||||||
| Hrivnák et al. (2009) | ||||||||||||||||||||||||
| Hrivnák et al. (2009) | ||||||||||||||||||||||||
| Hrivnák et al. (2009) | ||||||||||||||||||||||||
| Hopson and Zimba (1993) | ||||||||||||||||||||||||
- Note: Growth periods sorted in order of earliest date for significant growth being observed (pale gray cells = minor growth, dark gray cells = significant growth, empty cells = no growth).
- a Positive occurrence of study species in Central and/or Northern zones of European Union (CZ/NZ) concluded if study conducted on natural assemblages of aquatic macrophytes at a location in CZ/NZ, or, if study performed outside CZ/NZ, species presence recorded in CZ/NZ (Board of Trustees of the Royal Botanic Gardens, Kew, 2019).
From the 25 references, 43 individual growth periods were extracted. These are summarized in Table 3. The 43 growth periods were associated with different climatic classifications, waterbody types, categories of macrophytes, geographical locations, and sampling techniques.
Start of the growth period
Table 4 provides a condensed view of the growth periods shown in Table 3. It reveals that the main growth period for most aquatic macrophytes begins in April, because the largest number of major growth seasons commencing was observed in April (n = 16 or 37.2% of total growth periods), with 11 commencing in early April and five in mid-April. Only eight instances of major growth (18.6% of growth seasons) were observed in March. There were four growth periods with major growth in early March, with a further four commencing in mid-March. There is some evidence of minor macrophyte growth occurring in the first quarter of the year, with 10 growth periods indicating minor growth between January and April. However, none of the 43 growth periods collated indicated that any major macrophyte growth occurs before March. It was less common for major growth periods to begin in later months, with seven beginning in early to mid-May, four in early June (none in mid-June), and seven in early July (none in mid-July). Only one study observed major growth commencing after early July, with a second major growth period occurring in one species in November–December (Egeria densa; Getsinger & Dillon, 1984).
| Category | na | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage of growth periods where major growth beganb | |||||||||||||
| Monocotsc | 21 | 0 | 0 | 19 | 29 | 33 | 10 | 10 | 0 | 0 | 0 | 0 | 0 |
| Dicots | 12 | 0 | 0 | 25 | 50 | 0 | 8 | 17 | 0 | 0 | 0 | 0 | 0 |
| Ceratophyllales | 3 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Variousd | 6 | 0 | 0 | 17 | 17 | 0 | 17 | 50 | 0 | 0 | 0 | 0 | 0 |
| Percentage of growth periods where major growth endedb | |||||||||||||
| Monocotsc | 22 | 0 | 0 | 0 | 0 | 5 | 0 | 29 | 24 | 33 | 5 | 0 | 5 |
| Dicots | 12 | 0 | 0 | 0 | 0 | 0 | 8 | 33 | 8 | 33 | 17 | 0 | 0 |
| Ceratophyllales | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 0 | 67 | 0 | 0 |
| Variousd | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 83 | 0 | 0 | 0 |
| Percentage of major growth, observed over time | |||||||||||||
| Monocotsc | 22 | 0 | 0 | 19 | 48 | 76 | 86 | 67 | 43 | 10 | 5 | 5 | 0 |
| Dicots | 12 | 0 | 0 | 25 | 75 | 75 | 75 | 59 | 51 | 18 | 0 | 0 | 0 |
| Ceratophyllales | 3 | 0 | 0 | 0 | 100 | 100 | 100 | 100 | 67 | 67 | 0 | 0 | 0 |
| Variousd | 6 | 0 | 0 | 17 | 34 | 34 | 51 | 100 | 84 | 0 | 0 | 0 | 0 |
- a n, number of observations. Total = 42, not including Hopson and Zimba (1993).
- b Major growth as defined in Table 3. Beginning of major growth = first month where major growth occurred. End of major growth = last month where major growth occurred.
- c It must be noted that the growth period from Hopson and Zimba (1993) is not included in this table because no growth was considered “major.”
- d Growth periods where macrophytes were not differentiated by species.
End of the growth period
The main growth period for most aquatic macrophytes ends in September, as most of the major growth seasons ending were observed in September (n = 16 or 37.2% of total growth periods). The earliest that the major growth period ended was the end of May (n = 1). In later months, only one growth period ended in June, 11 ended in July (noting that in one instance a second growth period occurred later in the year) and eight ended in August. Only seven of the growth periods (16.2%) collated indicated that any major macrophyte growth occurs between October and December, with four indicating major growth until mid-October, one until end of October, and one with a second major growth period occurring in November–December. A single growth period indicated minor growth continuously between mid-July and December, and six indicated minor growth occurring in at least one month between September and December. Although plant matter may have been present after September, the growth occurring was minor and therefore this overwintering material would be dormant and would not be at risk from effects of herbicides that act on actively growing plants such as ALS-inhibitors.
DISCUSSION
Effect of climate classification on growth period
The 43 growth periods were collected from locations with several different climatic classifications. Fourteen of the growth periods were classified as Cfa (see Table 1 for definition), which is relevant to southeastern areas of the CZ. Twenty-six periods were classified as Cfb, which is relevant to most of the CZ and parts of the NZ. Finally, three growth periods were classified as Dfb, which is relevant to parts of the NZ. Based on the consolidated growth seasons in Table 3, there does not appear to be any consistent difference in the main start of the growth season between studies performed in Cfa and Cfb climates. This is demonstrated more clearly in Table 4, where the percentage of growth periods that began and ended in each month have been extracted. There is some suggestion that major growth periods begin later in Dfb climates (from mid-April) than Cfa and Cfb climates (from early March). This might be expected as Dfb climates have colder winters, which often feature ice and snow. Temperatures are thus expected to increase later in the season, so aquatic macrophytes may start growing later in spring. This contrasts with Cfa and Cfb climates. However, it is considered that there are only three growth periods for Dfb climates and thus additional data would be required to conclude this pattern with any confidence. Although there was no correlation between climate classification and growth season timing, this may be the result of variability in local climate conditions experienced by macrophytes during the sampling periods. Therefore, more detailed analysis of the influence of local temperature, light levels, and so forth, may uncover patterns in the abiotic factors that are influencing the start (and end) of aquatic macrophyte growth seasons that cannot be observed at the macroscale of climate classification.
Finally, it is noted that weather patterns are not static, and that there is the possibility of growth periods shifting as a result of climate change. However, the effects of climate change were not considered part of this review.
Effect of waterbody type on growth period
The aquatic macrophyte growth data were collected from macrophytes growing in different types of waterbodies; 14 were associated with ponds, 18 with streams and/or rivers, one with ditches, and 10 with other waterbody types (lakes, lochs, wetlands, marshlands). Based on the consolidated growth seasons in Table 3, there does not appear to be any consistent difference in the main start of the growth season for macrophytes growing in different waterbody types.
Effect of macrophyte category on the growth period
The 43 growth periods extracted were associated with at least 31 aquatic macrophyte species, although it is noted that macrophytes were not always identified to species level in the studies. Representatives of dicot (n = 12), monocot (n = 22), and Ceratophyllales (three) species were included. All the previously defined life-forms were also represented, with growth seasons for nine emergent (rooted), seven floating leaved (rooted), 22 submerged (rooted), and two free-floating (unrooted) aquatic macrophytes being available. Based on the consolidated growth seasons in Table 3, there does not appear to be any consistent difference in the main start of the growth season between monocots and dicots. This is more clearly demonstrated in Table 4, where percentages of growth period start and growth period end are presented. There also does not appear to be a consistent impact of species type on growth periods. In some cases, such as for Lemna gibba and Lemna minor, growth periods of similar species were similar or identical. In other cases, such as for species of the genus Potamogeton, growth periods demonstrated some differences in their ranges. It is difficult to analyze these data closely at the species level, because there are too few studies with identical species, and several studies where aquatic macrophytes were not identified to species level. Generally, the pattern of growth is similar across species—with some minor growth occurring at the start and end of the major growth period, the major growth period occurring primarily during the Spring, Summer, and autumn months, and no growth occurring outside this. There are a couple of exceptions to this, with Getsinger and Dillon (1984) reporting an unusual two months of major growth for E. densa in November and December. This may have been caused by the relatively mild winters of the climate classification Cfa, although unfortunately no temperature data were reported, so this cannot be confirmed.
Effect of the geographical location on the growth period
The studies from which the growth periods were extracted were performed both within (n = 22) and outside (n = 21) the CZ/NZs. The species for which growth was monitored in studies performed in the CZ/NZ were considered relevant species because these studies were all performed on naturally occurring assemblages of aquatic macrophytes. For those studies performed outside the CZ/NZ (e.g., Japan, USA, etc.), most of the species being monitored also occur within the CZ/NZ (see Table 3). Only five of these species are not found in CZ/NZ: Vallisneria denseserrulata (Kunii & Maeda, 1982), Blyxa echinosperma (Jiang & Kadono, 2001), Blyxa aubertii (Jiang & Kadono, 2001), Potamogeton illinoensis (Hopson & Zimba, 1993), and Vallisneria americana (Hopson & Zimba, 1993). The start of the major growth periods for the species range from early April to early July, and thus there is no consistent difference in the start of the main growth season in species that do not occur in the CZ/NZ compared with those that do. Although these particular species do not occur (or it is unknown whether they occur) in the CZ/NZ, some have closely related species that do occur in the CZ/NZ. Vallisneria denseserrulata occurs only in China and Japan, but V. spiralis is found in the CZ. Potamogeton illinoensis occurs only in America, whereas P. pectinatus (aka Stuckenia pectinata) is found all over the world, including in the CZ/NZ. Trapa japonica (Kunii, 1988) is not native to the CZ/NZ, but its close relative, T. natans, is found widely across the CZ/NZ. Blyxa echinosperma and B. aubertii are not found in the CZ/NZ, and no other Blyxa species occur either, being restricted to tropical and subtropical regions. Therefore, of the 43 growth periods extracted, only five are not for species that occur in the CZ/NZ, but of those, only two do not have close relatives that do occur in the region. However, even those (B. echinosperma and B. aubertii) were from studies in locations of climatic relevance to CZ/NZ, and thus may still act as representatives of species that do occur in the region. Therefore, it is considered that the species for which the growth periods have been extracted can be considered representative of those species occurring in the CZ/NZ.
Effect of sampling techniques on the growth period
The studies from which the growth periods have been extracted have been performed using a variety of sampling methods and are based on a range of growth metrics, such as area coverage, biomass, abundance, and so forth. Therefore, there is an element of variability in the extracted growth periods due to the differences in the methods by which the data have been collected and the parameters being measured. However, there is no apparent consistent influence on the extracted growth period in terms of the sampling method or the growth metric used. For example, major growth periods based on biomass measurements start as early as early March and as late as mid-May. Therefore, although several different methods and growth metrics have been used in the studies, the overall pattern in start and end of growth is relatively consistent.
Based on the data available, no clear causal factors for determining the main start of the growth periods of aquatic macrophytes can be identified; as such, all growth seasons from studies that were fully evaluated were considered together for the purpose of concluding an overall growth period.
Biological relevance of minor growth and effects on plant physiology
It is noted that further consideration of the ecological relevance of minor growth occurring outside the major growth period may be required for a regulatory submission. For example, if minor growth of a species occurred at a time when seeds or turions and/or hibernacula were being produced, this could theoretically affect population performance if this production were affected. Effects on plant physiology relating to overwintering organs or reproduction were not considered in the scope of this review, which only focused on studies measuring plant growth. The current standard risk assessment scheme for the effects of pesticides on aquatic plants also focuses on plant growth (within-season) as the relevant measured endpoint. Concerns over the potential impact of “carry-over” effects on populations would need to be considered on a case-by-case basis (but could involve specific testing or effects modeling, for example).
CONCLUSION
The 43 collated growth periods from 25 relevant publications provide a strong basis to conclude that general trends in growth periods of aquatic macrophytes in Central and Northern Europe (and areas with similar climates) are consistent.
Regulatory implications
The results of this review have implications for the risk assessment and authorization of PPPs whose reversible effects only affect macrophytes when they are actively growing. In pesticide active substances or product dossiers submitted for authorization, it may be possible to conclude a low risk for aquatic macrophytes if the predicted surface water exposure period for these PPPs is demonstrated to be outside the periods of active growth. This would require the support of exposure modeling or data to demonstrate when the aquatic macrophyte exposure period realistically occurs for a particular proposed use pattern.
This literature review supports a growth period for aquatic macrophytes in CZ and NZ EU beginning in April and ending in September. This period represents most species reviewed, and most growth that occurred for each species.
AUTHOR CONTRIBUTION
Helena Crosland: Data curation (equal); formal analysis (equal); writing—original draft preparation (lead); writing—review and editing (equal). Amy Brooks: Data curation (equal); formal analysis (equal); writing—original draft preparation (supporting); supervision (lead). Michael Hackett: Data curation (equal); formal analysis (equal); writing—original draft preparation (supporting). Johannes Witt: Conceptualization (supporting); writing—review and editing (equal); Thomas G. Preuss: Conceptualization (lead); writing—review and editing (equal).
ACKNOWLEDGMENT
The authors declare the following financial interests/personal relationships that may be considered potential competing interests: The research was funded by Bayer AG, and some of the listed authors are employed by Bayer AG. Bayer produces and sells agrochemicals. Thanks to S. Brewer at Text Mining Solutions for delivering the literature search results.
Open Research
DATA AVAILABILITY STATEMENT
All data used in this manuscript have been taken from published literature. All information used for this review was sourced from peer-reviewed journals via a data mining exercise. The peer-reviewed citations were sourced via PubMed, Toxnet (NLM), Science Direct, Springer Link, and Wiley Online Library. All citations for the articles used are provided in the reference list. To access data, please contact the relevant authors for the specific study.




